S50.5: Molecular evidence for relationships of Malagasy birds
Jon Fjeldså1, Steven M. Goodman2, Thomas S. Schulenberg3 & Beth Slikas4
1Zoological Museum, Universitetsparken 15, DK-2100 Copenhagen, Denmark, fax 45 35 32 10 10, e-mail jfjeldsaa@zmuc.ku.dk; 2Department of Zoology, Field Museum of Natural History, Roosevelt Road at Lake Shore Drive, Chicago Illinois 60605-2495, USA, e-mail sgoodman@fmnh.org and WWF, BP 738, Antananarivo (101), Madagascar, e-mail wwfrep@dts.mg; 3Department of Zoology, Field Museum of Natural History, Roosevelt Road at Lake Shore Drive, Chicago Illinois 60605-2495, USA, e-mail tschulenberg@fmnh.org; 4Molecular Genetics Laboratory, National Zoological Park, Smithsonian Institution, 3001 Connecticut Avenue NW, Washington, D.C. 20008, USA, e-mail bslikas@nzp.si.edu
Fjeldså, J., Goodman, S.M., Schulenberg, T.S. & Slikas, B. 1999. Molecular evidence for relationship of Malagasy birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 3084-3094. Johannesburg: BirdLife South Africa.The avifauna of Madagascar contains many elements of uncertain phylogenetic affinities and biogeographic origins. As is often the case with traditional avian taxonomy, morphological comparisons, and consequent biogeographic assumptions, can be very misleading. With new approaches to phylogenetic analysis and the use of molecular data, some patterns begin to emerge. Here we present new data demonstrating that the Malagasy 'Phyllastrephus' species are not Phyllastrephus and probably are not pycnonotids. We also review previous studies incorporating molecular data to resolve the phylogenetics of Malagasy birds.
INTRODUCTION
In 1972 Battistini & Richard-Vindard published a synthesis of the biogeography of Madagascar that included a chapter by Jean Dorst on 'The evolution and affinities of the birds of Madagascar.' Since the publication of this landmark work a considerable amount of new data has become available on natural history, species diversity and distribution of birds on the island. Furthermore, tissue has been collected from Malagasy birds for molecular studies. All these new data provide a fresh perspective and the means to propose explicit hypotheses on the relationships of Malagasy birds. At the same time, we know more about the sequence of events and time scale of the breakup of Gondwana, which is crucial to our understanding of when and how various plant and animal groups arrived on the Malagasy landmass. The non-marine fossil record on Madagascar, since the Cretaceous (Krause et al. 1997) is virtually non-existant, and provides clues only about the most recent past.
It is evident that a number of colonisations of Madagascar, including all those of now living birds, took place after Madagascar was separated from Africa in the Jurassic. The most recent reconstructions (Coffin & Rabinowitz 1992; see also Krause et al. 1997) show that Madagascar first slid southwards (from the pre-drift position in the Somali Basin) along the African coast, before it swung east to its present position by the end of the Cretaceous. Throughout this drifting period it was separated from Africa by a deep trench.
Because of the considerable depth of the Mozambique channel it seems that all avian colonisations must have been 'rare sweepstakes dispersal' by flying or rafting. Millot (1972: 750) claimed to have shown that great rivers, such as the Ruvuma and the Zambesi, may carry large floating masses of vegetation with a variety of animals in the direction of Madagascar, however for at least forest-dwelling birds this is not a conceivable means of dispersal. It has been assumed that the (young volcanic) Comoro Islands could have served as stepping stones. What is a more interesting idea is that, because of tectonic changes along the Davie Ridge strike-and-slip fault there may have been additional mid-Cenozoic stepping stones (Seamounts Paisley, Macua, Antandroy and Betsileo) in the middle of the Mozambique Channel. The presence of karstic Miocene limestones on three of these seamounts (Leclaire et al. 1989) suggests that they were not completely submerged during this period and provided a possible island-hopping route. Such island-hopping would have been aided by the suitable direction of the giant Gondwanan monsoon systems (Krause et al. 1997).
Unfortunately the interpretation of the biogeographic relationships of the Malagasy fauna is muddled by problems encapsulated in its classification. Considering the state of knowledge about species and subspecies of birds, there has been remarkably little progress in rationalising the composition of genera, tribes and families. This seems largely to be a historical accident of the parochial compartmentalisation that owes much to major regional faunistic studies. It was perhaps guided by a conviction that large water gaps would safely delimit the hierarchy of nature, which in the past could take little account of plate tectonics, as known today. Thus, the classification of Malagasy birds still is confounded by the historical approach of taxonomists treating a priori the origins of the island's vertebrate fauna as distant from Asia and Africa. The opposite problem also existed of trying to fit Malagasy species into African groups.
