S51.3: Phenotypic plasticity in Reed Warbler defence against brood parasitism
Anna K. Lindholm
Department of Zoology, University of Cambridge, Downing Street, Cambridge, CB2 3EJ, UK, fax 1 604 822 2416, e-mail akl1000@cam.ac.uk
Lindholm, A.K. 1999. Phenotypic plasticity in Reed Warbler defence against brood parasitism. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 3107-3124. Johannesburg: BirdLife South Africa.Frequencies of brood parasitism by the Cuckoo Cuculus canorus on populations of Reed Warblers Acrocephalus scirpaceus vary geographically in Britain. Frequencies of rejection of model cuckoo eggs were positively correlated with parasitism frequencies in four British populations of Reed Warblers. As evidence from natal dispersal distances suggest that there is considerable gene flow between British Reed Warbler populations, behavioural differences between populations are unlikely to reflect genetic differences. An alternative hypothesis is that the behavioural differences reflect individual flexibility in egg rejection according to the risk of parasitism. This would be adaptive in environments where the risk of parasitism fluctuates, so that in years when there is no or little parasitism, Reed Warblers could be more tolerant of odd eggs and therefore less likely to reject an egg of their own, while in times of high risk of parasitism, they could be less tolerant and thereby minimise the chance of rearing a Cuckoo. Such fluctuation in parasitism rates is predicted by a metapopulation model for the Cuckoo in Britain. Evidence for phenotypic plasticity in Reed Warbler egg rejection behaviour comes from experiments which indicated that Reed Warblers from two populations which reject model eggs at either a high or a low rate have a similar capability of recognising eggs, but differ in their rejection decisions. Reed Warblers were more likely to reject eggs when the risk of parasitism was relatively high or when experimental eggs were easy to reject.
INTRODUCTION
One of the main lines of defence of hosts against avian brood parasitism is recognising and rejecting a foreign egg in the nest. This defence is thought to have evolved to counter the costs of caring for a brood parasite (Rothstein 1990). In hosts of the Cuckoo Cuculus canorus, this cost usually includes the death of host young. The frequency of parasitism will influence the strength of selection for the evolution of egg rejection (Davies & Brooke 1989a,b; Takasu et al. 1993). Even within a species, populations subject to different frequencies of parasitism may exhibit different levels of defences. For example, Meadow Pipits Anthus pratensis in Britain are current hosts of the Cuckoo and reject model eggs more often than Meadow Pipits in Iceland, which are not parasitised (Davies & Brooke 1989a). Such population differences in behaviour have been assumed to reflect genetic differences between populations (Davies & Brooke 1989a; Soler & Møller 1990; Briskie et al. 1992). If genetic differences in fact underlie differences in rejection frequencies between populations, then similar frequencies of egg rejection should be found in populations, which are not isolated from each other. The aims of this study were 1) to test for differences in host defences between populations which are subject to different frequencies of parasitism but which are likely to share the same gene pool, through close proximity and high rates of dispersal, unlike British and Icelandic Meadow Pipits, and 2) to investigate how any population differences are maintained. The Reed Warbler Acrocephalus scirpaceus was a good candidate for study species as frequencies of parasitism of Reed Warblers vary across Britain (Fig. 1) and as it is a highly mobile migratory species. Reed Warblers are good dispersers, as their mean natal dispersal distance in Britain is nearly 50 km (Paradis et al. 1998) and population turnover is considerable (Gibbons et al. 1993). This paper represents a synthesis of the main findings from Lindholm (in press a & b) and Lindholm & Thomas (in press).
METHODS
Study sites
I selected two study sites in Wales where Reed Warblers were unparasitised (Fig. 2): Llangorse Lake, where the parasitism rate is 0% (n=329 nests) and Oxwich Marsh, where the parasitism rate is also 0% (n=119 nests). The parasitism frequency was determined by nest searches by this and previous authors, and nest and ringing records (Lindholm in press a). At both sites, Cuckoos were absent from the reedbed. In contrast, Cuckoos were commonly observed near the reedbed at Pannel Valley, a rarely parasitised site (0.3% parasitism of 327 nests). These Cuckoos must have had other host preferences, such as Dunnocks Prunella modularis, which have been parasitised in adjacent farmland. Wicken Fen was used as a regularly parasitised site (9.0% of 809 nests), which has been previously studied by Davies and Brooke (1988). These sites are within about 300 km of each other. Experiments were conducted in 1993 and 1994 at Llangorse Lake, 1993 at Oxwich, 1995 at Pannel Valley, and in 1993 and 1995 at Wicken Fen.
