S50.1: Composition of the West African lowland avifauna
Michel Louette
Royal Museum for Central Africa, B-3080 Tervuren, Belgium, fax 02 769 56 42, e-mail louette@africamuseum.be
Louette, M. 1999. Composition of the West African lowland avifauna. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 3042-3062. Johannesburg: BirdLife South Africa.The West African avifauna is impoverished in species numbers, although all African families (except Promeropidae) are represented and some (e.g., Estrildidae) are rich, but unique in taxonomical composition. Poverty in species is not evenly distributed over the habitats. The stenotopic forest avifauna lacks an endemic genus; most old equatorial elements are absent, the exceptional ones present are shared with central Africa. After extinction, sufficient long isolation and subsequent recolonisation is suspected, because species number in five genera is larger than (but includes those from) central Africa. The woodland and savanna fauna is more differentiated: it contains several endemic genera, but especially the mesic belts are impoverished; among groups, poor representation of canaries and the genus Accipiter and the absence of large fowl are striking. Zoogeographical causes suggested are: situated on a species-poor continent; outlying location; lack of montane or temperate habitats; and relatively small size. Colonisation problems from central Africa are due to habitat barriers near Cameroon ridge (forest bottleneck) and in South Sudan dry region (gap in woodland-savanna). Furthermore some tropical philopatric species are slow colonisers. Several birds are rarely recorded, possibly linked to recent spread, suggesting a scarcity in habitat diversity, compared to central Africa. Other ecological causes: low vegetation diversity, mostly lowland (altitudinal gradients would yield more species). Parallel vegetation belt structure versus mosaic vegetation structure elsewhere limits number of habitats, but it yields large ranges with poor local survival chances in case of climate change, with great opportunity for range dynamics, resulting in low fragmentation rate of bird species.
INTRODUCTION
Relevance of a zoogeographical study
Large-scale studies are needed to relate biogeographic patterns to environmental factors. Such studies require massive data sets, which should be obtained by Atlas work and linked by electronic means to ecological information (e.g., vegetation). Such studies would provide a very useful faunal analysis for purely scientific reasons, but even more so for application in wildlife surveys, conservation and management in a region as West Africa (WA hereafter). Historical distribution data (from specimens) used likewise could indicate the extent of habitat change that WA experiences. Birds would be excellent as a tool, but given the lack of resident ornithologists and ornithological study centres in WA, there is urgent need in funding initiatives for both Atlas work and exploitation of scientific collections. Because I was aware that it is unlikely that a fairly complete databank on biodiversity can be made in the short term, as a surrogate I advocated elsewhere the use of particular bird species as bioindicators for site quality, a methodology still in its infancy (Louette et al. 1995). Here I present another surrogate: a crude analysis of avifaunal composition, based on the fair amount of recent information.
The particularities of the West African avifauna
The composition of the African avifauna as a whole was studied by Moreau (1966) and by Snow (1980: non-passerines only); Fry (1980) analysed the avifauna of the northern tropical woodlands, but no purposeful analysis of the western Afrotropical avifauna ever was made. The most recent study of the African montane avifauna, for which only the Cameroon ridge is of concern in the present context (the isolated small massifs lack an endemic avifauna), is by Dowsett (1986); his paper is complementary to the present one on lowland WA.
The extra-African relationship study of WA’s birds is only briefly touched upon here. Suffice is to say that the scarcity of Palaearctic and Oriental elements highlights the fact that WA is quite closely related to the other African regions.
Snow (1980) found that among the non-passerines in his list, African birds of seasonal habitats are much more closely related to their Oriental relatives (examples: the shared genus Butastur, the shared species Rose-ringed Parakeet Psittacula krameri) than those from forest. Indeed, most stenotopic forest non-passerine monotypic genera with Oriental affinities do not occur in WA (Table 1), but e.g. the genus Sasia does (the polytypic genus Zoonavena, shared with the Oriental region, occurs on Sao Thomé Island near WA). This relationship with the Oriental region is of some importance in the biodiversity comparison here.