New morphological and molecular data have shown that many of these past taxonomic decisions were inappropriate, and confused the discussion about age and affinities of the Malagasy avifauna. The molecular data open new possibilities for defining monophyletic lineages and for providing some idea about the timing of events, despite the absence of fossils.
In the present paper we will review the molecular evidence relating to Malagasy birds, as well as some other phylogenetic studies which need to be followed up by DNA divergence data. We will review those few DNA hybridisation data (Sibley & Ahlquist 1990) that are of relevance for Malagasy birds, and a few studies using mtDNA and rDNA sequences. We present new data relating to the Malagasy greenbuls (genus Phyllastrephus) and their relationships to groups inhabiting other land masses.
RESULTS
The ancient Malagasy groups.
The following groups of bird are presumed to be relicts of ancient lineages.
Aepyornithidae, elephant birds
The relationships, origins, and species diversity of this recently extinct group is poorly known. Two genera are currently recognized, Aepyornis and Mullerornis, and there is complete confusion at the species level with names associated with isolated bones and eggshell fragments (Brodkorb 1963). Samples of recently excavated bone contain no retrievable or highly fragmented DNA (A. Cooper, pers. comm.) and the relationships of this group were not addressed in recent reviews of the biochemical systematics of ratites (Lee et al. 1997; Cooper 1997).
Mesitornithidae, Mesites
Although the anatomy of the Mesitornithidae, which are endemic to Madagascar, is highly specialized (Glenny & Friedmann 1954) they are usually regarded as 'primitive' relatives of rails, although sometimes placed in a separate order (review in Sibley & Ahlquist 1990). The interpretation of osteological characters of this group by Olson (1985) indicated that the mesites were part of an ancient group that included such diverse families as Ardeidae and Eurypygidae. Sibley & Ahlquist (1990) lacked material of this group for their DNA hybridization studies. Recent studies of 12S rDNA by Houde et al. (1997) provide inconclusive evidence of the origin and relationships of mesites. Their genetic distance from classically defined Gruiformes is considerable and they may not be part of this radiation.
Couinae, Couas
There is no common agreement on whether the couas of Madagascar are related to other (Asian, Neotropical) ground cuckoos or to the Old World malkohas, athough most often they are associated with malkohas, based on the similar soft plumage and platform type of nests. The phylogenetic analysis by Seibel (1988, reviewed in Payne 1997) fails to extract the phylogenetic component from the mixture of functional and genealogical information in osteological characters. Hughes (1996), using behavioural and ecological characters, suggest that couas are the sister taxon of Neotropical ground cuckoos (Neomorphinae). Couas were not included in the DNA hybridization data of Sibley & Ahlquist (1990), but given the very deep subdivision between typical cuckoos and the coucals/malkoas (T50H 13.0), and of the Cuculiformes as a whole (T50H 17.6), the couas could represent a lineage from as far back in time as the Oligocene (assuming 1.0 = 2.3 Mya for passerines and probably more for non-passerines).
Leptosomatidae, cuckoo-roller
Using varying characters such as the feeding apparatus, osteology, and muscle structure and DNA hybridization data (Cracraft 1971; Maurer & Raikow 1981; Burton 1984; Sibley & Ahlquist 1990), this endemic family of Madagascar and the Comoros is almost universally considered allied to the coraciiforms. Sibley & Ahlquist (1990) found its DNA divergence from the Coraciidae to be T50H 13.9, which suggests a separation in the Eocene. According to Olson (1985) rollers probably were widely distributed in the Eocene, and a fossil that is virtually indistinguishable from the living Eurystomus is known from the lower Eocene of Wyoming. Today, the African Broad-billed Roller Eurystomus glaucurus breeds in Madagascar and migrates to Africa (where the species also breeds widely).
Brachypteraciidae, ground-rollers
This group is in need of study. To our knowledge no biochemical research has been published. Older studies based largely on plumage or osteological characters seem to indicate that its divergence is probably a deep node within the Coraciiformes (Cracraft 1971). There is much good evidence to show that by the early Tertiary, members of the Coraciiformes were a dominant type of arboreal 'perching' birds (Olson 1985).
