S50.2: Weaving a story: The relationships of the endemic Ploceidae of Madagascar
Adrian J .F. K. Craig
Department of Zoology & Entomology, Rhodes University, Grahamstown, 6140, South Africa, fax 27 461 24377, e-mail zoac@giraffe.ru.ac.za
Craig, A.J.F.K. 1999. Weaving a story: The relationships of the endemic Ploceidae of Madagascar. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 3063-3070. Johannesburg: BirdLife South Africa.Four Ploceidae occur naturally on Madagascar: Forest Fody Foudia omissa and Madagascar Fody F. madagascariensis, and Nelicourvi Weaver Ploceus nelicourvi and Sakalava Weaver P. sakalava. A preliminary phylogenetic analysis using morphological characters and features of their breeding biology supports the view that the genus Foudia may be closest to the African genera Quelea and Euplectes. The Madagascan Ploceus species may be linked to the solitary, insectivorous African weavers, or the Asian Baya Weaver P. philippinus, rather than derived from the colonial savanna weavers of Africa.
INTRODUCTION
The avifauna of Madagascar can be broadly divided into three categories: endemic groups such as the Mesitornithidae, representatives of Afrotropical families (differing often only at the subspecific level) like the Hamerkop Scopus umbretta, and species with Asian affinities such as the Madagascar Bulbul Hypsipetes madagascariensis (Langrand 1990). Since Crook's (1964) monograph on the weavers, the genus Foudia, with two species in Madagascar and three (or four) on other Indian Ocean islands, has been linked with the African genera Quelea and Euplectes, primarily on the basis of their nesting and courtship behaviour. Crook (1964) tentatively assigned the two endemic Ploceus species, Sakalava Weaver P. sakalava and Nelicourvi Weaver P. nelicourvi, to a group that includes both African and Asian ploceids, noting that little information was available for them.
Previous accounts of the biogeography of the Madagascar avifauna have neglected the Ploceidae; they were not mentioned at all by Griveaud (1967) nor by Keith (1980). Apart from incidental observations, little has been recorded concerning the biology of these species, which are also of minor conservation concern compared to other Malagasy endemics. Recent research has focussed on endangered Foudia species on Mauritius (Mauritius Fody F. rubra), Seychelles (Seychelles Fody F. sechellarum) and Rodriques (Rodrigues Fody F. flavicans) (Collar et al. 1994), and much information on the Madagascar Fody F. madagascariensis also comes from populations introduced on other Indian Ocean islands (e.g., Crook 1961; Safford 1997). In this paper I have used published information and data from museum specimens to compare the four Madagascar ploceids, F. madagascariensis, Forest Fody F. omissa/eminentissima, Ploceus nelicourvi and P. sakalava, to possible relatives in Africa and Asia. From Asia, I selected only Baya Weaver P. philippinus, the most widespread and best-known species. From Africa, I chose four Ploceus species with a wide distribution in southeastern Africa: two non-colonial insectivores Forest Weaver P. bicolor and Spectacled Weaver P. ocularis, and colonial granivores Spotted-backed Weaver P. cucullatus and Masked Weaver P. velatus; also all three members of the genus Quelea: Red-billed Quelea Q. quelea, Cardinal Quelea Q. cardinalis, and Red-headed Quelea Q. erythrops; and four representatives of the genus Euplectes: two bishops Golden Bishop E. afer and Red Bishop E. orix and two widows Red-shouldered Widow E. axillaris and Yellow-rumped Widow E. capensis. The Madagascar and Asian species I know only as study skins, but I have field experience with all the African species except Q. cardinalis.
METHODS
Plumage details and nesting data were taken both from examination of specimens and from descriptions in the literature, supplemented by my own field notes for the African species. All skeletal data were recorded by me personally from museum collections (Table 1). The main published sources for Asian and Madagascan birds were Benson et al. (1977), Crook (1960, 1961, 1963, 1964), Frith (1976), Langrand (1990), Newton (1959) and Rand (1936). For Foudia omissa, which is often treated as conspecific with Red-headed Fody F. eminentissimma (cf. Dowsett & Forbes-Watson 1990), I have used biological data from populations of F. eminentissimma on other islands. A North American icterid, the Red-winged Blackbird Agelaius phoeniceus, was used as the outgroup, and data for the House Sparrow Passer domesticus also were included. Trees were generated using both PAUP (Swofford 1993) and Hennig86 (Farris 1988).