Model eggsTo simulate natural parasitism by the Cuckoo, model Cuckoo eggs were added to nests of Reed Warblers at each of the four sites, in the afternoon, during the laying or incubation periods. The model eggs were made of resin, and were of the same size and weight as real Cuckoo eggs. They were painted to resemble eggs of the Cuckoo gens that specialises on the parasitism of Pied Wagtails Motacilla alba. These speckled white eggs provided a good contrast to Reed Warbler eggs. If host eggs hatched, and the model egg remained in the nest, then the outcome was scored as acceptance. If the nest was active but the model egg was missing, or the eggs in the nest were cold for two successive days, then the outcome was scored as rejection. If the nest was depredated before hatch, then the nest must have been active for at least six days after experimental parasitism, or was excluded.
RESULTS & DISCUSSIONExperiment 1: Model Cuckoo eggs
The frequency at which model eggs were rejected from Reed Warbler nests differed between populations (Fig. 3; results from trials in the laying and incubation periods were pooled as the frequencies of each did not differ). The lower the parasitism rate, the more tolerant each population was of model eggs.
This association could be expected if the study populations were reproductively isolated from each other, but dispersal evidence (Paradis et al. 1998) suggests otherwise. Alternatively, phenotypic plasticity in Reed Warblers in response to the presence and behaviour of Cuckoos could explain these results. The two study populations in Wales were Cuckoo-free with no parasitism, and with no Cuckoos inhabiting the wooded edge of the reedbeds. The reedbed in Pannel Valley was unparasitised in 1995, but pairs of Cuckoos were often in close proximity to the reedbed. Cuckoos were both present and parasitising Reed Warblers at Wicken Fen. Thus the intensity of Cuckoo stimuli increases from Llangorse Lake and Oxwich to Pannel Valley and Wicken Fen as does the frequency of rejection of model eggs.
Davies and Brooke (1988) and Lotem et al. (1995) pointed out that Reed Warblers might have a particular problem in recognising Cuckoo eggs, because of variability in the appearance of eggs within a clutch. If Reed Warblers adopt recognition rules that are too stringent, then they are susceptible to mistakenly rejecting some of their own eggs. If they are too lax, they risk accepting any Cuckoo egg.
Model of rejection costsA simple model was used to identify the frequency of recognition errors committed by unparasitised Reed Warblers at which payoffs associated with an accepter phenotype balance those of a rejecter phenotype, at different probabilities of parasitism, following the approach of Davies and Brooke (1989b) and Lotem et al. (1995).
At parasitism, Cuckoos usually remove one egg from the host clutch, leaving a clutch size of x-1 (Wyllie 1981; Lotem et al. 1995). Host individuals may act as accepters or rejecters. Accepters do not discriminate between their own and Cuckoo eggs, while rejecters attempt to. For accepters, when parasitised (with a probability p), reproductive success is generally zero because the Cuckoo nestling evicts its nestmates after hatching. Exceptionally, hatch failure in Cuckoo eggs prevents the death of the host brood (Moksnes et al. 1993; Lotem et al. 1995; 1 case in 653 BTO nest record cards). When not parasitised, host clutch size x is unaffected. The accepter payoff A is therefore p(0) + (1-p)x. Rejecters correctly reject a mimetic Cuckoo egg at a probability of r, which has been measured at 0.19 for Reed Warblers at Wicken Fen (Davies & Brooke 1988). However, rejecting a Cuckoo egg entails a probability b of breaking eggs of one's own. The average number of host eggs broken in rejecting a Cuckoo egg is 0.3 eggs (Davies & Brooke 1988). The means of egg rejection is assumed to be ejection. The payoff to a parasitised rejecter which rejects a Cuckoo egg is (x-1-b) which occurs at a frequency of pr, whereas a rejecter which does not reject a Cuckoo egg has 0 at a frequency of p(1-r). If not parasitised, rejecters have an unknown probability e of erroneously rejecting one of their own eggs. The payoff is then (1-p) (e) (x-1). If no error is made the payoff is (1-p) (1-e)x. Thus the rejecter payoff R is pr(x-1-b) + p(1-r)(0) + (1-p) (e) (x-1) + (1-p) (1-e)x.
Solving, A is equal to R when:
![]() .
.
The equilibrium error probability for a given probability of parasitism can be read from the line (Fig. 4). For example, for a parasitism rate of 1%, the equilibrium rate of making errors equals 0.0052 for a clutch size of four eggs when r = 0.19 and b = 0.3. If a Reed Warbler is liable to make recognition errors at a rate greater than this, it would have higher fitness, on average, by being an accepter. The key point is that at very low levels of parasitism, even very small probabilities of recognition errors when unparasitised will favour accepters.