Possible mid-Tertiary colonisations
Philepittidae, Asities
On the basis of morphological characters, Prum (1993) demonstrated that this endemic Malagasy group, with two very different looking genera Philepitta and Neodrepanis, are specialised broadbills (Eurylaimidae) and should be considered a subfamily of that group. Their sister group was proposed to be the African Green Broadbill Pseudocalyptomena graueri, which inhabits a few humid forest sites in the Western (Albertine) Rift area. According to the tree topology suggested by Prum (1993), the asities are definitively younger than the divergence between Calyptomena and other Oriental broadbills at T50H 10.8 (Sibley & Ahlquist 1990). Probably, Madagascar could have been colonised from Africa after this continent 'collided' with Asia in the early Miocene, when there was a short period of humid forest connection between the two biogeographic regions (Axelrod & Raven 1978). Evolutionary tendencies within the asities is discussed by Prum & Razafindratsita (1997).
Vangidae, vangas
The vangas are endemic to Madagascar and the Comoro Islands. They exhibit a remarkable degree of morphological variation, particularly in bill shape, and are cited as one of the most striking examples of an island radiation in the bird world. DNA hybridization data placed Leptopterus (Cyanolanius) madagascariensis together with the African helmetshrikes Prionops and the Oriental Tephrodornis (divergence T50H 5.4) (Sibley & Ahlquist 1990). Thus, a new and diverse group (Vangini) was erected for vangas and various African and Oriental 'shrikes' and 'flycatchers' of woodland and savanna scrub; which were in turn joined with the African malaconotine bush-shrikes. The tree topology would support a rapid radiation in African forest habitats since the early Miocene (based on the split from the Oriental ioras, Aegithina, at T50H 7.0) and thereafter an adaptation to woodland and scrub, and colonisations to Madagascar and southeast Asia.
Schulenberg (1995) used DNA sequence data from the mitochondrial cytochrome b gene to investigate the phylogeny of 14 species of vangas (all species except for Red-shouldered Vanga Calicalicus rufocarpalis Goodman et al. 1997). Robust support was found for two clades of vangas, uniting two (Rufous Vanga Schetba rufa - Helmet Vanga Euryceros prevostii) and six species (the three species of stout-billed vangas Xenopirostris spp. - Sickle-billed Vanga Falculea palliata - White-headed Vanga Artamella viridis - Bernier's Vanga Oriolia bernieri) respectively. On the other hand, relationships between these two clades, or to any of the remaining six vanga species, remain unresolved. Thus, there is no support for the traditional view that vangas are monophyletic. Basal patterns of relationship within the corvid radiation, including potential sister-group relationships between any vangas and other corvids, likewise are unresolved. Until the relationships of these groups are worked out it is impossible to propose clear hypotheses on the origin and means of dispersal of ‘vangas’ to Madagascar.
Phyllastrephus, greenbuls
Some half-dozen Malagasy passerines were once placed (variously) in the genera Bernieria, Crossleyia, Xanthomixis and Oxylabes (in the Timaliidae). Salomonsen (1934) suggested that these, together with Crossley's Babbler Mystacornis crossleyi, and the jerys, Hartertula and Neomixis, may represent a single Malagasy radiation. Irwin (1983) suggested to add Thamnornis Warbler Thamnornis chloropetoides, and it also conceivable that other Malagasy forest 'warblers' and the recently discovered Cryptic Warbler Cryptosylvicola randrianasoloi (Goodman et al. 1996) belong here. However, Delacour (1943) moved the four first-mentioned genera to the African genus Phyllastrephus in the Pycnonotidae. Later, Benson & Irwin (1975) argued to move the species xanthophrys back to the Timaliidae in the monotypic genus Crossleyia. According to Olson (1989) the Malagasy P. madagascariensis and zosterops show no trace of the pycnonotid ossification in the nostril, and 'are almost certainly not bulbuls, much less members of the genus Phyllastrephus'.
Within Rand & Deignan's (1960) treatment of the Pycnonotidae five species of endemic Malagasy Phyllastrephus were recognized: Long-billed Greenbul madagascariensis with two subspecies (madagascariensis and inceleber), Spectacled Greenbul zosterops with four subspecies (fulvescens, andapae, zosterops, ankafanae), Dusky Greenbul tenebrosus, Yellow-browed Oxylabes xanthophrys, and Gray-crowned Greenbul cinereiceps. Subsequently, Colston (1972) described Appert's Greenbul P. apperti.