RESULTS
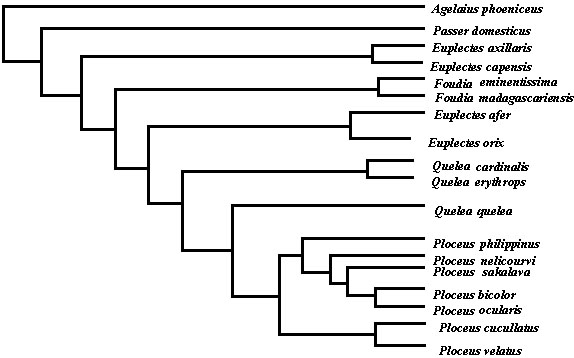
Three trees were obtained from both PAUP and Hennig86; these differed only in the sequence of species within the Ploceus clade. Bootstrap analysis indicated that the nodes were not strongly supported (52-55%), except for the associations between pairs of taxa such as P.velatus and P. cucullatus (77%) (Fig. 1). The cladogram does support the view that P. sakalava belongs with Ploceus rather than with Foudia in which it has been placed in the past, and that Foudia is closer to Euplectes and Quelea than to Ploceus. However, in this analysis both Euplectes and Quelea appear as paraphyletic taxa, whereas this selection of Ploceus species forms a monophyletic clade. The Madagascar members of Ploceus are associated with the solitary insectivorous weavers from Africa, not the savanna granivores, but the Asian species P. philippinus is included in the same cluster, so that any biogeographic inferences remain uncertain.
Table 2 shows the character states for the species in Fig. 1. The uninformative characters, which showed the primitive condition in all taxa, were disregarded in the analysis. With regard to skeletal characters, these are remarkably uniform in the three genera Foudia, Euplectes and Quelea. I have data for eight other Euplectes species, and all members of this genus share the same character states for these nine skeletal features. In contrast, the 17 species of Ploceus examined so far represent seven different combinations of skeletal characters. This implies that the current genus, which includes more than 60 species in most checklists, is likely to be paraphyletic.
DISCUSSION
The genus Ploceus
There is a long tradition of including many species of weavers in the genus Ploceus. Reichenow (1886) recognised 38 taxa in six subgenera. He included P. sakalava in the subgenus Ploceus alongside the Asian species Streaked Weaver P. manyar, Black-breasted Weaver P. benghalensis and Asian Golden Weaver P. hypoxanthus; P. velatus was placed in the subgenus Sitagra; and P. cucullatus in Hyphantornis. Ploceus ocularis and P. bicolor were assigned to the genus Symplectes (Reichenow 1887). In the most recent comprehensive Afrotropical checklist, Dowsett & Forbes-Watson (1990) recognise 56 species of Ploceus. Of the current world checklists, only Wolters (1979) has broken up this large genus. He restricted Ploceus to the five Asian species; placed sakalava and nelicourvi as the only two species making up Nelicurvius; ocularis in Hyphanturgus; bicolor in Symplectes; and velatus and cucullatus in Textor. Unfortunately Wolters did not publish any reasons for these decisions, but Morlion (1980) noted differences in pterolyosis within the genus Ploceus that could support such subdivisions.
Other data on the taxa investigated here
Basing his conclusions primarily on morphology and plumage characters, Moreau (1960) suggested that Foudia was closer to Euplectes than to Quelea. He also suggested that P. sakalava and P. nelicourvi had a recent common ancestor, and represented dry-country and forest versions of the same stock. Elsewhere he commented that Asian weavers appeared to have been better island colonists than African species, though he did not infer that Asia was therefore a more likely source for the Madagascar Ploceus species.
Crook (1963) examined nest structure and classified the nests of P. bicolor, P. ocularis, P. philippinus and P. cucullatus in separate types, while the nests of Euplectes, Quelea and F. madagascariensis all were placed in the same category. Later Schnell (1973) carried out a phenetic analysis of Crook's data. Clustering by correlation coefficients grouped Quelea and Euplectes together; F. sechellarum was linked with P. bicolor, F. madagascariensis with P. ocularis, and P. cucullatus with P. velatus. A slightly different arrangement resulted when he used distance coeeficients: Quelea, Euplectes, and F. madagascariensis formed one cluster, and P. cucullatus and P. velatus still were in the same cluster, but P. bicolor was isolated. In both these analyses, P. philippinus was not associated with any of the other species considered here.
