S51.4: Conditional host responses to Cuckoo Cuculus canorus parasitism
Ingar Jostein Øien1, Arne Moksnes3, Eivin Røskaft2, Erik Edvardsen3, Marcel Honza4, Oddmund Kleven3 & Geir Rudolfsen3
1Norwegian Ornithological Society, Seminarplassen 5, N-7060 Klæbu, Norway, fax 47 73591309, e-mail Eivin.roskaft@nina.nina.no; 2Norwegian Institute for Nature Research, Tungasletta 2, N-7005 Trondheim, Norway; 3Department of Zoology, Norwegian University of Science and Technology, NTNU, N-7034 Trondheim, Norway; 4Institute of Landscape Ecology, Academy of Sciences of the Czech Republic, Kvetná,Czech Republic
Øien, I.J., Moksnes, A., Røskaft, E., Edvardsen, E., Honza, M., Kleven, O. & Rudolfsen, G. 1999. Conditional host responses to Cuckoo Cuculus canorus parasitism. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 3125-3145. Johannesburg: BirdLife South Africa.Avian brood parasites that lay their eggs in the nest of other species are subject to a variety of defence mechanisms from the parasitised hosts in order to reject the egg. The rejection response or the lack of it has been described as a fixed genetic response to parasitism. However, several studies have proved a different response to the parasitic egg according to variation in the circumstances of parasitism.
Experimentally parasitised hosts of the Cuckoo Cuculus canorus are known to reject the foreign egg more frequently when they have seen a Cuckoo female near the nest, and the rejection rate may also vary with the degree of Cuckoo egg mimicry. Apart from these variables, very sparse data exist on conditional host responses to Cuckoo parasitism. Given this background we have investigated the degree of conditional responses to Cuckoo parasitism among parasitised Acrocephalus warblers in Middle Europe.
Nests of Reed Warblers Acrocephalus scirpaceus, Great Reed Warblers A. arundinaceus, Marsh Warblers A. palustris and Sedge Warblers A. shoenobaenus were investigated for conditional responses to parasitism. These responses were related to; (1) host nesting density, (2) Cuckoo density, (3) time of parasitism in the host's egg laying cycle, (4) timing of parasitism in the breeding season, (5) clutch size reduction by the Cuckoo, and (6) Cuckoo egg mimicry with the host's egg. Most of these variables affect the rejection response of the host. The importance of conditional responses towards Cuckoo parasitism is discussed.
INTRODUCTION
When a species experience different environmental 'conditions' within it’s range, changes in the phenotype may occur as an adaptation to heterogeneous environments (phenotypic plasticity). Such plastic phenotypic changes may or may not be adaptive. Adaptive phenotypic responses to the environment are considered to evolve in populations that encounter predictable environmental changes if the genetic variability is sufficient (Via et al. 1995).
It is reasonable to assume that the success of brood parasites will vary both temporally and spatially according to several environmental factors. Øien et al. (1998) have suggested that it could be adaptive for hosts to modify their responses to parasitism according to variation in these factors. Experimentally parasitised hosts of the Cuckoo Cuculus canorus are for instance known to reject the foreign egg more frequently when they have seen a Cuckoo female near the nest (Davies & Brooke 1989a; Moksnes et al. 1993b). The rejection rates of Cuckoo hosts also vary with the degree of Cuckoo egg mimicry with the host's egg (Brooke & Davies 1988; Davies & Brooke 1988; Moksnes et al. 1990). In addition to these studies, a study on Rufous Bush Chat Cercotrichas galactotes (Alvarez 1995) and a study on Reed Warbler Acrocephalus scirpaceus populations in Britain exist on conditional host responses to Cuckoo parasitism (Lindholm 1999).
The individuals in many Cuckoo host populations show intermediate reactions towards the Cuckoo egg since both rejection and acceptance occurs (Davies & Brooke 1989a; Moksnes et al. 1990). Such populations may be at an equilibrium between acceptance and rejection, as a compromise between the cost of parasitism and the cost due to recognition errors (Lotem et al. 1995; Takasu 1998, 1999). On the other hand, Rothstein (1982a, 1990) suggested that the rejection behaviour has not yet mutated for those species that accept the parasitic egg.
The aim of this paper is to uncover the degree of variation in reactions to brood parasitism within and between host populations, and to discuss the importance of conditional responses of parasitised hosts.
Variation in responses among populations may be genetic, depending on the differences in selection pressure from Cuckoo parasitism and the amount of gene flow between the populations. Recent optimality models, however, indicate that the host responds in a way that gives the best pay-off in any particular circumstance of parasitism (Røskaft et al. 1990; Moksnes et al. 1993b; Øien et al. 1998; Røskaft & Moksnes 1998). The rejection rate of a potential host species may therefore vary both among populations, and among the individuals within a population. In this paper 'phenotypic plasticity' refer to the variation in response between populations, while 'conditional responses' refer to the variation in responses between individuals within a population.
In order to explain variation in rejection rates among populations according to this view, it is necessary to consider the variation in rejection rate among the individuals in the respective populations. It follows from the model (Fig. 1) that a parasitised individual that either rejects or accepts the Cuckoo egg do so depending upon the circumstances of parasitism. This variation reflects individual conditional responses which means that many individuals within a population are able to both accept and reject the parasitic egg under certain conditions.
The results of these conditional responses among individuals give different rejection rates among populations, and these rates may fluctuate according to the present environmental circumstances of parasitism in each population (phenotypic plasticity).
Hypotheses
There are several possibilities for conditional responses towards the Cuckoo egg. This indicates that the rejection of a Cuckoo egg may not necessarily be genetically fixed, but rather results from a sum of different conditional responses to the particular circumstances of parasitism and to the variations in the costs of rejection.
We propose the following two hypotheses:
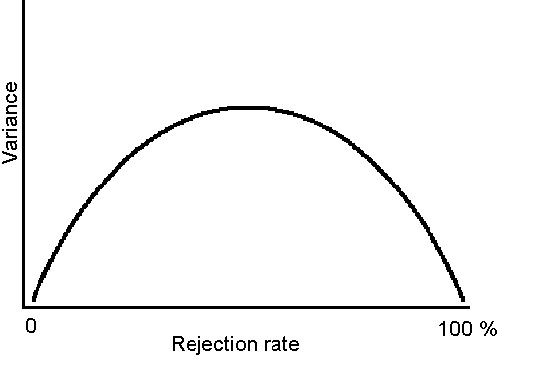
Because there is room for more flexible reactions among populations with medium rejection rates, we hypothesise (H 1) the variation in rejection rates towards a parasitic egg between these populations to be greatest (Fig. 1). Species with either 0 % or 100 % rejection rates have no variation between populations (their response is completely genetically fixed).
We hypothesise (H 2) to find conditional responses to be frequently observed among populations with medium rejection rates.
The aim of this study is to test Hypothesis 1 by comparing rejection rates of species where at least two different populations have been experimentally tested. Hypothesis 2 will be tested by use of field data from four Acrocephalus species in the Czech Republic.