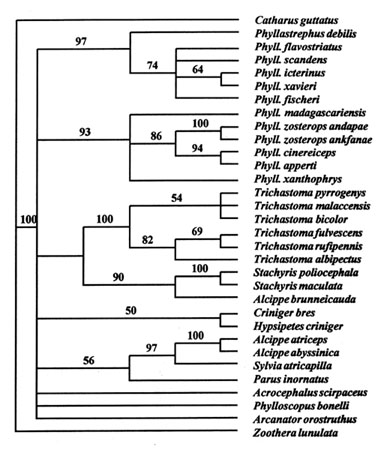
Excluding P. tenebrosus, tissue samples of all of these species were available for the study presented here. Standard procedures were used for cell lysis, proteinase digestion in SDS-based extraction buffer, organic purification using phenol/chloroform, and ethanol precipitation in the presence of ammonium acetate, and PCR sequencing. A 1065 base pair (bp) fragment of mitochondrial DNA cytochrome b was amplified and sequenced using either direct single-stranded or cycle sequencing. Sequences of Catharus, Zoothera, Parus, Acrocephalus, Phylloscopus and Sylvia were obtained from GenBank. Phylogenetic analyses were performed using PAUP* (version 4.0d64, Swofford 1998) and maximum-parsimony as well as maximum-likelihood analysis on a matrix of 1021 bp (positions 15000-16020 of Desjardins & Morais 1990). In the parsimony analysis, a step-matrix was imposed weighting transversions over transitions by factors of 5 and 10 to reduce the effect of saturated transitions. These two weightings gave similar results. A heuristic search yielded 2 most-parsimonious trees (CI=0.336, RI=0.551). However, the tree topology varied on certain points according to algorithm and choice of outgroup. For this reason we present a parsimony consensus tree with bootstrap values above 50% (Fig. 1).
The stable branches are: (1) The Malagasy Phyllastrephus species are always together and separated from Pycnonotidae and from Timaliidae. (2) The African greenbuls always forms a branch on its own (except for Criniger and Hypsipetes, which are unstable). (3) African Alcippe is always away from other babblers and together with a Sylvia warbler. The relationships of these groups to 'traditional warblers' (Acrocephalus, Phylloscopus, Sylvia) and to Parus are unstable. Dappled Mountain-Robin Arcanator orostruthus (currently classified as a thrush or as a timalliid babbler, and previously as a greenbul, see Benson & Irwin 1975) clusters with babblers in the most-parsimonious tree, but this position is unstable.
The average 3rd position transversion divergence amongst Malagasy Phyllastrephus is 11%, and the divergence towards African Phyllastrephus 18.4% and towards all Pycnonotidae 20.2%. The divergence from Timaliidae is 19.1% and from 'traditional warblers' 18.1%. Based on these and other divergence figures, there seems to have been a bush-like divergence amongst these groups 33-40 Mya (assuming 0.5% divergence per million years; Irwin et al. 1991). Similarly, the DNA hybridization data suggest a very dense packing of nodes among the warbler-like birds within the T50H interval 10.6-8.2 (Sibley & Ahlquist 1990: 865-6). However, this gives a somewhat lower age of 19-24 Mya, which is the lower Miocene. Except for very recent divergences, DNA hybridization dates are possibly more reliable than the cytochrome b dates (Slikas 1997). Given the high level of ts saturation, it is not surprising that these closely packed basal nodes cannot be resolved.
Because of our failure to identify the sister taxon of the Malagasy Phyllastrephus we cannot propose a specific hypothesis about the colonisation of Madagascar. However, it is clear that these Malagasy species were misplaced in Phyllastrephus and should be transferred to the genus Berniera. It also seems justified to erect a separate family for these species.
All of these species are forest-dwelling, and all except apperti and certain populations of madagascariensis and zosterops, occur in the eastern humid forests. Madagascariensis and zosterops also have subspecies that occur in areas of dry forest and apperti is only known from the southwest in the zone of transitional forest. At some eastern forest sites a maximum of five species occur on the slopes of the same mountain and up to four species occur in sympatry and often in the same mixed-species foraging flocks (Goodman et al. 1997). Within these sympatrically occurring forms there are distinct differences between the species in general body size, bill shape and length, and tarsus length. Some species such as xanthophrys and tenebrosus are largely terrestrial, while others such as zosterops and madagascariensis are almost exclusively arboreal. Also other marked differences are found, in feeding stratum and prey selection (Thompson 1987, Goodman & Parrillo 1997). Thus, these species represent another adaptive radiation within the Malagasy avifauna, with clear divisions of niche utilisation amongst sympatric species.