Experiment 2: A stuffed CuckooThe model predicts that if Reed Warblers could be made to perceive that they were more likely to be parasitised, they would reject more often. This is consistent with the finding that when commonly used Cuckoo hosts are shown taxidermic mounts of Cuckoos at the nest prior to experimental parasitism, rates of rejection of model eggs increase (Davies & Brooke 1988; Moksnes & Røskaft 1989). Thus showing a stuffed Cuckoo at the nest at Llangorse Lake and Pannel Valley might cause Reed Warblers to behave as though they were at a higher risk of parasitism, and reject more frequently. This was tested by placing one of two stuffed Cuckoos (of indeterminate sex) in the cups of Reed Warbler nests at Llangorse Lake and Pannel Valley, during the laying period. The mount was removed after Reed Warblers had responded to it, usually by mobbing, for five minutes. At Llangorse Lake and Pannel Valley, model eggs were no more likely to be rejected if a Cuckoo was shown prior to experimental parasitism (Fig. 5). This contrasts with the results that Davies & Brooke (1988) obtained for Wicken Fen in 1985-86 using one of the same Cuckoo mounts I employed, where Reed Warblers were significantly less likely to accept unlike model eggs had they seen a Cuckoo mount at the nest.
Experiment 3: More Cuckoos
Since there was no parasitism at Llangorse Lake or Pannel Valley during this study, a single exposure to a stuffed Cuckoo might not suffice to persuade Reed Warblers that they were at higher risk of parasitism. As a further test, I increased the exposure to Cuckoos at Llangorse Lake, choosing this site because of the absence of Cuckoos from the reedbed. Cuckoos were found in the area, but in different types of habitat.
In an isolated part of the reedbed, I placed a stuffed Cuckoo on a perch and put it into the reedbed for several hours per day from the start of the breeding season, accompanied by speakers which played calls of male and female Cuckoos. Each breeding pair was also shown a stuffed Cuckoo at the nest and then experimentally parasitised with a model egg during the laying period, as in experiment 2.
The experimental treatment of showing Cuckoos and playing their calls had no effect of the frequency of rejection of model eggs (Fig. 6). As birds respond differently to real and stuffed birds (Knight & Temple 1986), a possibility is that Reed Warblers did not respond to the mount as if it was real. Although Reed Warblers at Wicken Fen responded to the sight of a stuffed Cuckoo at the nest by increasing the frequency of rejection (Davies & Brooke 1988), the presence or attentions of real Cuckoos, which I was unable to simulate, may already have stimulated them.
Experiment 4: TranslocationAs Reed Warblers show high rates of natal and breeding dispersal (Paradis et al. 1998), they might respond to experimental parasitism according to cues from Cuckoos in their immediate breeding habitat. Thus if Reed Warblers were moved from an area of naturally high parasitism rates to a site with naturally low parasitism rates, or vice versa, they might respond to experimental parasitism by rejecting eggs at the same frequency as their neighbours. If the tendency to reject odd eggs was genetically determined, then the translocated Reed Warblers should reject eggs with the frequency of their home population. Thirty-five Reed Warblers from the parasitised site of Wicken Fen in England were caught early in the breeding season and transported to Llangorse Lake and released into the reedbed. The sex of many of the birds could not be determined by external examination. However, as both males and females reject eggs (Davies & Brooke 1988), either sex was suitable.
Despite extensive mist-netting in the reedbed, only five Reed Warblers originally from Wicken Fen were recaptured or resighted following release at Llangorse Lake. Only two nests were located; both were of pairs consisting of an ex-Wicken male and a Llangorse female. The nests were parasitised with a model egg during the laying period. Both pairs accepted the model egg until hatching. Although suggestive, the small sample sizes restrict interpretation of the results.
Experiment 5: Seasonal changesA further way of investigating the effects of changing parasitism rates is to examine natural variation. The breeding season of Cuckoos tends to end before that of Reed Warblers, so that Reed Warblers breeding at the end of the season have a lower risk of parasitism (Wyllie 1981; see also Alvarez 1996). If Reed Warblers respond to lower parasitism frequencies by reducing the frequency of egg rejection, then rejection frequencies would show a decline over time.
There was no significant seasonal change in rejection rates at Llangorse Lake and Pannel Valley, but a significant decline in rejection rates was found in analysing unpublished data from Davies and Brooke from 1984 to 1986 (Fig. 7; Lindholm in press b). Results from experiments at Wicken Fen in 1993 and 1995 were compatible with these results. They make sense, because Llangorse Lake and Pannel Valley were unparasitised during the study, so there was no seasonal change in parasitism rates. At Wicken Fen, parasitism rates decline seasonally, as does the frequency of rejection. An alternative hypothesis is that parasitism frequencies decline with nesting opportunities. If Reed Warblers were responding to this, rather than to parasitism rates, then a seasonal decline would be expected at all three sites. However, no hint of a decline was seen at Llangorse Lake.