MATERIAL AND METHODS Experimentally parasitised populationsIn Middle Norway, we supplied the rejection rates described by Moksnes et al. (1990) with some additional experiments. We introduced artificial non-mimetic Cuckoo eggs with the same weight and size as normal Cuckoo eggs into the nest in the host's laying period, and recorded whether the host accepted or rejected the parasitic egg. For those species where published data existed from another geographically site separated from our study area, we compared rejection rates between two or more areas for 17 potential Cuckoo host species in Europe (and one population in Japan).
The additional trials followed the procedure described in more detail by Moksnes and Røskaft (1988, 1989) and Moksnes et al. (1990), while the rejection rates from the other areas were in most cases those from Britain described by Davies and Brooke (1988). In all areas, the tested host species were sympatric with the Cuckoo (see Fig. 2 for references).
Out of the variation between the rejection rates and the mean rejection rates of each of the 17 potential Cuckoo host species, we calculated a rejection variance for each species. The rejection variance is defined as the variation (SD) in rejection rates of a species when at least two different populations have been tested (Fig. 2).
Naturally parasitised host populationsStudy area
The study of naturally Cuckoo-parasitised Acrocephalus populations was performed in Southern Moravia in the South-eastern part of the Czech Republic. The fieldwork was carried out during early May to middle July in the years 1992-1998.
Our study sites were near the villages of Luzice and Lednice (47o40'N, 16o48'E) about 40 and 70 km south of Brno. We searched for nests of Reed Warblers and Great Reed Warblers A. arundinaceus among the littoral vegetation surrounding several large fishponds. This vegetation consisted of Reed Beds Phragmites australis and Reedmace Typha angustifolia, which provided nesting sites for a dense population of Reed Warblers (see Moksnes et al. 1993a). Nests of Marsh Warbler A. palustris and Sedge Warblers A. shoenobaenus were found in nearby grass meadows and bush vegetation by observing the birds nest building activity or by occasional searches (for a detailed description of the field procedures, see Øien et al. 1998). Total numbers of nests found for each species in every year are shown in Fig. 3.
We recorded whether the nests were parasitised or not, and we further recorded the fate of the parasitised nest (accepted, or rejected by desertion or ejection). We assessed a Cuckoo egg as accepted if no rejection behaviour was performed by the sixth day after parasitism. For the parasitised nests, we collected data on the following circumstances of parasitism;
Host nesting density.-We used the number of host nests found in the area as an index of the breeding density of the host species. The field work was performed with approximately the same effort in the same geographically limited areas each year, and the field period stretched from the first nests was build in the spring until the last young fledged every season. Thus, the number of nests found gives a useful index of the breeding density of the hosts.
Density of the parasite.- The percentage of parasitised nests each year describes the hosts risk of being parasitised, and this parameter was used as an index of the density of Cuckoos in the study area. Another index of Cuckoo density is actually to use the number of Cuckoo eggs found each year. However, these two indexes correlated statistically significantly with each other (r=0.956, N=7, P=0.001). We therefore used parasitism rate as an index of Cuckoo density.
Sight of the parasite.- By video monitoring parasitised nests of the Reed Warblers only, we recorded whether the parasitised individuals were present near the nest during the act of parasitism and was able to detect the parasitism in this way. We assumed that individuals that detected the Cuckoo during egg laying performed distress calls (for a more detailed description; see Hagen 1996; Moksnes et al. MS).
Time of parasitism in egg laying cycle.- The time of parasitism in the host’s laying cycle was noted in many cases as many nests were visited every day during the egg laying of the host. When compared with the rejection rates, we pooled the first two days of egg laying with the day before the first host egg appeared (day 0–2 = early laying cycle). Likewise the days 3-6 of egg laying was pooled (day 3-6 = late laying cycle). Only a single Cuckoo egg was laid after clutch completion of the hosts.
Time of parasitism in season.- The days of parasitism throughout the breeding season were pooled in five 15-day periods between 1 May and 15 July.
Clutch size reduction by the Cuckoo.- In some cases of parasitism on Reed Warblers (N=14), the number of host eggs removed by the Cuckoo female during the procedure of parasitism was video-filmed (see Hagen 1996; Moksnes et al. MS). For the remaining parasitised nests, the number of eggs removed by the female Cuckoo was easily calculated, since the nests were checked every day, and the eggs marked in sequence as they were laid.
Cuckoo egg mimicry.- We assessed the degree of mimicry of the Cuckoo egg with the host eggs on a five-degree scale: (1) perfect mimicry, (2) good mimicry, (3) moderate mimicry, (4) poor mimicry and (5) non-mimetic (see Moksnes & Røskaft 1995, for more details).
We compared the rejection rates of the parasitised individuals according to the peculiarities of these parameters in each case of parasitism for the four species of Acrocephalus warblers investigated. All statistical tests were performed with SPSS for Windows (Version 8.0).
RESULTS AND DISCUSSIONExperimentally parasitised populations
Altogether 17 different species have been experimentally tested from two or three different geographical regions (mainly Norway and Great Britain). In those cases where more than two populations have been tested, we have in the following test used the two populations with the highest sample size. There is a statistically significant positive correlation between the rejection rates of the hosts in the different geographic regions (r=0.854, N=17, P=0.000, Fig. 4). The variance was greatest for those species with intermediate rejection rates (Fig. 2), however, more data is required before a firm conclusion can be made with regard to a critical test of Hypothesis 1.
The rejection rates for each species showed a significant correlation between the geographic areas, and we can therefore presume that each plot of a species represents a population that is in a similar stage of the evolutionary arms race with the Cuckoo.
According to Hypothesis 1, we expected the variation in rejection rates to be greatest in species that have rejection rates in the medium range (approximately 30-70%). Unfortunately, data on few species was available and the sample size (number of tested populations) is too tiny to create a correct picture. Although the few available data roughly fall within the hypothesised pattern, more data on geographic variation in rejection rates is needed before a complete test of this hypothesis is possible.
Naturally parasitised host populations
Density of parasites, hosts and the probability of seeing the Cuckoo at the nest
High densities of brood parasites increase the probability of being parasitised, and thus, the selection pressure on the host to reject the parasitic egg is increased.
Briskie et al. (1992) have found a much higher ejection rate among American Robins Turdus migratorius when sympatric with Brown-headed Cowbirds Molothrus ater than when allopatric. Similarly, Meadow Pipits Anthus pratensis and White Wagtails Motacilla alba on Iceland, where they are isolated from the Cuckoo, rejected non-mimetic model Cuckoo eggs significantly less frequent than the same species did in sympatry with the Cuckoo in Britain (Davies & Brooke 1989a). Soler and Møller (1990) also showed that Magpies Pica pica with no experience with the Great Spotted Cuckoo Clamator glandarius accepted all experimentally introduced cuckoo eggs.
