S51.2: Brood parasites: The advantages of being a different species
Juan J. Soler
Departamento de Biología Animal y Ecología, Facultad de Ciencias, Universidad de Granada. E-18071 Granada, Spain,
fax 34 9 58 243 238, e-mail jsolerc@goliat.ugr.es Soler, J.J. 1999. Brood parasites: The advantages of being a different species. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 3098-3106. Johannesburg: BirdLife South Africa.Avian brood parasites lay their eggs in the nests of other species (hosts) which incubate and rear the brood-parasite offspring. This reproductive strategy directly reduces the cost of reproduction (e.g. parental care) and, therefore, may be subject to natural selection. I discuss three additional advantages arising from the fact that host and parasite are different species and that, therefore, adult and nestling brood parasites apparently never have contact with each other. (1) In relation to the vertical transmission of pathogens, because some pathogens are host specific, the brood-parasite nestlings have a very low probability of being infected by the specific brood-parasite pathogens. Thus they have a reduced risk of being infected by pathogens compared with other nestlings which are reared in the nest of its own species. (2) In terms of the begging behaviour of brood-parasitic nestlings, because parental care is given by other species, there does not exist both genetic relatedness between foster parents and brood parasitic nestlings, nor a genetic correlation between exaggerated begging behaviour of an individual during the nestling stage and the costs when adult from the exaggerated begging behaviour of their offspring. Consequently, the level of begging behaviour in brood-parasitic nestlings is not constrained by the parent-offspring conflict or by such a genetic correlation. (3) Finally, brood parasites could also have the advantage of selecting foster parents for their offspring according to their parental quality. Brood parasites may be able to assess both the parental quality of the host pair and the quality of its territory using sexually selected traits when considering which nest to parasitise.
INTRODUCTION
Inter-specific avian brood parasites lay their eggs in nests of other species (hosts) which incubate and raise the parasitic offspring (Rothstein 1990). This reproductive strategy reduces the costs of reproduction because brood parasites receive no conspecific parental care (Payne 1974). The low cost of reproduction for brood parasites in comparison with other avian species (showing parental care) is a great advantage. This advantage has for example been related to the clutch size, clutch size of parasitic cuckoos being larger than that for non-parasitic cuckoos (Payne 1974).
On the other hand, because brood parasites cause enormous costs to their hosts, defence strategies against these parasites evolved in host populations. Among such counter-adaptations are recognition and rejection of parasitic eggs and nestlings (Rothstein 1990), thereby reducing the potential parasite reproductive success from clutch sizes (Payne 1974). Therefore, the large clutch size among brood parasites, resulting from the absence of parental care, could be counterbalanced by host defence against the brood parasites, and individual success (number of fledglings produced per breeding season) could be similar in parasitic and non-parasitic species, depending on the level of host defence. However, in any case, the total cost of breeding appears to be less in the parasitic than in the nesting cuckoos (Payne 1974).
Inter-specific brood parasitism has evolved several times in birds. It has been detected at least a single time in American cowbirds Molothrus spp. (Lanyon 1992) and finches (Vidua spp.; Klein & Payne 1998), and two times in Cuculidae (Aragon et al. in press; but see Hughes 1996). Moreover, it is likely that this reproductive strategy has evolved at least once in Honeyguides (Indicatoridae) and in Anatidae, because all the parasitic species are from the same genus and all other genera from the same family consist of non-parasitic species. In all the cases where brood parasitism has appeared, the most parsimonious evolutionary tree showed that this evolved from a non-parasitic species (Lanyon 1992; Klein & Payne 1998; Aragon et al. in press).