Experiment 6: Real eggsBehavioural observations at the unparasitised populations of Pannel Valley and Llangorse Lake indicated that many Reed Warblers were able to identify a foreign egg in the nest, but that they varied in their tendency to reject it. After parasitising nests, I returned to watch, and sometimes saw birds pecking at a model egg as though they were trying to break it. The pecks were sometimes very forceful, with a bird gripping supporting reeds with its feet and pounding vertically at the model egg, up to 100 times in half an hour. Birds, which pecked at the model egg in this way, had clearly identified the model egg as a foreign egg and were trying to break it. This suggests that if the experimental egg were easy to break, then birds at Llangorse Lake would be more likely to reject them than the solid model eggs.
To test this, one egg of the clutch was painted brown, on the day that the female finished laying, at Llangorse Lake and Wicken Fen. Birds at Llangorse Lake were significantly more likely to reject brown painted eggs than model eggs (Fig. 8), suggesting that more birds were capable of recognising unlike eggs than actually rejected them in earlier experiments. Moreover, the frequency of rejection of brown eggs did not differ between Llangorse Lake and Pannel Valley (Fig. 9), suggesting similar discriminatory abilities between birds of the two populations.
An interesting pattern emerges if the outcome of trials in which pecking at model eggs was seen is compared between populations (Fig. 10). At Llangorse Lake and Pannel Valley, birds, which peck a model egg, are no more likely to reject the egg than to accept it. Egg recognition and egg rejection appears decoupled. This differs from Wicken Fen, where birds which pecked at an egg went on to reject it. This is consistent with the idea that birds at Wicken Fen are willing to put more effort into rejecting an odd egg, which might well be a Cuckoo egg because Cuckoos are around, than at the unparasitised populations, where an odd egg is likely to be a Reed Warbler egg.
The observations of Reed Warblers pecking at eggs that they later tolerate, the higher frequencies of rejection of painted Reed Warbler eggs than model Cuckoo eggs at Llangorse Lake, the similar frequencies of rejection of these eggs between Wicken Fen and at Llangorse Lake, and a seasonal decline in rejection rates at Wicken Fen, provide evidence that many Reed Warblers recognise unlike model eggs, but do not necessarily reject them. Reed Warblers are capable of modifying their egg rejection behaviour, and appear to do so in line with the costs and benefits of rejection and the parasitism rate. In this way, Reed Warblers in different populations could reject eggs at different frequencies, according to the risk of parasitism, without genetic differentiation of populations. However, the main benefit of such a system is only apparent upon consideration of the ecology of the Cuckoo.
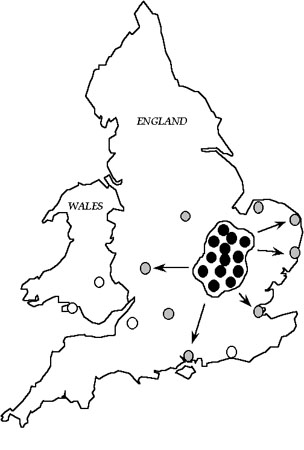
Models of Cuckoo ecologyReed Warblers in Britain have a patchy distribution (see Gibbons et al. 1993, p. 334), as reedbeds occur in discrete fragmented patches. Radio-telemetry evidence indicates that individual female Cuckoos are specific to just one host species, at least during a breeding season (Nakamura & Miyazawa 1997). Therefore, female Cuckoos, which specialise on Reed Warblers, will also have a patchy distribution (Fig. 11). The distribution map shows, curiously, that Reed Warbler Cuckoos have been found close to Wales, but not in it. This observation prompts the question of what would happen if a female Reed Warbler Cuckoo crossed the Welsh border and settled at Llangorse Lake.
Extinction model
A stochastic model (Renshaw 1991) was used to describe the growth of such a population. I assume that there is only one founder, and no further immigration, and that females show strict host fidelity. Furthermore I assume that genes for mimicry of Reed Warbler eggs passes from mother to daughter regardless of her choice of mate (Punnett 1933; Jensen 1966).