Recently Lindholm (1994, 1999) found that Reed Warblers in Britain have a higher rejection rate of non-mimetic model Cuckoo eggs in areas where the Reed Warbler is parasitised by the Cuckoo compared to nearby areas where Reed Warbles are sympatric with the Cuckoo, but not parasitised. These results were by Lindholm, explained as phenotypic plasticity (the host has the ability to assess the risk of being parasitised by estimating the density of Cuckoos in the area). Soler et al. (1994) have shown that the rejection rate of the Magpie hosts in Spain was positively correlated with the density of Great Spotted Cuckoo. In this population rejection of parasitic eggs in naturally parasitised nests of the Magpie increased at a rate of 0.5% per year during the period 1982-1992, - a period with a steady increase in the Great Spotted Cuckoo population. Finally, Nakamura (1990) found an increase of about 80% in the rejection rate of Azure-winged Magpies Cyanopica cyana parasitised by the Cuckoo over a 30-year period in Japan, just after the parasite started to utilise this species as a host. However, these authors (Soler et al. 1994, Nakamura 1990) concluded that the rapid increase in host response to cuckoo eggs could either be due to a micro evolutionary change or to a conditional response.
The parasitised individuals may modify their rejection behaviour depending on their experience of contact with Cuckoos. It is shown that Cuckoo eggs are rejected more often when the hosts have observed the Cuckoo near their nests than when the egg laying has occurred without the sight of the Cuckoo (experimental evidence; Davies & Brooke 1988; Moksnes et al. 1993b, evidence under natural conditions; Hagen 1996; Moksnes et al. MS). Rejection rates of non-mimetic model Cuckoo eggs in a parasitised population of Reed Warblers in Britain increased when a stuffed Cuckoo was placed near the nest, but in unparasitised Reed Warbler populations nearby, no such increase in rejection rates was observed (Lindholm 1994, 1999).
The density of the host population in an area may influence the rate at which the host population is parasitised. Yellow Warblers Dendroica petechia nesting synchronously with conspecifics had proportionally lower incidence of parasitism than asynchronous nests, suggesting a 'dilution effect' on the probability of being parasitised by the cowbird (Clark & Robsertson 1979). Similarly, Martinez et al. (1996) showed that there was an effect of Magpie breeding density and synchrony on brood parasitism by Great Spotted Cuckoos. Thus, a high density of breeding hosts may incur that a parasite is more easily detected by some of the breeding birds and the probability that a parasitised individual will detect the parasitism, and reject the egg because of alarming neighbours increases.
Density of the parasite.
There was no significant correlation between the rejection rate and the rate of parasitism for any of the species when all seven breeding years were used (1992-1998; Great Reed Warbler; r=0.634, N=6, P=0.176, Reed Warbler; r=0.390, N=7, P=0.387, Marsh Warbler; r=0.819, N=5, P=0.090, Sedge Warbler, r= ¸ 0.113, N=5, P=0.856). A partial correlation analysis between rejection rate and rate of parasitism was, however, statistically significant when controlled for species (rp= 0.458, N=27, P=0.013).
Sight of the parasite.
At 10 out of 14 parasitised Reed Warbler nest which were video-monitored, the host was close to the nest and performed alarm calls against the Cuckoo during the course of parasitism. In the remaining 4 cases, the Cuckoo female was undetected by the hosts during the act of parasitism (i.e. no alarm calls were recorded). In 5 out of the 10 nests where the Cuckoo was discovered at the nest by the host, the parasitic egg was rejected (Fishers exact probability test P=0.126) (data from Hagen 1996, Moksnes et al. MS). The Cuckoo egg was accepted in all the four cases where the Cuckoo remained undetected by the host.
Host nesting density.
The number of host nests was significantly higher in 1996 than in the other years (c 2=21.35, DF=9, P=0.011, Fig. 3). There was no statistically significant correlation between the rejection rate and available number of host nests (year) for any of the species (Great Reed Warbler; r=0.493, N=6, P=0.320, Reed Warbler; r=0.019, N=7, P=0.968, Marsh Warbler; r=0.191, N=5, P=0.758, Sedge Warbler, r=0.723, N=5, P=0.167). However, a partial correlation analysis between rejection rates and number of available host nests was statistically significant, when controlling for species (rp= ¸ 0.543, N=27, P=0.002), although in the opposite direction of what expected.
The rejection rates for the four Acrocephalus warbler populations in Southern Moravia did not show any positive correlation with their own population density in the study area. From a high density of potential hosts one should expect that individuals could share the information about Cuckoos, as well as a dilution effect on the risk of parasitism for each individual. This may explain why the rejection rate did not increase with increasing population density for any of the Czech Acrocephalus populations, and simply indicates that the rejection rate is more dependent upon the risk of being parasitised than of shared information.
Accordingly, there was a significant correlation between an increased risk of being parasitised (observed parasitism rate) and an increase in the rejection rate for the four study populations, when controlled for species. This result is in accordance with the experimental findings of Lindholm (1994) that rejection rates of non-mimetic model Cuckoo eggs was lower in Reed Warbler populations sympatric with Cuckoos with no history of Cuckoo parasitism, than in parasitised Reed Warbler populations.
Thus it seems likely that Cuckoo host populations modify their rejection behaviour depending on their experience of contact with Cuckoos. Our study confirms such alternation of host responses from one year to another within the same study area, although only for the pooled data. It is therefore, unlikely that genetic changes within the population may account for such rapid alternations. The warblers, in one way or another, are most likely able to assess the density of Cuckoos around their territories, probably because the Cuckoo activity in the surroundings of their nests is evident.
The phenotypic flexibility in host responses to parasitism is well expressed by the difference in rejection rate of hosts that have observed the female Cuckoo during the egg-laying process and those that have not. The sight of the Cuckoo near the nest seems to be a central cue to increase the likelihood of rejection. Our results from the Czech Reed Warblers is in accordance with the results from experiments where artificially parasitised Meadow Pipits (Moksnes & Røskaft 1989) and Reed Warblers (Davies & Brooke 1988) has been exposed to stuffed Cuckoos. Lindholm (1999) found that artificially parasitised Reed Warblers in areas where the risk of parasitism is low, failed to increase the rejection rate after being exposed to a Cuckoo mount.
Our study show that rejection rates of naturally parasitised Reed Warblers in an area where the risk of parasitism is a reality reject the Cuckoo egg at a higher rate when they have observed the Cuckoo female at their nest. However, 50% of the hosts that detected the Cuckoo female during its egg laying and made alarming signals still accepted the parasitic egg. Also among Brown-headed Cowbird hosts, such as the Least Flycatcher Epidomax minimus, well-defended nests are parasitised but the egg is still accepted (Robertson & Norman 1977). The hosts of Brown-headed Cowbirds, however, experience much lower costs by acceptance than do Cuckoo hosts. This because the cowbird young is reared together with the hosts own young. The individual Cuckoo hosts which detect the parasite but still accepts may be younger birds which are unable to unveil the intentions of the parasite due to lack of experience.
Another explanation which is advocated by Lotem et al. (1995), is that the costs due to recognition errors and the risk of damaging own eggs during ejection among young birds may outweigh the cost of rearing the Cuckoo chick. We have, however, investigated the costs of the different responses to Cuckoo eggs among this Reed Warbler population and found that acceptance always incur much higher costs than rejection (Øien et al. 1998).