Brood parasites have the advantage of reducing the cost of reproduction, and, in the interaction with hosts, it has been demonstrated that some characteristics increase the probability of success of parasitic offspring. Such characteristics are, (1) parasite responses to host defences (e.g. egg and nestling mimicry, adult mimicry of potential host predators; for reviews, see Payne 1977; Rothstein 1990), (2) a reduction in the duration of laying (Brooker & Brooker 1991; Sealy et al. 1995), (3) a reduction in the duration of the incubation period (Briskie & Sealy 1990; Soler & Soler 1991), (4) a faster growth rate than that of host nestlings (Soler & Soler 1991), and (5) thicker egg shells than those of the host (Spaw & Rohwer 1987; Rahn et al. 1988; Brooker & Brooker 1989, 1991; Soler 1990; Moksnes et al. 1993). Most of these characteristics have been explained as adaptations of brood parasites counteracting host defences and increasing survival probability of parasitic nestlings. Therefore, they are of advantage in the brood parasite-host coevolutionary process. On the other hand, Hamilton and Orians (1965) suggested some characteristics that are necessary in the non-brood parasitic ancestor for a successful evolution of brood parasitism. Such preadaptations are: short incubation period relative to egg size, nestlings being broadly adapted to a diversity of food and determined egg laying (eggs are laid until a certain clutch size is reached). These characteristics are not, therefore, the result of a coevolutionary process between parasites and hosts, but of advantage for the evolution of brood parasitism. However, such preadaptations were selected a posteriori, and different unique attributes are proposed for each group of brood parasites (Payne 1977).
In the present paper, I propose three different advantages for brood parasites that arise simply from the fact that brood parasites and their hosts are different species, and thus, with a different evolution behind their life-history traits. These are; (1) related to the risk of infection and immune-system of both brood parasites and hosts, (2) related to the co-adaptation of parental and offspring characters (i.e. the genetic relatedness and the genetic correlation between the parental and offspring traits), and (3) related to the probability of exploitation of sexually selected traits of hosts signalling good parental or territory quality. Point number 1 has previously been explicated and tested for the Great Spotted Cuckoo Clamator glandarius and its Magpie Pica pica host (Soler et al. In press), point number 3 has been suggested for the same brood parasite-host system (Soler, J.J. et al. 1995), but never experimentally tested. Point number 2 however has never been suggested or tested. In general, the advantage for brood parasites of being different species from their hosts, with different life-history traits evolved, is a rather neglected aspect in the study of the interaction between brood parasites and their hosts
.Risk of infection and immune-system
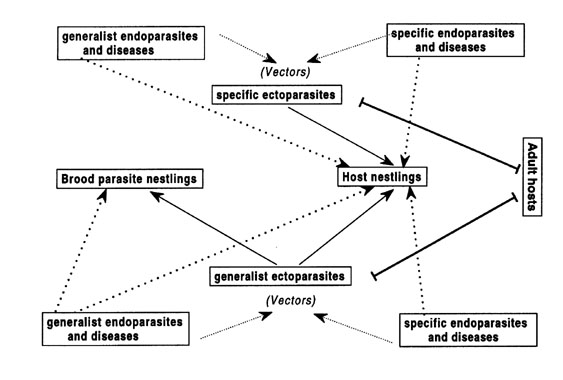
Most pathogens (general parasites) utilise fewer than 10 hosts species (Poulin 1998). Even though there are generalist pathogen species, many are highly host-specific (Brooks & McLennan 1993) that exploit only one or a few host species. Pathogen transmission from one host to another can occur between adults (horizontal transmission) or from parents to offspring (vertical transmission). On the basis of these pathogen generalities, Soler et al. (1998) hypothesised that nestling brood parasites experience restricted vertical transmission of pathogens because they have little or no contact with adult brood parasites (Fig. 1) (Payne 1977; Rothstein 1990).
By definition, a parasite (pathogen) is an organism living in or on another organism. The parasite feed on the host showing some degree of structural adaptation to it, and is frequently causing it some harm (Poulin 1998). It is clear that pathogens impose costs on their hosts (see examples in Clayton & Moore 1997, and, Poulin 1998). However, if the brood parasite nestling is reared by foster parents of a different species, the risk of being infected is greatly reduced compared to non-brood parasitic nestlings. Thereby, the cost of infections is reduced just because the foster parent and the brood parasite are different species. Some predictions from this hypothesis have been tested in the Great Spotted Cuckoo-Magpie system. Soler et al. (in press) found that the Great Spotted Cuckoo nestlings suffered less from specialist haematozoa than did magpie nestlings reared in the same nests, but no differences appeared with respect to the prevalence of a generalist ectoparasite (Fam. Carnidae).