METHODS
Area under consideration
I use as zoogeographical delimitation of WA: south of the Sahara desert; west of the Cameroon ridge for the forest district and west of the Ethiopian highlands for the non-forest districts (woodlands and savannas), hereafter for brevity, if appropriate, grouped under the term ‘savanna’. Fig. 1 shows a formal delimitation, based on a combination of ‘normalised difference green vegetation index’ (NDVI, a measurement mentioned below) and relief data.
The forest district east of the Cameroon ridge and south of the woodland district of WA is denominated central equatorial Africa (CEA); woodlands and savannas to the east are in East Africa (EA); not adjoining WA and not formally delimited from CEA and EA in this paper is southern Africa (SA). Only the tropical mesic areas are considered, temperate and dry districts elsewhere in Africa are ignored in the comparison. Due to the scarcity of high altitude in WA, the montane district is also excluded.
Zoogeographical situation
A study of the composition of the WA avifauna must take into account a multitude of factors, including the paramount zoogeographical factor ‘number of species in general on this continent’. Apart from that factor, those mentioned in the following sections need examination:
Location of WA
WA may be comparable to the rest of the African continent, but it could be different, merely due to the fact that it is situated at the periphery, with distance as a factor to consider. In fact, because of the geographical position, taxa evolved in WA must colonise first in an eastern and later in a southern direction and over rather long distances and vice versa for colonists to WA from other regions. Size is another factor of importance, as is the absence of oceanic islands.
Altitude
Generally speaking, WA is lowland, compared to the rest of the continent (Fig. 2). Even when the real montane species are ignored, this factor must still be taken into account when the numbers of species in WA are considered, because the presence of some species may be linked to altitudinal gradients. Conversely, birds occurring at low elevation in EA and SA should not a priori be absent from WA (data compared in the Kenyan atlas - Lewis & Pomeroy 1989 for EA and in the Southern African atlas - Harrison et al. 1997 for SA). The main source for altitudinal limits of bird species is Hall & Moreau (1970) and Snow (1978), and in a few cases, Stattersfield et al. (1998).
Habitat
Habitat structure and richness of vegetation need the following comments. Position and altitude no doubt result in climatic differences from the rest of Africa. Variation in the amount of rainfall in WA provokes a gradual change in vegetation structure from the wettest (south) to the driest (north) regions, resulting in parallel vegetation districts. The southernmost belt near the coast, from Sierra Leone to easternmost Ghana, catches enough rainfall in order to support a rainforest, called the ‘Upper Guinea forest block’. Further east, this vegetation type is interrupted until coastal Nigeria, where a second rainforest region called ‘Lower Guinea forest block’ begins, stretching till the foothills of the escarpment in eastern Congo and somewhat beyond, in isolated patches in Uganda and Kenya. While part of the avifauna is similar, the forest is definitely no longer ‘lowland’ in this eastern outlier. The whole of Upper Guinea and the westernmost outlier of Lower Guinea are included in WA. This region is about one third the size of the forest in CEA.
To the north, the forest gives way to woodland and savanna. Fry (1980) found that the Isoberlinia woodlands typical for the intermediate rainfall areas of this region are only about half the size of the corresponding southern African Brachystegia woodlands and also that they were more disturbed through human activities, especially during the last decades. The whole of the Isoberlinia belt is included in WA as here defined.
Up to now, when bird distribution was linked with vegetation in large-scale studies, the arbitrary division in districts (as exemplified in Fig. 3) was used (Louette 1981; Louette 1983 for Cameroon); in the future, electronic devises should enable one to compare (historic) distribution data and several parameters of vegetation structure. NDVI is a contemporary nonlinear function, obtained from satellites and derived from reflected solar radiation; its value is in most cases correlated to photosynthesis and therefore vegetation cover. The African data are available over the Internet (Tucker 1997). To illustrate the variation in this factor, 5 different categories were differentiated (Fig. 4), resulting in a division in districts not identical to the classical vegetation districts. However, in this paper, only a general comparison is made.
Barriers
The possible effect of habitat barriers must be taken into account, and also the recent history of climate and vegetation changes.