B. apperti and cinereiceps are each others closest relatives (corrected sequence divergence 7.8%) and occur in allopatry. The latter species has a broad distribution across the eastern humid forest and occurs in montane forest generally above 1200 m, while apperti is known from a limited geographic range in the southwest in the zone of transition between humid and deciduous forest. There is good evidence that portions of southwestern Madagascar were once distinctly more mesic in the recent geological past (Burney 1993) and several typically eastern humid forest species have either sibling species or disjunct ranges between this region and the southwest. The separation of these populations and subsequent differentiation is almost certainly a result of natural habitat changes that gave rise to vicariantly separated populations.
Distinctive taxa in need of phylogenetic assessment
The (extinct?) Madagasar Pochard Aythya innotata belongs to a clade that also includes the Ferruginous White-eye A. nyroca, widespread in Eurasia, and the Australian White-eye A. australis of southeast Asia and Australia, based on DNA sequences from the mitochondrial control region (Sorenson and Fleischer 1996; see also Livezy 1997). Additional data may be able to resolve this trichotomy.
The Madagascar Partridge Margaroperdix madagascarensis has been proposed to be a near relative of the quail Coturnix or to represent an ancesteral francolin type (Crowe and Crowe 1985). mtDNA sequence data support a close relationship with Coturnix, based on cytochrome b sequence data (Bloomer and Crowe 1998) and sequence data from the d-loop (E. Randii in litt.).
The Madagasar Wood Rail Canirallus kioloides is assumed related to the Guineo-Congolian Grey-throated Rail C. oculeus based on habitat, the patterned downy young, and skeletal characters shared with the assumed most primitive extant rail Nkulengu Rail Himantornis haematopus (Olson 1973). Canirallus possibly also is linked with the Oriental/New Guinea Rallicula. DNA hybridization data are lacking, but these species would presumably represent branches from below the T50H level 8.6 (Sibley & Ahlquist 1990: 848, see also Sibley et al. 1993).
The Madagascar Serpent-Eagle Eutriorchis astur may be related to the Congo Serpent-Eagle Dryotriorchis spectabilis (Olson 1973). The two peculiar Coracopsis parrots traditionally are grouped with Afrotropical Psittacus and Poicephalus, but a phylogenetic analysis is lacking.
In addition to the taxa discussed above, among the Malagasy passerines are a large number of genera endemic to Madagascar or to the Madagascan region: Nesillas, Thamnornis, Dromaeocercus, Randia, Newtonia, Cryptosylvicola, Pseudobias, Neomixis, Hartertula, Oxylabes, and Mystacornis. The majority of these are classified as ‘warblers’ (‘Sylviidae’) or ‘babblers’ (‘Timalliidae’) by traditional morphological characters, which provide insufficient data; the phylogenetic positions all of these genera should be re-examined, preferably with molecular characters.
CONCLUSION
Our study of the Malagasy 'Phyllastrephus' species shows, together with the recent studies of vangas and asities, that groupings based on traditional morphological comparison and various biogeographic assumptions may be very misleading. Because of new approaches to phylogenetic analysis, and the use of molecular data, a clearer picture begins to emerge. Some Malagasy birds could well be of a similar age as the lemurs, and their relationships remain unclear. Other groups represent Mid-Tertiary events, and could well be associated with the appearance of stepping stones in the middle of the Mozambique Channel (Krause et al. 1997). These happened at a time when there was continuous humid forest across tropical Africa (Axelrod & Raven 1978) and a brief faunal exchange with the Oriental region in connection with the closure of the Tethys Sea.
Although a large proportion of the members of the endemic Malagasy groups inhabit rainforest habitats, some adapt well to degraded forest and some members inhabit dry forest and various kinds of woodland and scrub. Judging from this variation, we cannot exclude that many colonisers (e.g. in the vanga/bush-shrike group) were birds of rather open woodlands, which tend to be more dispersive than true forest birds. Malagasy endemics that have close relatives in Africa are mainly birds of wetlands and grassland (e.g., two Sarothura, Pterocles, Cisticola), scrub and woodlands (e.g., many raptors, Streptopelia, Agapornis, Nectarinia, Lonchura). A few rather ubiquitous taxa could equally well have arrived from Asia (Treron, Cuculus, Terpsiphone, Dicrurus, Monticola), or quite certainly from Asia (Ninox, Hypsipetes, Copsychus), from the Malesian archipelago (Alectroenas, see Stoddart & Benzon 1970) and even from Australia (Turnix nigricollis, and possibly Caprimulgus madagascariensis; see Marshall 1978:31). Most species shared with the African mainland comprise waterbirds and rather ubiquitous species (Keith 1980).