Moreover, Møller (1997) suggested that parasitism influences the evolution of life histories. Pathogens often reduce the reproductive success of their hosts (e.g. Møller et al. 1990; Loye & Zuk 1991; Lehmann 1993) and parental effort should be adjusted to the reproductive value of their offspring (Schaffer 1974; Pianka & Parker 1975; Forbes 1993). Life-history theory predicts that parents should allocate less effort to offspring that show a lower reproductive value because of parasitism (Møller 1997). Thus, in the absence of brood-parasite nestling recognition, and because brood parasite nestlings are expected to be less often infected by pathogens (see above) compared with their foster siblings, parent hosts should pay more attention to pathogen-free nestlings (brood parasites) than to the – probably – infected ones (the parents’ own offspring).
However, no data have been available to test this hypothesis. The generally healthy appearance of the brood parasitic nestlings could, on the other hand, partially explain why they are preferably fed over the foster parents’ own nestlings. Both when the two nestling species are sharing the same nest (Soler, M. et al. 1995; Lichtenstein & Sealy 1998), and when only the brood-parasite nestling remains in the nest (Brooke & Davies 1989; Davies et al. 1998).
Co-adaptation of parent and offspring characters
In many organisms, traits expressed in one individual can also be influenced by, and function together with, traits that are expressed in different individuals in the previous generation. These so-called maternal effects (or more generally, indirect genetic effects) are present when the environment provided by parents influences the expression of traits in offspring (Cheverud & Moore 1994; Rossiter 1996; Wolf et al. 1998; Wolf & Brodie 1998). On the other hand, offspring traits are also able to influence the expression of parental traits, thereby provoking coadaptation of parental and offspring characters (Wolf & Brodie 1998). In altricial birds, parents have to feed their young in the nest during the nestling period. Thus, a conflict appears between the amount of food required by nestlings and that which the parents are willing to provide. This is known as the parent-offspring conflict (Trivers 1974), and its study and modelling have received increased attention recently (Godfray 1995a; Kilner & Johnstone, 1997). Most models suggest the existence of an equilibrium between levels of offspring begging and parental food provision, based on the genetic relationship between parents and offspring (Godfray 1991; Johnson & Grafen 1992; Godfray 1995b). This equilibrium is based on the fact that begging is a reliable (costly) signal by which parents obtain accurate information about the resource needs of their nestlings (Godfray 1995b). However, despite the fact that energetic costs are basic assumptions for most models predicting an equilibrium between offspring and parental traits related to begging behaviour, three recent studies have detected the absence of an energetic cost of begging (McCarty 1996; Leech & Leonard 1996; Soler et al. MS). The level of begging behaviour can be limited by the coadaptation between parental and offspring characters. If the level of begging is a genetically inherited trait (see Mock & Parker 1998), and if begging at a high rate is of selective advantage for an individual nestling because it increases the probability of reaching the adult stage (Mock & Parker 1998 and references therein), all individuals should be selected to beg at the maximum level. However, an exaggerated offspring begging behaviour implies an extra-effort for their parents and therefore a decrease in expected lifespan (or fitness) of their parents (or siblings). This could therefore prevent the evolution of this exaggerated offspring behaviour (Johnson & Grafen 1992; Briskie et al. 1994; Godfray 1995b). In addition, when an offspring with the exaggerated begging genotype reaches the reproductive age, their offspring would beg at the same level as their parents. The exaggeration of begging for food during the nestling stage would therefore have negative effects on the fitness of adults. This results in an negative genetic correlation between different individual stages (nestling and adult), which prevents the evolution of an exaggerated begging behaviour (Alexander 1974).