In this model there are only the parameters of birth and death. Because so little field data is available due to the difficulty of studying Cuckoos, these parameters were estimated. Each female, starting from the founder, breeds from her first year. Each was assigned a random probability of producing a daughter, which recruits to the breeding population, and of dying (assumed to occur after the end of the breeding season). The fertility of female Cuckoos is poorly known, but estimates were made from field studies that aimed to identify female Cuckoos by their distinctive egg types. Wyllie (1981) made an estimate of an average of 9.2 eggs per female per year. Based on studies of Reed Warblers, Cuckoos were estimated to lay an average of 5.8 and 4.1 eggs per year, with the latter estimate including records of only one or two eggs laid, since these could represent first year females (Lindholm in press a). Adult females were assumed to lay a random number of female eggs per year, with an overall mean corresponding to half of 9.2, 5.8 and 4.1. Each egg was given a random chance to survive and return to the natal site of 0.11, an estimate based on studies of the fledging success of Cuckoos and ringing records (Brooke & Davies 1987; Lindholm in press a). The probability of dying was set at 0.36 per year for adult females, which is the estimated average annual rate of adult mortality (Lindholm in press a). The population was allowed to grow to a size of fifteen females. Above this population size, surplus females were assumed to emigrate and breed elsewhere.
The model was run for 50 years, or to extinction, whichever came first, 500 times. Half of the simulations ended in population extinction within 5 years, regardless of the fertility rate (Fig. 12). However, these results are somewhat conservative. Patches of reedbed tend to be rather small, and Cuckoos tend to have rather large territories. A site like Llangorse Lake, with about 200 pairs of Reed Warblers, would hold about five female Cuckoos (Lindholm in press a). Running the model with a population ceiling of five further increases the probability of population extinction (Lindholm in press a). Evidence from empirical studies (Thomas 1990) suggests that a population of five females is likely to go extinct rapidly, through random variation in birth and death rates, and environmental variation, such as the odd hard winter increasing the mortality of Cuckoos or Reed Warblers. Without further immigration, a population founded by a single female at Llangorse Lake is likely to go extinct quickly.
Metapopulation modelFrequent immigration of Reed Warbler Cuckoos to a site such as Llangorse Lake is unlikely, as ringing data indicate that the mean distance between birthplace and breeding site is 27 km in Cuckoos (Lindholm in press a). Thus Reed Warbler Cuckoo populations in Britain may be described as a metapopulation, with a core cluster of small Cuckoo populations which are linked by frequent dispersal and which therefore rarely go extinct, in an area of high density of Reed Warbler populations (Fig. 13). As long-distance dispersal is rare, more distant populations will receive fewer migrants and will be more vulnerable to extinction. Once extinct, recolonisation would be slow. Populations of Reed Warblers distant from the core area would experience intermittent unpredictable cycles of parasitism. Even near or within the core, parasitism frequencies would fluctuate, because of the low carrying capacity of reedbed patches and demographic stochasticity in Cuckoo populations (Lindholm in press a).
CONCLUSIONS
Because birth and death rates are variable and unpredictable in Cuckoos, Reed Warblers are unable to predict from one year to the next how heavily they will be parasitised. Flexibility in egg rejection would allow Reed Warblers to adjust to their current situation, so that they would be less likely to mistakenly reject an egg of their own in years where there is no or little parasitism, and when Cuckoo numbers are high, to minimise the chance of rearing a Cuckoo young. Such a system would lead to differences in frequencies of egg rejection between populations, depending on the local parasitism rate. This is a feasible alternative hypothesis to that of genetic differences for differences in rejection behaviour between populations.
ACKNOWLEDGEMENTSI thank Robert Thomas and Mike Brooke for assistance in the field. The Brecon Beacons National Park, Oxwich National Nature Reserve, Wicken Fen Nature Reserve, the Llangorse Ringing Group, Stephen Rumsey and Roger and Rosanne Raikes provided access to the field sites and accommodation. I am grateful to Nick Davies and Mike Brooke for helpful discussions and for providing unpublished data. This work was financially supported by the Association of Commonwealth Universities, the Association of Field Ornithologists and the Association for the Study of Animal Behaviour.
REFERENCES
Alvarez, F. 1996. Model Cuckoo Cuculus canorus eggs accepted by Rufous Bush Chats Cercotrichas galactotes during the parasite’s absence from the breeding area. Ibis 138: 340-342.
Briskie, J.V., Sealy, S.G. & Hobson, K.A. 1992. Behavioral defenses against avian brood parasitism in sympatric and allopatric populations. Evolution 46: 334-340.
Brooke, M de L. & Davies, N.B. 1987. Recent changes in host usage by Cuckoos Cuculus canorus in Britain. Journal of Animal Ecology 56: 873-883.
Davies, N.B. & Brooke, M. de L. 1988. Cuckoos versus Reed Warblers: adaptations and counteradaptations. Animal Behaviour 36: 262-284.
Davies, N.B. & Brooke, M. de L. 1989a. An experimental study of co-evolution between the Cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. Journal of Animal Ecology 58: 207-224.