Hosts age and time of parasitism in season
It is difficult to separate the effect of season from the effect of age, because the average age of the breeders usually decreases throughout the breeding season. Because the appearance of an individual’s own eggs need to be learned during the first breeding (Rothstein 1978), acceptance of Cuckoo eggs may occur mainly among the young naïve breeders during a learning period as shown by Lotem et al. (1992) for the Great Reed Warbler. In an experimental study of Cuckoo parasitism in this species, Lotem et al. (1995) proposed that an evolutionary equilibrium is not developed in a dimorphic population with acceptor and rejecter genotypes equally adapted (see also May & Robinson 1985). They suggested that rather a phenotypically plastic response in the host population cause such equilibrium. Lotem et al. (1995) argued that these naïve birds might tend to accept Cuckoo eggs also later on in life.
Robertson and Norman (1976) noted that experience might influence the host's response to Brown-headed Cowbird, and Smith et al. (1984) found that older female Song Sparrows Melospiza melodia responded more strongly to a cowbird near their nest than did yearlings breeding at the same time. Also Røskaft, Rohwer and Spaw (unpublished) have collected similar data on Northern Orioles Icterus galbula showing that naïve birds tended to accept cowbird eggs at a higher rate than did old birds.
The tendency to accept a parasitic egg increases throughout the breeding season. This is supported by studies on Reed Warblers in the Czech Republic (Øien et al. 1998), and in Britain (Lindholm 1999). A higher acceptance of Cuckoo eggs late in the season (when Cuckoos have left the area) has also been observed in the Rufous Bush Chat (Alvarez 1995). This can be interpreted as a conditional response either to a lower density of Cuckoos and/or a conditional response to the time of parasitism in the season as part of a 'best of a bad situation' (Dawkins & Krebs 1979). This because the possibilities for re-nesting decreases as the breeding season proceeds (Alvarez 1995). A similar trend of higher acceptance of Brown-headed Cowbird eggs by Yellow Warblers later in the season has also been recorded by Burgham and Picman (1989) and was interpreted as an attempt to reproduce when there is not enough time to re-nest. A similar explanation is used for Meadow Pipits that rarely reject Cuckoo eggs in mountainous areas, even if they are able to recognise the Cuckoo egg in their nest, probably because the short breeding season limits the possibilities for re-nesting (Røskaft & Moksnes 1998).
The rejection rate may be higher when parasitism occurs early in the breeding cycle (egg-laying and early incubation) as compared with later stages of incubation when there is a lower probability that the Cuckoo eggs will hatch (Moksnes et al. 1990, 1993b; Braa et al. 1992; but see also Rothstein 1990).
A great number of experiments and observations among Brown-headed Cowbird hosts support that the timing in the laying cycle is an important variable. Yellow Warblers were more likely to reject parasitic eggs when they were parasitised during the first half of the clutch initiation period (Clark & Robertson 1981; Sealy 1995). Least Flycatchers, which is classified as an acceptor species by Rothstein (1975), desert the nest only when parasitised by the Brown-headed Cowbird during nest building (Briskie & Sealy 1987).
A weakened effort exerted in rejection throughout the incubation period is further described for Northern Orioles (Rothstein 1977), Eastern Kingbirds Tyrannus tyrannus (Sealy & Bazin 1995) and Cedar Waxwings Bombycilla cedorum (Rothstein 1976). On the other hand, Grey Catbirds Dumetella carolinensis artificially parasitised early in the laying cycle, showed greater tolerance towards Brown-headed Cowbird eggs than nests experimentally parasitised later in the laying cycle (Rothstein 1974). Among Cuckoo hosts, Moksnes et al. (1990, 1993b) found a significant lower rejection of non-mimetic Cuckoo eggs late as compared to early in the breeding period among Bluethroats Luscinia svecica and Meadow Pipits in Norwegian mountain areas. Similar results are described for Chaffinches Fringilla coelebs and Bramblings F. montifringilla (Braa et al. 1992).
Three of the host species showed a tendency to reject more frequently during the first two days of egg laying than during the last three days. However, this was not statistically significant for any of the individual species (Great Reed Warbler; Fishers exact probabilities test, P=0.515, Reed Warbler; c 2= 0.032, P=0.858, Marsh Warbler; c 2= 0.588, P=0.443, Sedge Warbler; Fishers exact probabilities test, P=1.000, Fig. 5). When pooled, there was still a tendency, although not statistically significant, towards a higher rejection of Cuckoo eggs laid in the early stage (42%) than among those laid in late stage (36%; c 2=0.452, P=0.355).
There was a tendency towards a higher parasitism rate early and late in the breeding season when the number of breeding individuals was low. In late May and early June when most nests were initiated, the parasitism rate was lowest. This was however, only significant for Reed – and Sedge Warblers, individually, and for the total sample (Great Reed Warbler; c 2= 4.66, DF=4, P=0.324, Fig. 6a, Reed Warbler; c 2= 17.91, DF=4, P=0.001, Fig. 6b, Marsh Warbler; c 2= 3.73, DF=4, P=0.443, Fig. 6c, Sedge Warbler; c 2= 15.96, DF=3, P=0.001, Fig. 6d, all four species; c 2= 29.17, DF=4, P=0.000, Fig. 7). There was however, no tendency that the rejection rate of the parasitic eggs followed this pattern, for any individual species or all four species pooled (Fig. 7).
No nests in our Acrocephalus populations were ever parasitised later than the second day of incubation after the clutch was completed. All four warbler species had similar rejection rates when parasitised in early and late stages of the laying cycle. None of the species therefore, follow a pattern of higher rejection rates when parasitised early in the laying cycle. However, as only one individual host was parasitised after clutch completion it was not possible to test whether they tended to accept more after the clutch was completed.
Even if the selection pressure from Brown-headed Cowbird parasitism is much less than from Cuckoo parasitism, changes in the response to parasites over the cycle (laying versus incubation) is described for several host species. Yellow Warblers (Hobson & Sealy 1989) and Least Flycatchers (Briskie & Sealy 1989) responses to cowbird eggs depends upon the stage when the nest was parasitised. Yellow Warblers also reacted most strongly to stuffed cowbird models during egg laying when they where most susceptible to parasitism and they used egg burial as anti parasite strategy primarily when the parasite's egg was deposited early in the laying cycle (Clark & Robertson 1981).
Older Yellow Warblers also perform stronger aggression and more intensive nest-guarding when exposed to a model female Brown-headed Cowbird in the early stage of the breeding cycle. However, yearling American Robins did not show any difference in response in the early stage compared to the late stage in the nesting cycle (McLean et al. 1985).
In general, first time breeders in passerine populations tend to breed later in the season than do older, more experienced birds. Thus, earlier findings on decreasing rejection rates throughout the breeding season for Reed Warblers and for cowbird hosts like the Yellow Warbler (Clark & Robertson 1981), may as well be a phenotypic flexible behaviour. This behaviour depends upon the age of the parasitised host as well as a conditional response to the timing of parasitism. In our material, we found no evidence that the rejection rate decreased throughout the season for any of the four Acrocephalus species.
The explanation for a lower rate of rejection among naïve breeders may simply be that young breeders are inexperienced. They are therefore unable to realise the intention of the brood parasite observed near it's nest, or unable to reveal a parasitic egg in it's nest due to lack of experience with the appearance of own eggs. Another explanation is that of the equilibrium theory, which suggests that acceptance of a brood parasite's egg could be as adaptive as rejection. This theory was first proposed by Zahavi (1979) and later developed by Rohwer and Spaw (1988).