This genetic correlation does not, however, appear in obligate brood parasites, where the parents not feed their offspring. The brood parasite offspring is neither genetically related to their foster parents or siblings. It could therefore be predicted that the level of begging behaviour is higher in brood-parasites than in non- parasitic nestlings because an exaggerated begging behaviour does not have negative indirect genetic effects (maternal effects), or reduces fitness of its genetic relatives in obligate brood parasite species.
In accordance with this prediction, all available literature on begging behaviour of brood-parasite nestlings report that brood-parasitic nestlings beg more loudly and intensely than do their nest mates of the host species (Molothrus ater and M. bonariensis: Nice 1939; Gochfeld 1978; Cuculus canorus: Brooke & Davies 1989; Davies et al. 1998; Clamator glandarius: Redondo 1993; Soler et al. MS). Most of the theoretical models predict these results on the basis of the absence of a genetic relatedness between parasitic nestlings and their foster parents (Godfray 1991; Johnson & Grafen 1992; Godfray 1995b). Thus, being a different species is an inherent advantage for brood-parasite nestlings when eliciting feeding from their foster parents.
The probability of exploitation of sexually selected traits of hosts signalling good parental qualitySome adult traits are reliable signals of genetic or parental quality, and thereby selected through a sexual-selection process (good-gene, or good-parent sexual-selection process) (Andersson 1994; Møller 1994). Traits signalling good parental quality are common in most avian species. Thus, such traits probably also appear in the hosts of brood parasites. Theory on signalling evolution, mostly that considering sexually selected traits, is mainly based on conspecific communication. Selection favours individuals whose displays are more efficient at eliciting beneficial responses from the receptors (Johnstone 1997). However, it is known that signals can also be detected by undesired receptors, such as predators or parasites (Cade 1979; Turttle & Ryan 1981; Sakaluk & Belwood 1984). These are the costs which prevent the continuous evolution of exaggerated signals. Therefore, the signals we observe in nature should be those that strike the optimum balance between these two conflicting pressures for greater effectiveness and lower fitness costs (Wiley 1983, 1994; Endler 1992, 1993).
Adult brood parasites have the possibility of choosing foster parents for their offspring, not only at the species level, but also at the individual level. Parental quality of an individual bird is a variable trait, and, for brood parasites, it would be of selective advantage to select hosts of good parental quality to rear their offspring (Soler et al. 1995).
Thus, for brood parasites, it would be of great selective advantage to recognise those kind of sexually selected signals in host species, and then base host selection on such traits. This would be the case when there is no relationship between the degree of parental quality, reflected by sexually selected traits, and the degree of defence against brood parasitism. Lotem et al. (1992) found a lower defence level in one year old than in adults Great Reed Warblers Acrocephalus arundinaceus against the European Cuckoo. They thereby detected that the parasitism rates in those first years breeding warblers (low parental quality) were higher than in adults. However, it has been demonstrated that the level of host defence depends on time of sympatry between the brood parasite and the host population (Soler & Møller 1990; Briskie et al. 1992). Thus, in a recently parasitised host population, or when parental quality and level of host defence are not related, brood parasites should select good-parental-quality hosts simply because this would improve their fitness. Accordingly, Soler et al. (1995) found that the Great Spotted Cuckoo selects Magpie nests on the basis of nest volume, a characteristic indicating territory quality and willingness to invest in reproduction.
If brood parasites exert differential selection pressures on individual hosts with an exaggerated sexually selected trait signalling good parental quality, we would expect a selection for a decrease in that signal to reach the optimum balance between the conflicting pressures in parasitised, and not parasitised areas. In a study of 15 different European magpie populations we (Soler et al. in prep.) found that volume of magpie nests was smaller in areas suffering from parasitism that in those where the Great Spotted Cuckoo was absent.