The only strong indicators of forest-adapted colonisers (judging from similar habitat requiremennts of island and mainland relatives) would be Coracina cinerea and Dromaeocercus brunneus (Parker 1984) and some of the mid-Tertiary colonisers (asities, forest rails, Eutriorchis). Other probable candidates include various 'warblers' (Randia, Newtonia, Cryptosylvicola), 'babblers' (Mystacornis, Oxylabes) and the Malagasy Phyllastrephus/Berniera. However, a more comprehensive study is needed to determine (1) how many of the above-mentioned 'warblers', 'babblers' and 'greenbuls' belong to the same clade, and (2) a mainland sister taxon.
Within Africa, 'Lemurian' elements often have a relict distribution, in the Guineo-Congolian rainforest or the adjacent Western Rift mountains (e.g., Pseudocalyptomena graueri) or in the Eastern Arc Mountains of Tanzania (examples in Lovett & Wasser 1993) or further south along the eastern coast. Considering the direction of the Gondwanan monsoon systems (Krause et al. 1997) it is not likely that these taxa colonised Africa from Madagascar but more likely that they went the opposite way. But while they survived well and even diversified in Madagascar (where the biotic community would have been stable, see Cronk 1997), they vanished in Africa (as the biotic communities changed) and survived only in specific places where conditions were extremely predictable. While some of the endemic birds of the Eastern Arc Mountains have been identified as relict species (Dinesen et al. 1994) no 'Lemurian' relicts have been identified. More comprehensive DNA work is needed to identify African sister taxa of all the Malagasy forest birds.
ACKNOWLEDGEMENTS
Slikas' laboratory work on Phyllastrephus was conducted in the Molecular Genetics Laboratory at the National Zoological Park, with the consent of lab director Robert Fleischer. Schulenberg's laboratory work on vangas was supported by grants from Sigma Xi, the University of Chicago (Neirman Fund), the American Museum of Natural History (Frank M. Chapman Memorial Fund), and the Field Museum of Natural History (Reichelderfer Fund).
Fieldwork in Madagascar was authorised by the Direction des Eaux et Forêts and the Commission Tripartite; we are extremely grateful for the support of these agencies, and to Célestine Ravaoarimanga for issuing the necessary permits.
REFERENCES
Axelrod, D.I. & Raven, P.H. 1978. Late Cretaceous and Tertiary vegetation history of Africa. In: Werger, M.J.A. (ed.) Biogeography and ecology of Southern Africa. The Hague; Dr. W. Junk Publishers: 77-130.
Battistini, R. & Richard-Vindard, G. (eds). 1972. Biogeography and ecology in Madagascar. The Hague; Dr. W. Junk Publishers.
Benson, C. W. & Irwin, M.P.S. 1975. The systematic position of Phyllastrephus orostruthus and Phyllastrephus xanthophrys, two species incorrectly placed in the family Pycnonotidae (Aves). Arnoldia 17 (7): 1-10.
Bloomer, P. & Crowe, T.M. 1998. Francolin phylogenetics: molecular, morphobehavioural, and combined evidence. Molecular Phylogenetics and Evolution 9: 236 - 254.
Brodkorb, P. 1963. Catalogue of fossil birds. Part 1 (Archaeopterygiformes through Ardeiformes). Bulletin of the Florida State Museum 7: 179-293.
Burney, D. 1993. Late Holocene environmental changes in arid southwestern Madagascar. Quaternary Research 40: 98-106.
Burton, P.J.K. 1984. Anatomy and evolution of the feeding apparatus in the avian orders Coraciiformes and Piciformes. Bulletin of the British Museum (Natural History) Zoology Series 47: 331-443.
Coffin, M.F. & Rabinowitz, P.D. 1992. The Mesozoic East African and Madagascan conjugate continental margins. In: Watkins, J. S., Zhiqiang, F. & McMillen, K. J. (eds) Geology and geophysics of continental margins. American Association of Petroleum Geologists Memoir 53: 207-246.
Colston, P.R. 1972. A new bulbul from southwestern Madagascar. Ibis 114: 89-92.