Davies, N.B. & Brooke, M. de L. 1989b. An experimental study of co-evolution between the Cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. Journal of Animal Ecology 58: 225-236.
Gibbons, D.W., Reid, J.B. & Chapman, R.A. 1993. The New Atlas of Breeding Birds in Britain and Ireland: 1988 - 1991. BTO, SOC, IWC. T & A D Poyser, London.
Harrison, S. 1991. Local extinction in a metapopulation context: an empirical approach. Biological Journal of the Linnean Society 42: 73-88.
Jensen, R.A.C. 1966. Genetics of cuckoo egg polymorphism. Nature, London 209: 827.
Knight, R.L. & Temple, S.A. 1986. Methodological problems in studies of avian nest defence. Animal Behaviour 34: 561-566.
Lindholm, A.K. Brood parasitism by the Cuckoo on patchy Reed Warbler populations in Britain. Journal of Animal Ecology, in press.
Lindholm, A.K. Tests of phenotypic plasticity in Reed Warbler defences against Cuckoo parasitism. Behaviour, in press.
Lindholm, A.K. & Thomas, R.J. Differences between populations ofReed Warblers in defences against brood parasitism. Behaviour, in press.
Lotem, A., Nakamura, H. & Zahavi, A. 1995. Constraints on egg discrimination and cuckoo-host co-evolution. Animal Behaviour 49: 1185-1209.
Moksnes, A. & Røskaft, E. 1989. Adaptations of Meadow Pipits to parasitism by the Common Cuckoo. Behavioural Ecology and Sociobiology 24: 25-30.
Moksnes, A., Røskaft, E. & Korsnes, L. 1993. Rejection of Cuckoo (Cuculus canorus) eggs by Meadow Pipits (Anthus pratensis). Behavioral Ecology 4: 120-127.
Nakamura, H. & Miyazawa, Y. 1997. Movements, space use and social organization of radio-tracked common cuckoos during the breeding season in Japan. Japanese Journal of Ornithology 46: 23-54.
Paradis, E., Baillie, S.R., Sutherland, W.J. & Gregory, R.D. 1998. Patterns of natal and breeding dispersal in birds. Journal of Animal Ecology 67: 518-536.
Punnett, R.C. 1933. Inheritance of egg-colour in the ‘parasitic’ cuckoos. Nature, London 132: 892-893.
Renshaw, E. 1991. Modelling Biological Populations in Space and Time. Cambridge; Cambridge University Press.
Rothstein, S.I. 1990. A model system for coevolution: avian brood parasitism. Annual Review of Ecology and Systematics 21: 481-508.
Soler, M. & Møller, A.P. 1990. Duration of sympatry and coevolution between the Great Spotted Cuckoo and its Magpie host. Nature, London 343: 748-750.
Takasu, F., Kawasaki, K., Nakamura, H., Cohen, J.E. & Shigesada, N. 1993. Modeling the population dynamics of a cuckoo-host association and the evolution of host defenses. American Naturalist 142: 819-839.
Thomas, C.D. 1990. What do real population dynamics tell us about minimum viable population sizes? Conservation Biology 4: 324-327.
Wyllie, I. 1981. The Cuckoo. London; B.T. Batsford.
Fig. 1. A county by county breakdown of parasitism frequencies of Reed Warblers in Britain from the British Trust for Ornithology (BTO) nest record scheme from 1932 to 1996 (n=10491 nest records). The national average is 6% (Lindholm in press a).
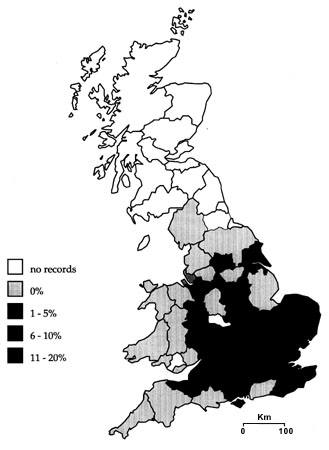
Fig. 2. Locations of the four study sites in Great Britain.