In conclusion, avian brood parasites have a potential advantage over non-brood-parasite species, simply because brood parasites are different species than their foster parents. The brood parasite gains several benefits, since (1) the risk of infection in brood-parasite nestlings is lower, (2) the costs of an exaggerated begging behaviour is low for brood parasite nestlings, and (3) brood parasite adults are able to use host traits signalling good parental quality to choose foster parents for their young. Studies on these aspects are needed for a better understanding of the brood-parasite / host systems and their coevolutionary interactions.
ACKNOWLEDGEMENTS
I am most grateful to Juan Gabriel Martínez, Anders Pape Møller and Manuel Soler (coauthors of the papers) for our fruitful collaboration. The ideas to this article have emerged from our work with Magpies and Great Spotted Cuckoos, and distilled and clarified through our cooperation. Eivin Røskaft kindly commented and corrected a previous version of the article. Funds were provided by a post-doctoral grant by the Spanish Research Council (DGICYT, PB 94-0785).REFERENCES
Alexander, R.D. 1974. The evolution of social behaviour. Annual Review of Ecology and Systematics. 5: 325-383.
Andersson, M. 1994. Sexual selection. Princeton University Press: 599pp.
Aragon, S., Møller, A.P., Soler, J.J. & Soler, M. In press. Molecular phylogeny of cuckoos supports a polyphyletic origin of brood parasitism. Journal of Evolutionary Biology.
Briskie, J.V. & Sealy, S.G. 1990. Evolution of short incubation periods in the parasitic cowbirds, Molothrus spp. Auk 107: 789-794.
Briskie, J.V., Naugler, C.T. & Leech, S.M. 1994. Begging intensity of nestling birds varies with sibling relatedness. Proceedings of the Royal Society of London Series B 258: 73-78.
Briskie, J.V., Sealy, S.G. & Hobson, K.A. 1992. Behavioral defenses against avian brood parasitism in sympatric and allopatric populations. Evolution 46: 334-340.
Brooke, M. de L. & Davies, N.B. 1989. Provisioning of nestling cuckoos Cuculus canorus by reed warbler Acrocephalus scirpaceus host. Ibis 131: 250-256.
Brooker, M.G. & Brooker, L.C. 1989. The comparative breeding behaviour of two sympatric cuckoos, Horsfield's bronze-cuckoo Chrysococcyx basalis and the shining bronze-cuckoo C. lucida, in western Australia: A new model for the evolution of egg morphology and host specificity in avian brood parasites. Ibis 131: 528-547.
Brooker, M.G. & Brooker, L.C. 1991. Eggshell strength in cuckoos and cowbirds. Ibis 133: 406-413.
Brooks, D.R. & McLennan, D.A. 1993. Parascript: Parasites and the Language of Evolution. Smithsonian Institution Press.
Cade, W.H. 1979. The evolution of alternative male reproductive strategies in crickets. In: Blum, M.S. & Blum, N.A. (eds) Sexual selection and reproductive competition in insects. Academic Press: 343-379.
Clayton, D.H. & Moore, J. (eds.) 1997. Host-parasite evolution. General principles & avian models. Oxford University Press: 473pp.
Cheverud, J.M. & Moore, A.J. 1994. Quantitative genetics and the role of the environment provided by relatives in the evolution of behavior. In: Boake, C.R.B. (ed) Quantitative genetic studies of behavioral evolution. University of Chicago Press, pp: 67-100.
Davies, N.B., Kilner, R.M. & Noble, D.G. 1998. Nestling cuckoos, Cuculus canorus, exploit hosts with begging calls that mimic a brood. Proceedings of the Royal Society of London Series B 265: 673-678.
Endler, J.A. 1992. Signals, signal conditions and the direction of evolution. American Naturalist 139: S125-S153.
Endler, J.A. 1993. Some general comments on the evolution and design of animal signalling systems. Philosophical Transactions of the Royal Society of London Series B 340: 215-225.
Forbes, M.L.R. 1993. Parasitism and host reproductive effort. Oikos 67: 444-450.
Gochfeld, M. 1978. Begging by nestling shiny cowbirds: adaptive or maladaptive. Living Bird 17: 41-50.