This explanation for a lower rejection rate late in the breeding season need not necessarily be linked to the age of the parasitised host. Even if the parasitised host recognise a Cuckoo egg in their nest, they may accept the parasitic egg if there is a limited possibility for re-nesting when the breeding season is well proceeded. Among Meadow Pipits, which have a short breeding season in mountainous areas, ejection of the Cuckoo egg is rare because it might be better to accept the Cuckoo egg and 'hope' that it will fail to hatch. Thus the host's decision to accept or reject is based on a trade-off between the cost of acceptance, and the cost of desertion without possibilities for re-nesting (Lotem et al. 1995; Davies et al. 1996; Røskaft & Moksnes 1998).
Reduction of clutch size
The host's reaction towards the Cuckoo egg is also influenced by the clutch reduction caused by the number of eggs taken by the female Cuckoo during egg laying. Among Reed Warblers in the Czech Republic the probability of rejection by desertion increased significantly when a Cuckoo female removed more than one of the host’s egg (Øien et al. 1998). However, this response may be directed to the reduction in clutch size, and not to the Cuckoo egg (Rothstein 1975, 1977, 1982 a, b; Hill & Sealy 1994; Øien et al. 1998).
In a study of 144 parasitised Reed Warblers in the same area, the Cuckoo removed a mean of 1.69 eggs from the nests of rejecters, while 1.33 eggs were removed from the nests of acceptors (P=0.015; Øien et al. 1998). A mean of 1.15 host eggs was left in the nest of the acceptors after parasitism, while the corresponding value for the rejecters was 0.62 (Øien et al. 1998). Thus, the Cuckoo removed more eggs from rejecters and more eggs remained in the nests after the course of parasitism in acceptors. When including the other three species a similar pattern was found (Fig. 8a). More eggs were removed by the Cuckoo from rejecter nests than from those of the acceptors (Great Reed Warbler; F=0.14, N=18, P=0.709, Reed Warbler; F=11.89, N=106, P=0.001, Marsh Warbler; F=3.23, N=26, P=0.085, Sedge Warbler; F=7.56, N=8, P=0.033), while more eggs remained in the acceptor nests than in those from rejecters (Great Reed Warbler; F=6.98, N=19, P=0.017, Reed Warbler; F=6.11, N=113, P=0.015, Marsh Warbler; F=0.85, N=30, P=0.365, Sedge Warbler; F=0.622, N=9, P=0.456, Fig. 8b).
Our results from these four Acrocephalus warblers show that the parasitic eggs in the nests where the Cuckoo female removed most host eggs during laying had a higher probability of being rejected.
However, in those nests where the female Cuckoo left more host eggs, such rejection could also be a reaction towards the reduction in clutch size alone. Acceptors may abandon clutches where the total number of eggs falls below a critical level (Rothstein 1975, 1977, 1982a, b). Clutch size reduction may therefore as well be the ultimate cause of desertion, although, the response by the host will be interpreted as conditional. Under such conditions the selection pressure on the Cuckoo is to eat as few and leave as many host eggs as possible in the nest.
Parasite egg mimicry
We would expect that Cuckoo hosts reject non-mimetic eggs at a higher frequency than mimetic eggs, and this is shown experimentally by Davies and Brooke (1989a). From a naturally Cuckoo-parasitised population of Reed Warblers in Czech Republic, we have no indications that the degree of mimicry is important for elucidating rejection (Moksnes et al. 1993a; Øien et al. 1998).
Brown-headed Cowbird hosts do not usually reject eggs that deviates from their own only by one difference, but eggs that differ in two out of three parameters are usually rejected. This built-in tolerance reduces the likelihood that the host will reject their own eggs if these are untypical in size or coloration (Rothstein 1982b; Ortega & Cruz 1988; Ortega et al. 1993). In African Village Weaverbirds Ploceus cucullatus, however, the incidence of rejection was proportional to the degree of difference between the parasite and the host eggs (Victoria 1972).
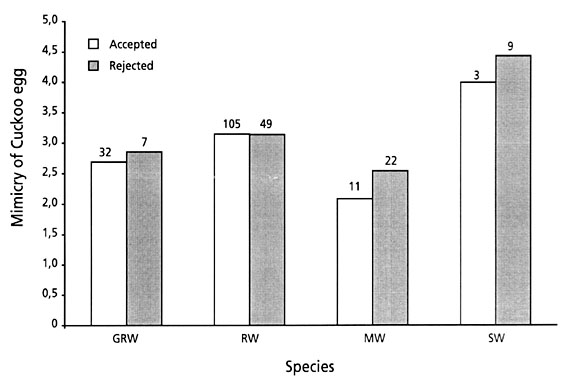
Moksnes et al. (1993a) found no difference in the degree of mimicry of Cuckoo eggs rejected by Reed Warblers and those that accepted the parasitic eggs. A similar pattern was found for the other species in the area, although, there was a tendency towards better mimicry of the eggs that were accepted for the Marsh – and Sedge Warblers (Great Reed Warbler; F=0.28, N=39, P=0.599, Reed Warbler; F=0.01, N=154, P=0.946, Marsh Warbler; F=3.50, N=33, P=0.060, Sedge Warbler; F=1.05, N=12, P=0.329, Fig. 9).
As described by Moksnes et al. (1993a) and Øien et al. (1998) for the Reed Warbler, the degree of Cuckoo egg mimicry did not influence the rejection rate in the studied population. With a possible exception for the Marsh Warbler, a similar pattern was found for the three other species in the present study.
The egg mimicry of the Cuckoo egg to the host eggs is evidently evolved as part of the evolutionary arms race between the Cuckoo and the host. When a host population has evolved recognition of own eggs, followed by development of rejection behaviour towards non-mimetic Cuckoo eggs, the Cuckoo responds by evolving egg mimicry (Dawkins & Krebs 1979; Davies & Brooke 1989b; Moksnes et al. 1990). The warblers in this area seem to be parasitised by a generalist Cuckoo gens (Edvardsen 1998). The lack of a relation between the rejection rates and the degree of mimicry may be due to the fact that the Cuckoo egg gens in this area is the best one adapted to these four Acrocephalus species. Our assessment of the egg mimicry may therefore be slightly different from what is assessed by these four host species.
CONCLUSIONFrequently parasitised host populations living in ancient sympatry with the parasite are found to show a more frequent rejection of model eggs than populations of the same species that have not experienced such contact with the parasite (Davies & Brooke 1989a; Soler & Møller 1990; Briskie et al. 1992; Dufty 1994; Lindholm 1994). Such behavioural differences have been explained as being a result of differences in allele frequencies between populations (local host evolution).
Models have suggested that if parasitism is sufficiently rare, even a low rate of erroneous rejections of parasitic eggs may favour acceptance (Davies & Brooke 1989b; Lotem et al. 1995; Takasu 1998, 1999). In Brown-headed Cowbird hosts, rejection is relatively costly, and therefore there may be a trade off between the costs of rejection and acceptance (cf. Rohwer et al. 1989; Spaw & Rohwer 1987; Røskaft et al. 1990). Selection for acceptance versus rejection will fluctuate over time as the costs and benefits of these strategies vary with the environmental conditions (Sealy 1995; Takasu 1998, 1999). In the Cuckoo-host system, the costs of acceptance are much higher, and the benefit of rejection usually greatly outweighs the rejection costs (see Røskaft & Moksnes 1998; Øien et al. 1998).