Nest structure remains a potentially useful source of information in the Ploceinae. However, there can be significant variations related to nest site within the same species, and the length of the entrance tunnel also is variable (cf. Crook 1961; Davis 1971). The distinctive roof in some Foudia nests evidently has been overlooked in the past (Safford 1997). The contribution of the sexes to nest construction also may be difficult to categorise; I observed male P. ocularis as the builders (Craig 1984), but an experienced bird photographer reported that the female of the pair did most of the work at several other nest sites (N. Myburgh, pers. comm.). The primary selective force acting on weaver nest structure is likely to be predation, with some features related to structural constraints, and with climatic factors playing a minor role (Craig 1995).
In a comparative study of limb musculature Bentz (1979) dissected specimens of P. ocularis, P. cucullatus, Q. quelea, F. madagascariensis and E. afer. Four characters of the forelimb muscles and six on the hindlimb were shared by all these species. Although F. madagascariensis had one autapomorphy on the forelimb and E. afer one on the hindlimb, comparison with the Estrildidae suggested that the most significant variation in limb muscle morphology was at the family or subfamily level, rather than at the generic level.
The waxy preen-gland secretions have not been studied extensively in passerine birds. Poltz & Jacob (1974) analysed samples from four Ploceinae, including Q. quelea and P. cucullatus. They found that the fatty acids were identical in three Ploceus species, while the dominant compound in Q. quelea was different. The alcohol component was almost exclusively one type in Ploceus, and this was also the major constituent in Quelea, with significant contributions from other alcohols. This suggests that that these secretions may be informative at the generic level.
Karyotyping has not been used widely in bird studies. Christidis (1986) commented that F. madagascariensis appeared to have a karyotype identical to that of P. velatus and P. philippinus. Thus this character may not provide useful information on relationships within the sub-family. DNA studies have yet to include any Madagascar ploceids. DNA-hybridization showed Q. quelea and Q. erythrops as close relatives, with Euplectes their sister taxon, and Ploceus species, including P. cucullatus, forming part of the same branch (Sibley & Ahlquist 1990).
CONCLUSIONS
While molecular techniques currently enjoy a high profile, the most cost-effective approach seems to be to frame a phylogenetic hypothesis using other characters, which can then be tested and refuted or supported by molecular data. Comparative behaviour studies, as demonstrated by Crook (1964), remain an accessible option. A recent analysis concluded that behavioural characters are just as reliable as morphological characters in phylogenetic studies (De Queirez & Wimberger 1993). Clearly we are not yet able to identify the closest relatives of the Madagascar ploceids with any confidence, but the fodies most likely are African in origin, whereas for the two Ploceus species an Asian ancestry also is plausible. I hope that fieldworkers in Madagascar will contribute new data to these questions.
ACKNOWLEDGEMENTS
My original field studies of ploceids in South Africa were stimulated and guided by the late Profs. G. J. Broekhuysen and K. Immelmann, and by Prof. G. L. Maclean and Dr. C. J. Skead. For access to museum specimens, I am grateful to D. Allan, T. Cassidy, K. Garrett, J. Hinshaw, A. C. Kemp, W. E. Lanyon, M. Louette, R. B. Payne, and D. Willard. Research funding has been provided by Rhodes University, and the Foundation for Research Development. N. Barker assisted with the systematic analysis, and P. E. Hulley and C. J. Whittington-Jones commented on a draft of the paper.
REFERENCES
Benson, C., Colebrook-Robjent, J.F.R. & Williams, A. 1977. Contribution à l'orithologie de Madagascar. L'Oiseau et le Revue Française d'Ornithologie 47: 167-191.
Bentz, G.D. 1979. The appendicular myology and phylogenetic relationships of the Ploceidae and Estrildidae (Aves: Passeriformes). Bulletin of the Carnegie Museum of Natural History 15: 1-25.
Christidis, L. 1986. Chromosomal evolution in finches and their allies (families: Ploceidae, Fringillidae, and Emberizidae). Canadian Journal of Genetics and Cytology 28: 762-769.
Collar, N.J., Crosby, M.J. & Stattersfield, O.J. 1994. Birds to watch 2. Cambridge; BirdLife International.
Craig, A.J.F.K. 1984. The Spectacled Weaver Ploceus ocularis and monogamy in the Ploceinae. Proceedings of the Fifth Pan-African Ornithological Congress: 477-483.
Craig, A.J.F.K. 1995. Adaptation and evolution in ploceid weaver nests. Ostrich 66: 100-102.
Crook, J.H. 1960. Studies on the reproductive behaviour of the baya weaver Ploceus philippinus (L). Journal of the Bombay Natural History Society 57: 1-44.
Crook, J.H. 1961. The fodies (Ploceinae) of the Seychelles Islands. Ibis 103a: 517-548.