Godfray, H.C.J. 1991. Signalling of need between parents and offspring. Nature 352: 328-330.
Godfray, H.C.J. 1995a. Evolutionary theory of parent-offspring conflict. Nature 376: 133-138.
Godfray, H.C.J. 1995b. Signalling of need between parents and young: parent-offspring conflict and sibling rivalry. American Naturalist 146: 1-24.
Hamilton, W.J. & Orians, G.H. 1965. Evolution of brood parasitism in altricial birds. Condor 67: 361-382.
Hughes, J.M. 1996. Phylogenetic analysis of the Cuculidae (Aves, Cuculiformes) using behavioral and ecological characters. Auk 113: 10-22.
Johnson, R.A. & Grafen, A. 1992. The continuous Sir Philip Sydney game: a simple model of biological signalling. Journal of Theoretical Biology 156: 215-234.
Johnstone, R.A. 1997. The evolution of animal signals. Krebs, J.R. & Davies, N.B. (eds) Behavioural ecology. An evolutionary approach. Blackwell Science 155-176.
Kilner, R. & Johnstone, R.A. 1997. Begging the question: are offspring solicitation behaviours signals of need? Trends in Ecology and Evolution 12: 11-15.
Klein, N.K. & Payne, R.B. 1998. Evolutionary associations of brood parasitic finches (Vidua) and their host species: Analyses of mitochondrial DNA restriction sites. Evolution 52: 566-582.
Lanyon, S.M. 1992. Interspecific brood parasitism in blackbirds (Icterinae): A phylogenetic perspective. Science 255: 77-79.
Leech, S.M. & Leonard, M.L. 1996. Is there an energetic cost to begging in nestling tree swallows (Tachicineta bicolor)? Proceedings of the Royal Society of London Series B 263: 983-987.
Lehmann, T. 1993. Ectoparasites: direct impact on host fitness. Parasitology Today 9: 8-13.
Lichtenstein, G. & Sealy, S.G. 1998. Nestling competition, rather than supernormal stimulus, explains the success of parasitic brown-headed cowbird chicks in yellow warbler nests. Proceedings of the Royal Society of London Series B 265: 249-254.
Lotem, A., Nakamura, H. & Zahavi, A. 1992. Rejection of cuckoo eggs in relation to host age: a possible evolutionary equilibrium. Behavioral Ecology 3: 128-132.
Loye, J.E. & Zuk, M. (eds) 1991. Bird-parasite interactions: ecology, evolution and behavior. Oxford University Press: 406pp.
McCarty, J.P. 1996. The energetic cost of begging in nestling passerines. Auk 113: 178-188.
Mock, D.W. & Parker, G.A. 1998. The evolution of sibling rivalry. Oxford Series in Ecology and Evolution. Oxford University Press: 464pp.
Moksnes, A., Røskaft, E & Braa, A.T. 1993. Rejection behavior by common cuckoo hosts towards artificial brood parasite eggs. Auk 108: 348-354.
Møller, A.P. 1994. Sexual selection and the barn swallow. Oxford Series in Ecology and Evolution. Oxford University Press: 365pp.
Møller, A.P. 1997. Parasitism and the evolution of host life history. In: Clayton, D.H. & Moore, J. (eds) Host-parasite evolution. General principles & avian models. Oxford University Press: 105-127.
Møller, A.P., Allander, K. & Dufva, R. 1990. Fitness effects of parasites on passerine birds: a review. In: Blondel, J., Gosler, A., Lebreton, J.-D. & McCleery (eds) Population biology of passerine birds: an integrated approach. Springer-Verlag: 269-280.
Nice, M.M. 1939. Observations on the behavior of a young cowbird. Wilson Bulletin 51: 233-239.
Payne, R.B. 1974. The evolution of clutch size and reproductive rates in parasitic cuckoos. Evolution 28: 169-181.
Payne, R.B. 1977. The ecology of brood parasitism in birds. Annual Review of Ecology and Systematics 8: 1-28.