Bird ranges
All bird species ranges were examined (reference works: Hall & Moreau 1970; Snow 1978, whose nomenclature in conjunction with the maps is used here). Newly described forms are based on Morel & Chappuis (1992), recent updates on distribution (mainly as listed in Dowsett & Dowsett-Lemaire 1993 by country; and from numerous recent papers). The aim was to count numbers of resident or intra-African migratory species among all the bird families in the different areas of Africa and also to compare particular distributions in WA with CEA (forest) and with the woodland and savanna regions in EA and SA. The comparison among the regions is tested at the species level for the whole avifauna but also at higher taxonomical levels, especially for the localisation of endemism and in the ecological comparison within speciose groups (see case studies). The aim of the present analysis is to suggest causes for the differences between WA and other regions.
RESULTS
Number of species
The counts in Table 2 show that the avifauna of WA in general is rather poor in species in particular groups, equivalent in many and has a surplus in only few. Generally speaking, forested WA is an impoverished version of CEA, but the northern districts are more differentiated. Fry (1980) calculated the number of species for the northern and southern woodlands of Africa and found a lack of endemism in the north (= in WA), but a general and essential similarity in numbers of other species that are characteristic of a particular woodland type. The present analysis demonstrates that this similarity is less convincing when one includes all vegetation belts in the comparison. The EA and SA avifaunas contain a greater number of species than WA, suggesting either a greater number of speciation centres and/or better survival there.
The forest district of WA does not contain endemic genera. Endemic genera in CEA are listed in Table 1. Some of these could be suspected as belonging to a (peri)montane avifauna (and therefore a fortiori being absent from WA, because of the relief) but this cannot be the case for the ones marked ‘lowland’. So, no important traces of exclusive old relationships remain, except that WA shares with CEA the peculiar Afro-endemic forest genera Agelastes and Picathartes within which speciation took place (WA and CEA having an endemic species each in both genera), and these regions also share e.g. the monotypic Brown Nightjar Veles binotatus. The number of stenotopic forest birds in general is lower in WA than in CEA. Most of them do have a vicariant (subspecies or allospecies) in CEA. Nevertheless, the WA forest avifauna has a surplus of species in four or five genera (Ceratogymna, Bleda, Trichastoma, Melaenornis and less strictly also Tauraco), compared to CEA.
WA is also deficient in the number of savanna and woodland birds (Table 2), but in contrast to the forest, it contains more species endemic or near-endemic to the area. Surprisingly they often belong to monotypic genera: the woodlands contain the localised Emerald Starling Coccycolius iris (close to Lamprotornis: Sibley & Ahlquist 1990) and the widespread Oriole Warbler Hypergerus atriceps (belonging to the Palaeofauna: Sibley & Ahlquist 1990). Other WA ‘specialities’ as the monotypic Egyptian Plover Pluvianus aegyptius are near-endemic; I consider they spilled over into EA as a result of recent colonisation.
Distribution
Forest fragmentation no doubt occurred in historical times at different locations. An important one is apparent at the break between Upper and Lower Guinea with scars for many bird taxa, but in certain other cases, the Cameroon ridge is the break between allospecies, subspecies or the range limit, given a certain ‘overflow’. New data constantly are correcting the picture: e.g., Sjöstedt’s Barred Owlet Glaucidium sjoestedti is not bordered on the west by the Cameroon montane area (as indicated by Louette 1981) but it does occur in Nigeria (Ash et al. 1989). For most cases, it is impossible to know if these corrections are new discoveries of an ancient situation or if they are the results of expansion. The distribution of some other birds does not follow classical zoogeographical breaks, but this is also the case in CEA: not all distribution patterns in the African equatorial forest are easily explained by historical fragmentation (Louette 1990). Also difficult to explain is the occurrence of a forest race of the widespread ‘savanna’ Barred Owlet Glaucidium capense in an isolated pocket in Ivory Coast (Dowsett & Dowsett-Lemaire 1993).