It seems obvious that conditional responses are involved in many aspects of the host reactions to brood parasitism. In connection with egg-recognition, several conditional strategies seem to be involved, and even acceptor species of Brown-headed Cowbird hosts show rejection behaviour towards own broken eggs, thus showing that they are capable of the critical behaviours needed to reject parasitic eggs (Rothstein 1982a; Kemal & Rothstein 1988). Individuals of several species of both Brown-headed Cowbird and Cuckoo hosts accept the parasitic egg(s) even when, according to optimality models they should benefit from deserting the parasitised nest (Røskaft & Moksnes 1998). Studies like this one enlighten the complexity of host reactions to avian brood parasitism, and indicate that we are in the very beginning of understanding the mechanisms underlying host responses. Phenotypic flexibility in reactions towards parasitism seems to be adaptive for the host populations, and the conditional responses among individuals attributable to genetic differences therefore depends on both genotype frequencies and the additive effects of alleles on the phenotype. In further work on geographic variation in responses within species, poly-loci models must be employed, and data on several aspects of the conditions of parasitism should be collected. We have shown that four species with median rejection rates incurs many conditional responses towards brood parasitism as indicated by Hypothesis 2.
ACKNOWLEDGEMENTS
This work was made possible through funding by the Nansen Foundation, the Commission of the European Community, the Cultural Agreement between the Czech Republic and Norway and a grant from Grant Agency of the Czech Republic (no.206/970168). Thanks are due to Alice L. Clarke, Kathrin Forster,Tomas Grim, Lise Greger Hagen, Eva Hanssen, Karel Janko, Jarmila Madrova, Dorte Meilvang, Oldrich Mikulica, Cecilie Mørch, Per H. Olsen, Bård G. Stokke, Barbara and Michael Taborsky, Yvonne Teuschl and Wolfgang Vogel who participated in the fieldwork, and to Manuel Soler for comments improving the paper.
REFERENCESAlvarez, F. 1995. Model Cuckoo Cuculus canorus eggs accepted by Rufous Bush Chats Cercotrichas galactotes during the parasite’s absence from the breeding area. Ibis 138: 340-342.
Braa, A.T., Moksnes, A. & Røskaft, E. 1992. Adaptations of Bramblings and Chaffinches towards parasitism by the Common Cuckoo. Animal Behaviour 43: 67-68.
Briskie, J.V. & Sealy, S.G. 1987. Responses of Least Flycatchers to experimental inter- and intraspecific brood parasitism. Condor 89: 899-901.
Briskie, J.V. & Sealy, S.G. 1989. Changes in nest defence against a brood parasite over the breeding cycle. Ethology 82: 61-67.
Briskie, J.V., Sealy, S.G. & Hobson, K.A. 1992. Behavioral defences against avian brood parasitism in sympatric and allopatric host populations. Evolution 46: 334-340.
Brooke, M. de L. & Davies, N.B. 1988. Egg mimicry by Cuckoos Cuculus canorus in relation to discrimination by hosts. Nature, London 335: 630-632.
Burgham, M.C.J. & Picman, J. 1989. Effect of Brown-headed Cowbirds on the evolution of Yellow Warbler anti-parasite strategies. Animal Behaviour 38: 298-308.
Clark, K.L. and Robertson, R.J. 1979. Spatial and temporal multi-species nesting aggregations in birds as anti-parasite and anti-predator defenses. Behavioural Ecology and Sociobiology 5:359-371.
Clark, K.L. & Robertson, R.J. 1981. Cowbird parasitism and evolution of anti-parasite strategies in the Yellow Warbler. Wilson Bulletin 93: 249-258.
Davies N.B & Brooke M de L. 1988. Cuckoos versus Reed Warblers: adaptations and counteradaptations. Animal Behaviour 36: 262-284.
Davies, N.B. & Brooke ,M de L. 1989a. An experimental study of co-evolution between the Cuckoo Cuculus canorus, and its hosts. I. Host egg discrimination. Journal of Animal Ecology 58: 207-224.
Davies, N.B. & Brooke, M. de L. 1989b. An experimental study of co-evolution between the Cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. Journal of Animal Ecology 58: 225-236.
Davies, N.B., Brooke, M. de L. & Kacelnik, A. 1996. Recognition errors and probability of parasitism determine whether Reed Warblers should accept or reject mimetic model Cuckoo eggs. Proceedings of Royal. Society London, B 263: 925-931.
Dawkins, R. & Krebs, J.R. 1979. Arms race between and within species. Proceedings of Royal Society London, B 205: 489-511.
Dufty, A.M.Jr. 1994. Rejection of foreign eggs by Yellow-headed Blackbirds. Condor 96: 799-801.
Edvardsen, E. 1998. Host preferences in the Cuckoo Cuculus canorus. Cand scient thesis, Norwegian Univiversity of Science and Technology.
Haartman, L. von. 1981. Co-evolution of the Cuckoo Cuculus canorus and a regular Cuckoo host. Ornis Fennica 58: 1-10.
Hagen, L. 1996. Gjøkens Cuculus canorus egglegging i forhold til Rørsangerens Acrocephalus scirpaceus aktivitet ved reiret. Cand scient thesis, Norwegian University of Science and Technology, NTNU.
Hill, D. P & Sealy, S.G. 1994. Desertion of nests parasitized by cowbirds: have Clay-coloured Sparrows evolved anti-parasite defence? Animal Behaviour 48: 1063-1070.
Hobson, K.A. & Sealy, S.G. 1989. Responses of Yellow Warblers to the threat of cowbird parasitism. Animal Behaviour 38: 510-519.
Järvinen, A. 1984. Relationship between the Common Cuckoo (Cuculus canorus) and its host, the Redstart (Phoenicurus phoenicurus). Ornis Fennica 61: 84-88.
Kemal, R.E. & Rothstein, S.I. 1988. Mechanisms of avian egg recognition: adaptive responses to eggs with broken shells. Animal Behaviour 36: 175-183.
Lindholm, A. 1994. Absence of defences against brood parasitism in unparasitized Reed Warblers. Journal für Ornithologie 135: 134.
Lindholm, A. 1999. Phenotypic plasticity in Reed Warbler defence against brood parasitism. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 3107-3124. Johannesburg: BirdLife South Africa.
Lotem, A., Nakamura, H. & Zahavi, A. 1992. Rejection of Cuckoo eggs in relation to host age: a possible evolutionary equilibrium. Behavioural Ecology 3: 128-132.
Lotem, A., Nakamura, H. & Zahavi, A. 1995. Constraints of egg discrimination and Cuckoo-host coevolution. Animal Behaviour 49: 1185-1209.
Martinez, J.G., Soler, M. & Soler, J.J. 1996. The effect of Magpie breeding density and synchrony on brood parasitism by Great Spotted Cuckoos. Condor 98: 272-278.