Crook, J.H. 1963. A comparative analysis of nest structure in the weaver birds (Ploceinae). Ibis 105: 238-262.
Crook, J.H. 1964. The evolution of social organisation and visual communication in the weaverbirds (Ploceinae). Behaviour Supplement 10: 1-178.
Davis, T.A. 1971. Variation in nest-structure of the common weaverbird Ploceus philippinus (L.) of India. Forma Functio 4: 225-239.
De Queirez, A. & Wimberger, P.H. 1993. The usefulness of behavior for phylogeny estimation: levels of homoplasy in behavioral and morphological characters. Evolution 47: 46-60.
Dowsett, R.J. & Forbes-Watson, A.D. 1993. Checklist of birds of the Afrotropical and Malagasy regions. Vol. 1: Species limits and distribution. Liege; Tauraco Press.
Farris, J.S. 1988. Reference manual for Hennig86, version 1.5. New York; The author.
Frith, C.B. 1976. A twelve-month field study of the Aldabran fody Foudia eminentissima aldabrana. Ibis 118: 155-178.
Griveaud, P. 1967. Le peuplement ornithologique de Madagascar: origines - biogéographie. Cahiers ORSTROM, Serie Biologie, number 4: 53-76.
Keith, S. 1980. Origins of the avifauna of the Malagasy region. Proceedings of the Fourth Pan-African Ornithological Congress: 99-108.
Langrand, O. 1990. Guide to the birds of Madagascar. New Haven & London; Yale University Press.
Moreau, R.E. 1960. Conspectus and classification of the ploceine weaver-birds. Ibis 102: 298-321, 443-471.
Morlion, M.L. 1980. Pterylosis as a secondary criterion in the taxonomy of the African Ploceidae and Estrildidae. Proceedings of the Fourth Pan-African Ornithological Congress: 27-41.
Newton, R. 1959. Notes on the two species of Foudia on Mauritius. Ibis 101: 240-243.
Poltz, J. & Jacob, J. 1974. Bürzeldrüsensekrete bei Ammern (Emberizidae), Finken (Fringillidae), und Webern (Ploceidae). Journal für Ornithologie 115: 119-127.
Rand, A.L. 1936. The distribution and habits of Madagascar birds. Bulletin of the American Museum of Natural History 72: 143-499.
Reichenow, A. 1886. Monographie der Gattung Ploceus Cuv. Zoologisches Jahrbuch 1: 113-164.
Reichenow, A. 1887. Monographie der Gattung Symplectes Sw. Zoologisches Jahrbuch 2: 625-638.
Safford, R.J. 1997. The nests of sympatric native and introduced fody Foudia species on Mauritius. Ostrich 68: 27-30.
Schnell, G.D. 1973. A reanalysis of nest structure in the weavers (Ploceinae) using numerical taxonomy techniques. Ibis 115: 93-106.
Sibley, C.G. & Ahlquist, J.E. 1990. Phylogeny and classification of birds. New Haven & London; Yale University Press.
Swofford, D.L. 1993. PAUP: Phylogenetic analysis using parsimony, version 3.1.1. Champaign; Illinois Natural History Survey.
Wolters, H.E. 1979. Die Vogelarten der Erde, Lieferung 4. Hamburg & Berlin; Paul Parey.
Table 1. Character states of male birds used for a phylogenetic comparison of selected Ploceidae; the presumed primitive condition is coded as 0.

Table 2. Character states recorded for these species.
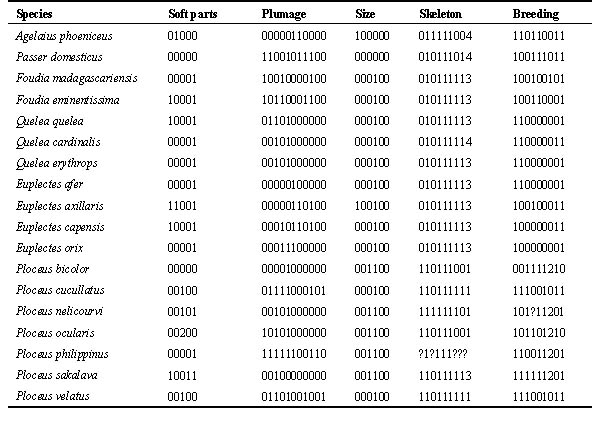
Fig. 1. A cladogram of selected species of Foudia, Euplectes, Quelea and Ploceus, with Agelaius phoeniceus and Passer domesticus