Pianka, E.R & Parker, W.S. 1975. Age-specific reproductive tactics. American Naturalist 109: 453-464.
Poulin, R. 1998. Evolutionary Ecology of Parasites. From individual to communities. Chapman & Hall: 212 pp.
Rahn, H., Curran-Everett, L. & Booth, D.T. 1988. Eggshell differences between parasitic and non-parasitic Icteridae. Condor 90: 962-964.
Redondo, T. 1993. Exploitation of host mechanisms for parental care by avian brood parasites. Etología 3: 235-208.
Rossiter, M.C. 1996. Incidence and consequences of inherited environmental effects. Annual Review of Ecology and Systematics 27: 451-476.
Rothstein, S.I. 1990. A model system for coevolution: avian brood parasitism. Annual Review of Ecology and Systematics 21: 481-508.
Sakaluk, S.K. & Belwood, J.J. 1984. Gecko phonotaxis to cricket calling song – a case of satellite predation. Animal Behaviour 32: 659-662.
Schaffer, W.M. 1974. Selection for optimal life histories: the effects of age structures. Ecology 55: 291-303.
Sealy, S.G., Neudorf, D.L. & Hill, D.P. 1995. Rapid laying by brown-headed cowbirds Molothrus ater and other parasitic birds. Ibis 137: 76-84.
Soler, J.J., Møller, A.P., Soler, M. & Martínez, J.G. Interactions between a brood parasite and its host in relation to parasitism and immune defence. Evolutionary Ecology In press.
Soler, J.J., Soler, M., Møller, A.P. & Martínez, J.G. 1995. Does the great spotted cuckoo choose magpie hosts according to their parenting ability? Behavioral Ecology and Sociobiology 36: 201-206.
Soler, M. 1990. Relationship between the great spotted cuckoo, Clamator glandarius, and its corvid hosts in a recently colonized area. Ornis Scandinavica 21: 212-223.
Soler, M., Martínez, J.G., Soler, J.J. & Møller, A.P. 1995. Preferential allocation of food by magpies Pica pica to great spotted cuckoo Clamator glandarius chicks. Behavioral Ecology and Sociobiology 37: 7-13.
Soler, M. Møller, A.P. 1990. Duration of sympatry and coevolution between great spotted cuckoo and its magpie host. Nature 343: 748-750
Soler, M. & Soler, J.J. 1991. Growth and development of great spotted cuckoos and their magpie host. Condor 93: 49-54.
Soler, M., Soler, J.J., Martínez, J.G. & Moreno, J. MS. Begging behavior and its energetic cost in great spotted cuckoo and magpie host chicks. Auk.
Spaw, C.D. & Rohwer, S. 1987. A comparative study of eggshell thickness in cowbirds and other passerines. Condor 89: 307-318.
Trivers, R.L. 1974. Parent-offspring conflict. American Zoology 14: 249-264.
Turttle, M.D. & Ryan, M.J. 1981. Bat predation and the evolution of frog vocalisations in the Neotropics. Science 214: 677-678.
Wiley, R.H. 1983. The evolution of communication: information and manipulation. In Halliday, T.R & Slater, P.J.B. (eds) Communication. Blackwell Scientific Publications: 82-113.
Wiley, R.H. 1994. Errors, exaggeration and deception in animal communication. In: Real, L.A. (ed.) University of Chicago Press: 157-189.
Wolf, J.B. & Brodie III, E.D. 1998. The coadaptation of parental and offspring characters. Evolution 52: 299-308.
Wolf, J.B., Brodie III, E.D., Cheverud, J.M., Moore, A.J. & Wade, M.J. 1998. Evolutionary consequences of indirect genetic effects. Trends in Ecology and Evolution 13: 64-69.
Fig. 1. Risk of directly transmission of pathogens in host and brood parasite nestlings. continuous arrows represent direct transmission from adult hosts and discontinuous arrows represent transmission mediated by ectoparasite vectors. Bars represent ectoparasites on adult hosts.