May, R.M. & Robinson, S.K. 1985. Population dynamics and avian brood parasitism. American Naturalist 126:475-494.
McLean, I.G., Smith, J.N.M. & Stewart, K.G. 1986. Mobbing behaviour, nest exposure, and breeding success in the American Robin. Behaviour 96:171-186.
Moksnes, A. & Røskaft, E. 1988. Responses of Fieldfares Turdus pilaris and Bramblings Fringilla montifringilla to experimental parasitism by the Cuckoo Cuculus canorus. Ibis 130: 535-539.
Moksnes, A. & Røskaft, E. 1989. Adaptations of Meadow Pipits to parasitism by the Common Cuckoo. Behavioural Ecology and Sociobiology 24: 25-30.
Moksnes, A. & Røskaft, E. 1995. Egg-morphs and host preference in the Common Cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. Journal of Zoology, London 236: 625-648.
Moksnes, A., Røskaft, E., Bicìk, V., Honza, M. & Øien, I.J. 1993a. Cuckoo Cuculus canorus parasitism on Acrocephalus warblers in Southern Moravia in the Czech Republic. Journal für Ornithologie 134: 425-434.
Moksnes, A., Røskaft, E., Braa, A.T., Korsnes, L., Lampe, H.M. & Pedersen, H.C. 1990. Behavioural responses of potential hosts towards artificial Cuckoo eggs and dummies. Behaviour 116: 64-89.
Moksnes, A., Røskaft, E. & Korsnes, L. 1993b. Rejection of Cuckoo (Cuculus canorus) eggs by Meadow Pipits (Anthus pratensis). Behavioural Ecology 4: 120-127.
Nakamura, H. 1990. Brood parasitism by the Cuckoo Cuculus canorus and the start of new parasitism on the Azure-winged Magpie Cyanopica cyana. Japan Journal of Ornithology 39: 1-18.
Øien, I.J., Moksnes, A., Røskaft, E. & Honza, M. 1998. Costs of Cuckoo Cuculus canorus parasitism to Reed Warblers Acrocephalus scirpaceus. Journal of Avian Biology 29: In press.
Ortega, C.P. & Cruz, A. 1988. Mechanisms of egg acceptance by marsh-dwelling blackbirds. Condor 90: 349-358.
Ortega, J.C., Ortega, C.P. & Cruz. A. 1993. Does Brown-headed Cowbird egg coloration influence Red-winged Blackbird responses towards nest contents?- Condor 95: 217-220.
Robertson, R.J. & Norman, R.F. 1976. Behavioural defences to brood parasitism by potential hosts of the Brown-headed Cowbird. Condor 78: 166-173.
Robertson, R.J. & Norman, R.F. 1977. The function and evolution of aggressive host behavior towards the Brown-headed Cowbird (Molothrus ater). Canadian Journal of Zoology 55: 508-518.
Rohwer, S. & Spaw C.D. 1988. Evolutionary lag versus bill size constraints: a comparative study of the acceptance of cowbird eggs by old hosts. Evolutionary Ecology 2: 27-36.
Rohwer, S., Spaw, C..D. & Røskaft, E. 1989. Costs to Northern Orioles of puncture-ejecting parasitic cowbird eggs from their nest. Auk 106: 734-738.
Røskaft, E. & Moksnes, A. 1998. An optimality analysis of coevolution between parasitic cuckoos and cowbirds and their hosts. In: Parasitic Birds and Their Hosts: Behavioral, Ecological and Evolutionary Interactions (Rothstein, S.I. & Robinson, S.K. eds.). Oxford: Oxford University Press (in press).
Røskaft, E., Orians, G.H. & Beletsky L.D. 1990. Why do Red-winged Blackbirds accept eggs of Brown-headed Cowbirds. Evolutionary Ecology 4: 35-42.
Rothstein, S.I. 1974. Mechanisms of avian egg recognition: possible learned and innate factors. Auk 91: 796-807
Rothstein, S.I. 1975. An experimental and teleonomic investigation of avian brood parasitism. Condor 77: 250-271.
Rothstein, S.I. 1976. Experiments on defences Cedar Waxwings use against cowbird parasitism. Auk. 93: 675-691.
Rothstein, S.I. 1977. Cowbird parasitism and egg recognition of the Northern Oriole. Wilson Bulletin 89: 21-32.
Rothstein, S.I. 1978. Mechanisms of avian egg recognition: additional evidence for learned components. Animal Behaviour 26: 671-677.
Rothstein, S.I. 1982a. Success and failures in avian egg recognition with comments on the utility of optimality reasoning. American Zoologist 22: 547-560.
Rothstein, S.I. 1982b. Mechanisms of avian egg recognition: Which egg parameters elicit responses by rejecter species. Behavioural Ecology and Sociobiology 11: 229-239.
Rothstein S.I. 1990. A model system for coevolution: avian brood parasitism. Annual Review of Ecological Systematics 21: 481-508.
Sealy, S.G. 1995. Burial of cowbird eggs by parasitized Yellow Warblers: An empirical and experimental study. Animal Behaviour 49: 877-889.
Sealy, S.G. & Bazin, R.C. 1995. Low frequency of observed cowbird parasitism on Eastern Kingbirds: host rejection, effective nest defence or parasite avoidance? Behavioural Ecology 6: 140-145.
Soler, M. & Møller, A.P. 1990. Duration of sympatry and coevoultion between Great Spotted Cuckoo and its Magpie host. Nature, London 343: 748-750.
Soler, M., Soler, J.J., Martinez, J.G. & Møller, A.P. 1994. Micro-evolutionary change in host response to a brood parasite. Behavioural Ecology and Sociobiology 35: 295-301.
Smith, J.N.M., Arcese, P. & McLean, I.G. 1984. Age, experience and enemy recognition by wild Song Sparrows. Behavioural Ecology and Sociobiology 14: 101-106.
Spaw, C.D. & Rohwer, S. 1987. A comparative study of eggshell thickness in Cowbirds and other Passerines. Condor 89:307-318.
Takasu, F. 1998. Why do all host species not show defence against avian brood parasitism: Evolutionary lag or equilibrium? American Naturalist 151:193-205.
Takasu, F. 1999. The role of theoretical models explaining the relationships between brood parasites and their hosts. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 3146-3164. Johannesburg: BirdLife South Africa.
Via, S., Gomiulkiewicz, R., De Jong, G., Scheiner, S.M., Schlichting, C.D. & Van Tienderen, P.H. 1995. Adaptive phenotypic plasticity: consensus and controversy. Trends. Evolutionary Ecology 10: 212-217.
Victoria, J.N. 1972. Clutch characteristics and egg discriminative ability of the African Village Weaverbird Ploceus cucullatus. Ibis 114: 367-376.
Zahavi, A. 1979. Parasitism and nest predation in parasitic cuckoos. American Naturalist 113: 157-59.
Fig. 1. A hypothetical model of mean rejection rates of Cuckoo
eggs in different host populations, in relation to variation in rejection rates between
populations of the same species.