Cooper, A. 1997. Studies of avian ancient DNA: from Jurassic Park to modern island extinctions. In: Mindell, D.P. (ed.) Avian molecular evolution and systematics. San Diego; Academic Press: 345-373.
Cracraft, J. 1971. The relationships and evolution of the rollers: families Coraciidae, Brachypteraciidae, and Leptosomatidae. Auk 88: 723-752.
Cracraft. J. 1981. Toward a phylogenetic classification of the recent birds of the world (Class Aves). Auk 98: 681-714.
Cronk, Q.C.B. 1997. Islands: stability, diversity, conservation. Biodiversity and Conservation 6: 477-493.
Crowe, T.M. & Crowe, A.A. 1985. The genus Francolinus as a model for avian evolution and biogeography in Africa. Relationships among species. In: Schuchmann, K.L. (ed) African Vertebrates: systematics, phylogeny and evolutionary ecology. Bonn; Zoologisches Forschungsinstitut und Museum Alexander Koenig.
Delacour, J. 1943. A revision of the genera and species of the family Pycnonotidae (bulbuls). Zoologica 28: 17-27.
Desjardins, P. & Morais, R. 1990. Sequence and gene organisation of the chicken mitochondrial genome, a novel gene order in higher vertebrates. Journal of Molecular Biology 212: 599-634.
Dinesen, L., Lehmberg, T., Svendsen, J.O., Hansen, L. & Fjeldså, J. 1994. A new genus and species of perdicine bird (Phasianidae, Perdicini) from Tanzania: a relict form with Indo-Malayan affinities. Ibis 136: 3-11.
Dorst, J. 1960. A propos des affinités systematiques de deux oiseaux malgaches: Tylas eduardi et Hypositta corallirostris. L'Oiseau et Revue Française d'Ornithologie 30: 259-269.
Frost, P.G.H. 1975. The systematic position of the Madagascan partridge Margaroperdix madagascarensis. Bulletin of the British Ornothologists' Club 95: 64 - 68.
Goodman, S.M., Langrand, O. & Whitney, B.M. 1996. A new genus and species of passerine from the eastern rain forest of Madagascar. Ibis 138: 153-159.
Goodman, S. M., Hawkins, A.F.A. & Domergue, C.A. 1997. A new species of vanga (Vangidae, Calicalicus) from southwestern Madagascar. Bulletin of the British Ornithologists' Club 117: 5-10.
Goodman, S.M., Pidgeon, M., Hawkins, A.F.A. & Schulenberg, T.S. 1977. The birds of southeastern Madagascar. Fieldiana: Zoology, New Series 87: 1-132.
Goodman, S.M. & Parrillo, P. 1997. A study of the diets of Malagasy birds based on stomach contents. Ostrich 68:104 - 113.
Houde, P., Cooper, A., Leslie, E., Strand, A.E. & Montaño, G.A. 1997. Phylogeny and evolution of 12S rDNA in Gruiformes (Aves). In: Mindell, D.P. (ed) Avian molecular evolution and systematics. San Diego; Academic Press: 121-158.
Hughes, J.M. 1996. Phylogenetic analysis of the Cuculidae (Aves, Cuculiformes) using behavioral and exological characters. Auk 113: 10-22.
Irwin, D.M., Kocher, T.D. & Wilson, A.C. 1991. Evolution of the cytochrome b gene of mammals. Journal of Molecular Evolution 32: 128-144.
Irwin, M.P.S. 1983. The Malagasy species of Timaliidae (Babblers). Honeyguide 116: 26-31.
Keith, S. 1980. Origins of the avifauna of the Malagasy region. Proceedings of the Fourth Pan-African Ornithological Congress: 99-108.
Krause, D.W., Hartman, J.H. & Wells, N.A. 1997. Late cretaceous vertebrates from Madagascar. In: Goodman, S. M. & Patterson, B. D. (eds) Natural change and human impact in Madagascar. Washington, D. C.: Smithsonian Institution Press: 3-43.
Leclaire, L., Bassias, Y., Clocchiatti, M. & Ségoufin, J. 1989. La Ride de Davie dans le Canal de Mozambique: approche stratigraphique et géodynamique. Comptes Rendus de l'Académie des Sciences (Paris) (série 2) 308: 1077-1082.
Lee, K., Feinstein, J. & Cracraft, J. 1997. The phylogeny of ratite birds: resolving conflicts between molecular and morphological data sets. In: Mindell, D. P. (ed) Avian molecular evolution and systematics. San Diego; Academic Press: 173-211.