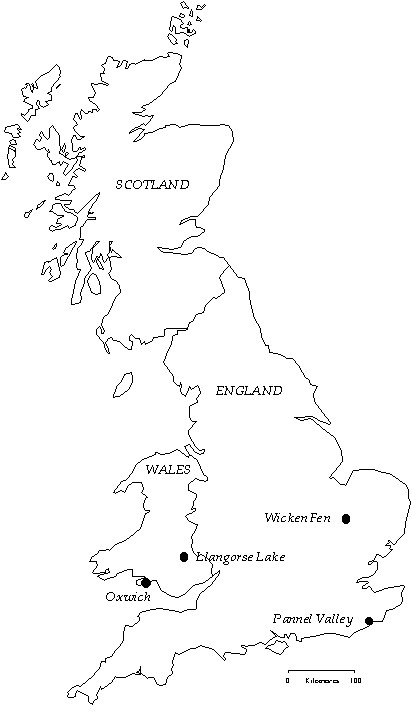
Fig. 3. Outcomes of experimental parasitism at each of the four study sites, from Lindholm and Thomas (in press). Results from Wicken Fen include data from Davies and Brooke (1988). Sample sizes are indicated above each column and asterisks indicate significant differences by pairwise G tests. Model eggs were deserted (3 cases at Llangorse Lake, 11 at Pannel Valley and 4 at Wicken Fen), ejected (2 cases at Llangorse Lake and 3 at Wicken Fen), or buried in the nest lining (1 case at Pannel Valley and 2 at Wicken Fen).
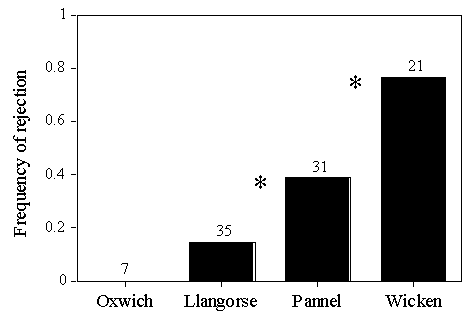
Fig. 4. A cost-benefit model of egg rejection in Reed Warblers.
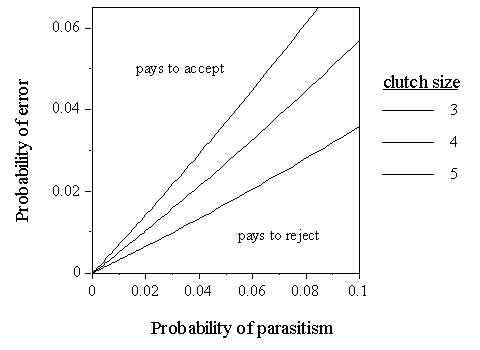
Fig. 5. Frequency of rejection of model eggs with (in black) and without (in white) presentation of a stuffed Cuckoo at the nest, from Lindholm (in press b) and Davies and Brooke (1988), for data from Wicken Fen.
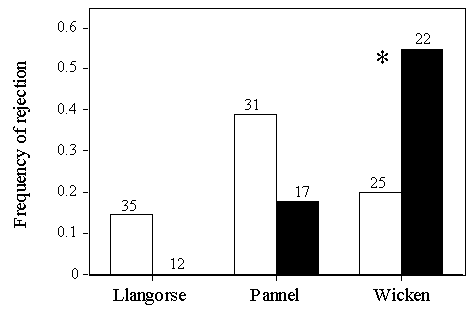
Fig. 6. Frequency of rejection of model Cuckoo eggs at Llangorse Lake without presentation of a stuffed Cuckoo, from experiment 1, and in response to the experimental treatment of showing a Cuckoo at the nest once, from experiment 2, and of showing Cuckoos in the habitat repeatedly (many times), as well as at the nest once, from experiment 3, from Lindholm (in press b).
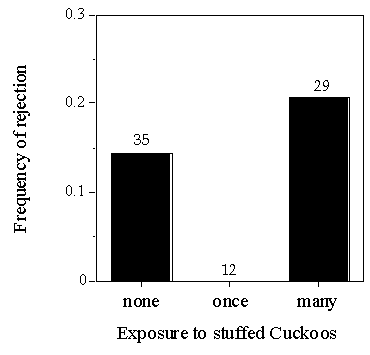
Fig. 7. Effect of clutch initiation date on the probability of rejecting unlike model eggs, at three sites, by weeks starting on the 10th May. In the bottom graph, filled circles indicate data from N.B. Davies and M. Brooke (unpublished) for 1984 to 1986, while open circles indicate results of experiments from 1993 (sample size in boldface) and 1995 (each trial is shown by one circle). Data for Llangorse Lake include eleven additional trials with unlike model eggs with outcomes that did not differ significantly from earlier trials.