In most cases, the woodland and savanna bird species are not affected by the Cameroon ridge – Lake Chad zoogeographical break, but a substantial number of them is affected (Louette 1981). In fact, the Cameroon montane area nowadays acts as a weak barrier for the expansion and possible intermixture of the fauna of all the lowland districts (and this area may well have been a more formidable barrier in the past: Louette 1981). However, avifaunal colonisation in both directions is in progress as a dynamic process, making the actual range limits to differ among species (Fry 1980 lists among nonforest birds their eastern limit or their eastern allospecies contact). Indeed, presence of hostile habitat (such as forest to savanna birds) is a powerful factor limiting expansion, more so than topography and distance. Some ranges demonstrate the origin of birds in WA and their expansion, which is limited by forest. In a few cases species seem to have made use of a temporary passage. Louette (1992) discussed the occurrence of the Western Grey Plantain-eater Crinifer piscator in a savanna corridor near the middle Congo river in CEA/SA; this colonisation pattern is not restricted to the birds originating north of the equatorial forest, the opposite occurred recently from the south to WA for the Rufous-tailed Palm Thrush Cichladusa ruficauda (Germain & Cornet 1994). In these two examples, this process involves bird genera originally absent in the other region. These distribution patterns and their suspected dynamics must in the long run lead towards a longer species list in both areas. It reinforces the impression that the avifauna is locally incomplete.
The same colonisation pattern is presumed in the following more complex example, involving range dynamics and exclusion by vicariants, a situation which surely occurs regularly. The distribution of allospecies in the superspecies Dendropicos (Mesopicos) goertae let Louette & Prigogine (1982) presume a further step, whereby the northern lowland woodland taxon (Grey Woodpecker D. goertae) spilled over to the south of the Lower Guinea forest. There, the expansion was limited by the presence of the Olive Woodpecker D. griseocephalus, a southern bird, adapted to high ground (Fig. 5).
In contrast, several species are limited to forest in WA, whereas they do enter woodland and even savanna elsewhere (Table 3; see also the genus Guttera in case study, below), only one species (Blue-breasted Kingfisher Halcyon malimbica) is in the opposite situation. These are cases of habitat shift and habitat restriction. The structure of the vegetation is probably more complex in the periforest belt outside WA (compare Fig. 3 and Fig. 4: classical vegetation classes to NDVI data). However, generalisation would be premature, because other (biological) factors may be involved: the Thick-billed Cuckoo Pachycoccyx audeberti is a brood parasite with specific hosts (Allport & Fanshawe 1994). In other species habitat choice differs by region, but does not follow the pattern ‘WA versus the rest’: e.g. the Yellow White-eye Zosterops senegalensis enters forest, in some localised areas in- and outside WA.
Several birds are rarely recorded in WA (Table 2), possibly indicating recent colonisation from the east, suggesting WA was even poorer in species during a preceding period. This includes forest as well as savanna birds. An alternative explanation, that rarity of birds could indicate existence of particular, localised habitats in WA, is unsatisfactory, due to the simple situation of parallel apparently uniform and large vegetation belts, all situated in lowland.
Large parallel vegetation belts yield large bird ranges. These birds have poor local survival chances in case of climate change, but with great opportunity for range dynamics, resulting in low fragmentation rate of bird species. Models indicate that extinction becomes a frequent event, hence a net result of fewer species. It is remarkable that the most used qualification for the status of a great many species in the species-rich family Estrildidae is ‘local’ in Nigeria, a well-studied country in WA (Elgood et al. 1994).
Differences in abundance may indicate habitat preference, but the example of the Hooded Vulture Necrosyrtes monachus, much more common in WA than elsewhere, possibly qualifies as a case of recent spread from WA.
The following are examples of the qualification for several types of temporary Afrotropical visitors to WA:
Erratic: Ovambo Sparrowhawk Accipiter ovampensis
Expansion of population: Black Cuckoo Cuculus clamosus
Intra-African ‘real’ migration towards another region in the non-breeding season: Rufous-cheeked Nightjar Caprimulgus rufigena
Why are these birds only visitors to WA and did not become resident breeders? Lack of niche during part of the year or migratory philopatry? Are the individuals of erratic and expansion type really lost to the pan-African breeding population? This is not the case for the real migrants to WA nor e.g. for migrants to EA and SA from the Madagascar breeding grounds.
Consideration will be given in the discussion chapter to the possibility that the presence of migrant visitors limits the number of resident species in WA.
In conclusion, more data and more research are needed in order to determine the causes for bird distribution in WA.
Case studies
For several taxa, the general information on distribution seems adequate enough to demonstrate particular patterns in WA avifaunal composition. (When names of species are given, in fact a member of the corresponding superspecies is intended; therefore, no English names are given in this list).