Fig. 2. Rejection rates (in percentage) of artificial, non mimetic Cuckoo eggs for 17 different species of potential cuckoo host species in Europe, in relation to the variance (SD) in rejection rate of different populations of the same species. (Data from Davies and Brooke 1989a; Lindholm 1999; Moksnes et al. 1990; Brown et al. 1990; Haartman 1981; Järvinen 1984; Lotem et al. 1995; Moksnes & Røskaft unpublished).
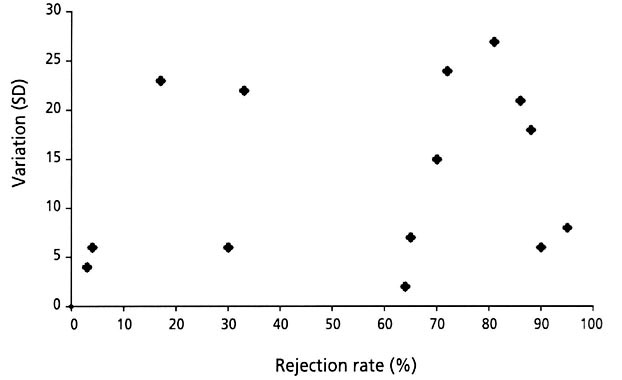
Fig. 3. The correlation between rejection rates of two different host populations from different European Cuckoo host species, tested by non-mimetic Cuckoo eggs (n =17 species).In those cases where more than two populations have been tested, we used the two populations with the highest sample sizes (references as in Fig. 2).
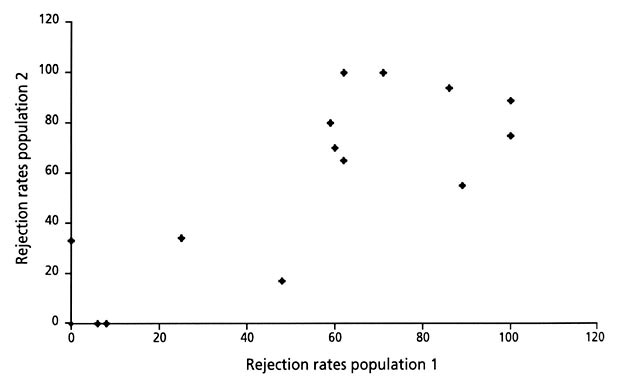
Fig. 4. Number of recorded nests of Great Reed Warblers, Reed Warblers, Marsh Warblers and Sedge Warblers in Lednice (1992 – 1994) and Luzice (1995 – 1996).
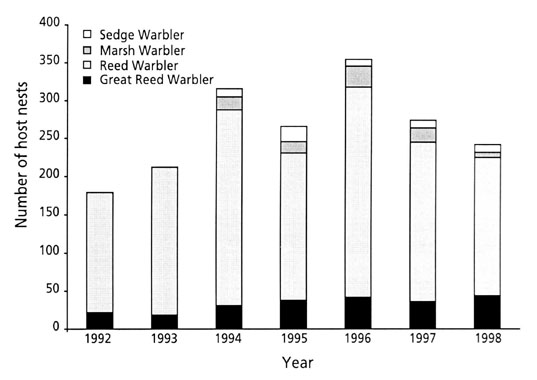
Fig. 5. Rejection rates of birds parasitised during day 0 – 2 (early stage) and those parasitised during day 3 – 6 (late stage), for four Acrocephalus species in Southern Moravia. None of the differences between early and late stages were statistically significant.
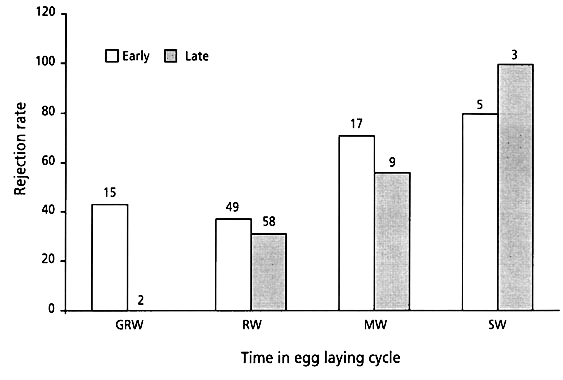
Fig. 6. Parasitism rate in relation to time in breeding season (1= 1 – 15 May, 2= 16 – 31 May, 3= 1 – 15 June, 4= 16 – 30 June, 5= 1 – 15 July), for a) Great Reed Warblers, b) Reed Warblers, c) Marsh Warblers and d) Sedge Warblers.
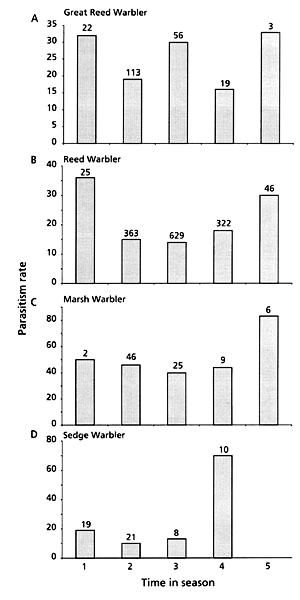
Fig. 7. The rejection rates of all four Acrocephalus species pooled (open bars), and the rate of parasitism (filled bars) for the same species in relation to breeding period (breeding period as in Fig. 6).
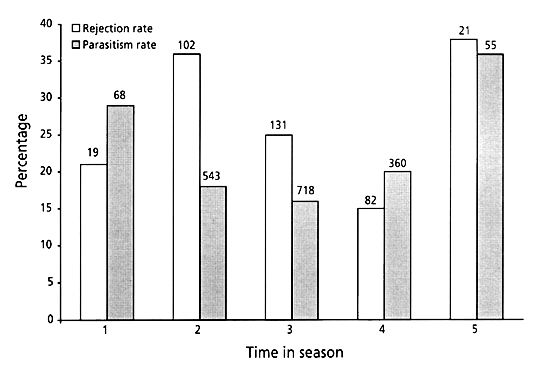
Fig. 8 (a). Number of eggs removed by the Cuckoo when parasitising the four Acrocephalus species, in relation to whether the egg was later accepted (open bars) or rejected (filled bars). (b) Number of host eggs left in the nest by the Cuckoo after parasitism in relation to whether the egg was later accepted (open bars) or rejected (filled bars).
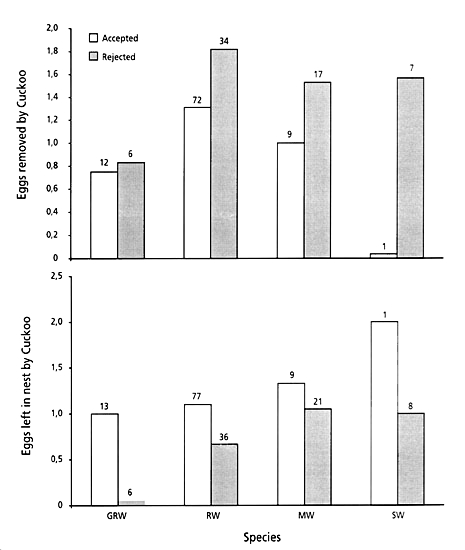
Fig. 9. The mimicry of Cuckoo eggs laid in nests of four Acrocephalus species in Southern Moravia in relation to whether they were later accepted (open bars) or rejected (filled bars).