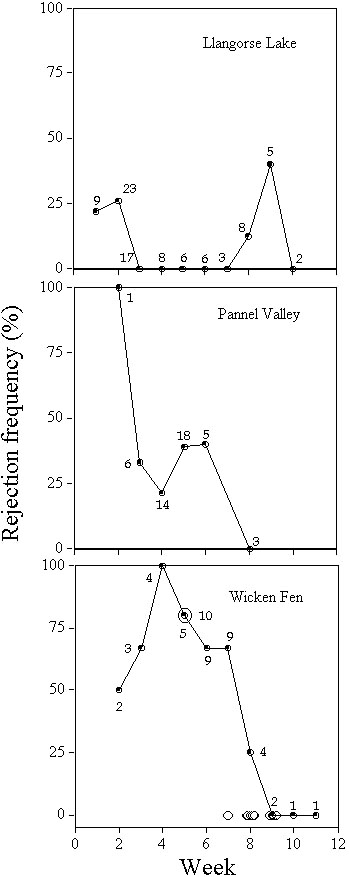
Fig. 8. Frequency of rejection of brown painted eggs versus model Cuckoo eggs at Llangorse Lake, from Lindholm (in press b).
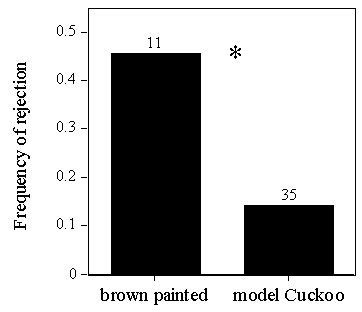
Fig. 9. Frequency of rejection of brown painted eggs at Llangorse Lake and Wicken Fen, from Lindholm (in press b).
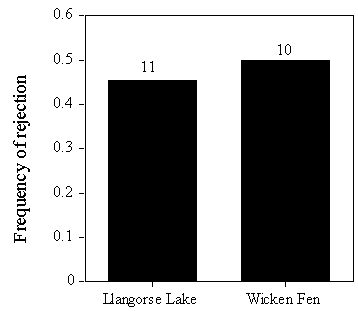
Fig. 10. Frequency of rejection of model eggs in relation to whether pecking was seen (in white) or not seen (in black), from Lindholm (in press b) and Davies and Brooke (1988). The methods of rejection were desertion at Llangorse Lake, burial (1 case) and desertion at Pannel Valley, and ejection (2 cases, of which one was from a nest where pecking was seen) and desertion at Wicken Fen.
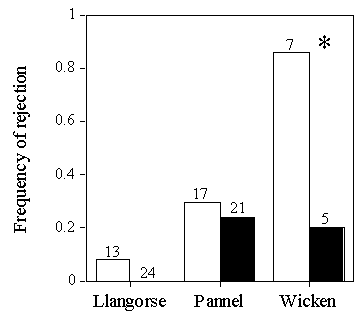
Fig. 11. The locations of all records of Cuckoo parasitism of Reed Warblers in Great Britain, from BTO nest record cards from 1932 - 1996 (Lindholm in press a). Larger circles indicate more records.
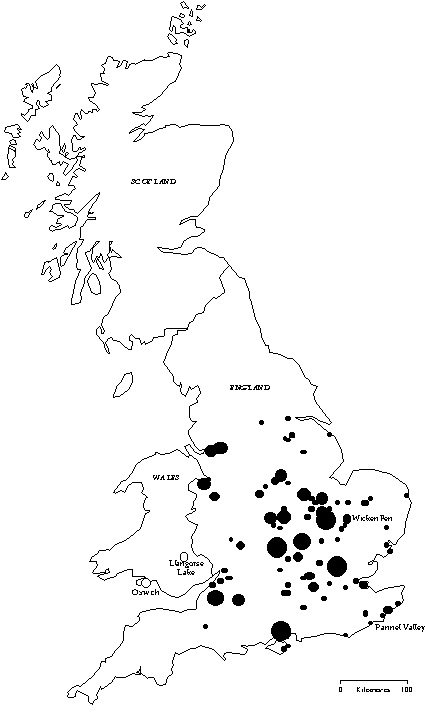
Fig. 12. Cumulative proportion of runs of the extinction model which ended in extinction, for three estimates of annual fertility (adapted from Lindholm in press a).
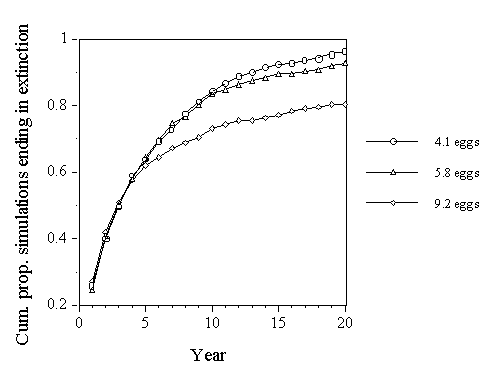
Fig. 13. Hypothesised metapopulation model of Reed Warbler Cuckoos in Britain, from Lindholm (in press a) and after Harrison (1991). Black dots represent populations which are linked by frequent dispersal and have a low probability of extinction. Hatched dots represent populations which receive occasional immigrants, while white dots represent extinct populations.