Level of order: Galliformes
Equivalent between WA and CEA or EA: Forest: Francolinus squamatus, F. lathami, and Agelastes meleagrides; Woodland: F. bicalcaratus, F. coqui; Savanna: Numida meleagris (Migratory Coturnix spp.). Deficient in WA: General: F. shelleyi; Forest: Afropavo congensis; Woodland: F. afer; Savanna: F. sephaena, Acryllium vulturinum. Surplus in WA: Ptilopachus petrosus (rocky outcrops in savanna: a specific habitat, this is not a generalist, its absence outside WA is surprising; but its habitat is taken by Francolinus spp. in SA). Habitat shift: Guttera edouardi (limited to forest in WA, from which it is eliminated by G. plumifera in the region east of the Sanaga river - Louette 1981, and in all CEA. Elsewhere it lives in woodland).
Level of family: Estrildidae
Table 2 highlights deficiencies and surpluses in the different genera. This family is the best illustration that WA is not an impoverished region for all systematic groups. This case is all the more interesting because harsh conditions in the dry season are thought to select for food generalists among the residents (Lack 1986), which would limit the number of species per group in savanna (contra forest). However, while this may help to explain the limited number of species in several groups in WA, it does not in the Estrildidae, a family not reported to have developed extreme food specialisation between species (Goodwin 1982).
Level of genus: Serinus.
This and the following case, to the contrary of the previous example, demonstrate the poverty of WA in particular groups. Ignoring the Cape endemics and the temperate and montane species, WA is: Equivalent: All non-forest districts: S. mozambicus; Woodland: S. gularis; Savanna: S. leucopygius; Deficient: Woodland: S. capistratus, S. sulphuratus, and S. mennelli; Surplus: No examples.
Level of genus and species: Accipiter.
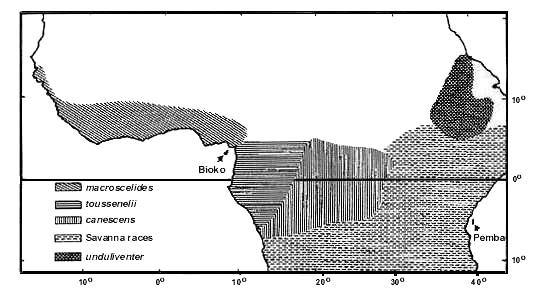
Equivalent: The only common species in WA savanna is A. badius which has two races in Africa: one in WA (shared with the Oriental region), the other in EA and SA. Both are very similar in ecology; both are unaccountably rare in Kenya (Lewis & Pomeroy 1989) where they meet. The southern race is definitely of local African origin; it is sympatric with a whole array of other Accipiter. The northern race has no sympatric congeneric species over most of its range. Deficient: Forest: A. castanilius (absent from Upper Guinea); Savanna: A. minutus: this species occurs at low altitude in EA. A. tachiro does occur in the forest district of WA, but is absent in savanna in WA. One would expect a local race similar or identical to the race sparsimfasciatus, morphologically a savanna adapted taxon, which occurs in montane as well as in lowland environment in EA. The morphologically forest adapted WA race macroscelides does not enter WA savanna in general, except locally, e.g. in Gambia (Louette in press: see Fig. 6). Localised: A. melanoleucus is present in forest in WA, but is localised in woodland here. A. ovampensis is a transient, erratically breeding in savanna in WA and in EA, more common in SA (though even there an enigmatic bird). Surplus: None.
DISCUSSION
Number of species in WA
The rather low total number of species in a tropical region of Africa is a permanent cause for surprise. Therefore, Fjeldså (1993) compared African and South American avifaunas. He suggested as reason why Africa in general is depauperate: the fragmentation of forests was less complex on this continent. Karr (1980) has demonstrated that the number of forest undergrowth birds is lower in Africa than in South America or Southwest Asia because of historical and some biotic (coevolution, priority of arrival and diversification of several taxa) effects.