Livezey, B.C. 1997. A phylogenetic classification of waterfowl (Aves: Anseriformes), including selected fossil species. Annals of Carnegie Museum 66: 457-496.
Lovett, J.C. & Wasser, S.K. (eds). 1993. Biogeography and ecology of the rain forests of Eastern Africa. Cambridge; Cambridge University Press.
Marshall, J.T. 1978. Systematics of smaller Asian night birds based on voice. Ornithological Monographs 25.
Maurer, D.R. & Raikow, R.J. 1981. Appendicular myology, phylogeny, and classification of the avian order Coraciiformes (including Trogoniformes). Annals of Carnegie Museum 50: 417-34.
McCall, R.A. 1997. Implications of recent geological investigations of the Mozambique Channel for the mammalian colonization of Madagascar. Proceedings of the Royal Society of London, Series B (Biological Sciences) 264: 663-665.
Millot, J. 1972. In conclusion. In: Battistini, R. & Richard-Vindard, G. (eds) Biogeography and ecology in Madagascar. The Hague; Dr. W. Junk Publishers: 741-756.
Olson, S.L. 1973. A classification of the Rallidae. Wilson Bulletin 85: 381-416.
Olson, S.L. 1985. The fossil record of birds. In: Farner, D. S., King, J. R. & Parkes, K. C. (eds) Avian Biology. Vol. VIII; Orlando: Academic Press: 79-252.
Olson, S.L. 1989. Preliminary systematic notes on some old world passerines. Rivista italiano di Ornitologia 59: 183-195.
Parker, S.A. 1984. The relationships of the Madagascan genus Dromaeocercus (Sylviidae). Bulletin of the British Ornithologists' Club 104: 11-18.
Payne, R.B. 1997. Family Cuculidae (Cuckoos). In: de Hoyo, J., Elliot, A. & Sargatal, J. (eds) Handbook of the birds of the world. Vol. 4; Barcelona; Lynx Editions: 508-610.
Prum, R.O. 1993. Phylogeny, biogeography, and evolution of the broadbills (Eurylaimidae) and asities (Philepittidae) based on morphology. Auk 110: 304-324.
Prum, R.O. & Razafindratsita, V.R. 1997. Lek behavior and natural history of the velvet asity (Philepitta castanea: Eurylaimidae). Wilson Bulletin 109: 371-560.
Raikow, R.J. 1987. Hindlimb myology and evolution of the Old World suboscine passerine birds (Acanthisittidae, Pittidae, Philepittidae, Eurylaimidae). Ornithological Monographs 41.
Salomonsen, F. 1934. Revision of the Madagascar timaliine birds. Annals and Magazine of Natural History 14: 60-79.
Schulenberg, T.S. 1995. Evolutionary history of the vangas (Vangidae) of Madagascar. PhD Thesis, University of Chicago, Chicago, USA.
Sibley, C.G. & Ahlquist, J.E. 1990. Phylogeny and classification of birds. New Haven; Yale University Press.
Sibley, C.G., Ahlquist, J.E. & DeBenedictis, P. 1993. The phylogenetic relationships of the rails, based on DNA comparisons. Journal of the Yamashina Institute for Ornithology 25: 1-11.
Slikas, B. 1997. Phylogeny of the avian family Ciconiidae (storks) based on cytochrome b sequences and DNA-DNA hybridization distances. Molecular Phylogenetics and Evolution. 8: 275-300.
Sorenson, M.D. & Fleischer, R.C. 1996. Multiple independent transpositions of mitochondrial DNA control region sequences to the nucleus. Proceedings of the National Academy of Sciences USA 93: 15239-15243.
Stoddart, D.R. & Benson, C.W. 1970. An old record of a blue pigeon Alectroenas species and sea-birds on Farquhar and Providence. Atoll Research Bulletin 136: 35-36.
Swofford, D.L. 1998. PAUP: Phylogenetic analysis using parsimony. Washington, D.C. ; Smithsonian Institution.
Thompson, P.M. 1987. Zahamena Forest (Madagascar) expedition 1985. Study report number 20. Cambridge; International Council for Bird Preservation.
Fig. 1. A parsimony bootstrap consensus tree for five Malagasy "Phyllastrephus" species and other sylvoid birds, based on a mitochondrial DNA cytochrome b sequence, with transversion substitutions weighted 5 times over transition substitutions