The reason why, in the two major vegetation districts (Table 2), on a continent which is already poor in number of species, WA is equivalent to the other African regions in a whole array of groups, but not in others will be considered briefly. Groups poorly represented in Africa in general (large woodpeckers, parrots), for which one would perhaps expect a world-wide tropical niche to be available, are in each case non-existent or at best rare in WA. In view of the pan-African situation, this is not unexpected. But among particular groups, which are well represented elsewhere in Africa, such as large members of fowl (not compensated e.g., by Otidae), several species (size classes) in the genus Accipiter and canaries (not compensated by look-alikes of any kind) WA has striking deficiencies.
Among WA surpluses are a few supernumerary species in polytypic genera in the forest and the more distinct composition in the savanna with some unique genera. One would have expected a priori more differentiation in the forest (compare Gaston & Williams 1996), so we must presume poor speciation and/or low survival in WA in this biome. For the savanna, poor dispersal could be invoked, but this is the contrary to the supposed factor named above as favourable for dispersal, namely the structure of WA in parallel vegetation belts!
Interpretation of difference among regions in Africa can take into account (zoo)geographical (such as philopatry for some species with restricted ranges) and ecological factors. We must not forget, however, that the position of each subcontinent being unique, a number of differences (in avifauna) no doubt cannot be explained by simply comparing all the subcontinents.
Possibly the fauna was in a preceding period depleted in WA. In savanna, the number of species seems to be growing by colonisation from the east, where an ecological equivalent region is situated, and only slowly because it is not in immediate contact. In the forest, a more rapid colonisation from the touching CEA towards WA is suspected. It is probable, given the number of species and also the presence of supernumerary species in some forest bird genera in WA, that colonisation in the opposite direction was less frequently successful, possibly because of general saturation in species of CEA.
Causes
Climate or competition?
Given the fact that WA is apparently deficient, it is remarkable that birds from the Palaearctic failed to colonise mesic WA as breeding birds, although many of them occur as non-breeding migrants. Indeed, in several groups, nonbreeding Palaearctic migrants augment numbers of species (and even more individuals!). On the other hand, do these migrants possibly limit the number of resident species? No general study is available for WA. From SA comes the information that the ranges of migrants sometimes are remarkable complementary to those of residents (examples: Lanius spp. in the Kalahari, Harrison et al. 1997; general patterns, Underhill et al. 1998). In EA, no significant differences were detected by Lack (1986) between migrant visitors and residents as to general habitat choice, but Leisler (1992) claims that migrants exploit different (parts of) the habitats than residents. In line with studies that Palaearctic migrants prefer open and secondarised habitats in Africa, WA hosts many Palaearctic migrants in savanna and very few in the forest district (e.g., Louette 1981 for Cameroon).
Among bird groups that are otherwise well represented in Africa (mainly in SA) as well as in the Palaearctic and that are considered to be good dispersers, I want to mention the genera Anas (many species in SA) and Circus (2 species in SA). Is the absence of residents in WA in those genera due to the presence of migrants? In Kenya, the African Marsh Harrier C. ranivorus attains its northern limit and it is not so common at the coast, suggesting relief or climate are possible factors of importance determining its distribution.
Climate can be invoked, because in the temperate district of SA, several breeding birds are of recent Palaearctic origin, e.g., the White Stork Ciconia ciconia, the Booted Eagle Hieraaetus pennatus and the European Bee-eater Merops apivorus (none of which breed in WA). In WA, at most could such an ubiquitous species as the Stonechat Saxicola torquata (with a preference for cooler microhabitats in Africa) be suspected as being possibly a colonist from the Palaearctic! It is equally possible (though less convincing) that this is also the case e.g. for the Adamawa Turtle Dove Streptopelia hypopyrrha, possibly an offshoot of a Palaearctic species. Conversely, also a few typical Afrotropical birds were able to colonise the Palaearctic (e.g. the Helmeted Guineafowl Numida meleagris, the Black-crowned Tchagra Tchagra senegala, and the Fan-tailed Cisticola Cisticola juncidis) in the region just north of the Sahara. The origin of the few other breeding birds shared in the two continents over wide areas (including water birds as the Purple Heron Ardea purpurea, the Grey Heron A. cinerea, the Common Squacco Heron Ardeola ralloides, the Black-crowned Night Heron Nycticorax nycticorax, the Greater Flamingo Phoenicopterus ruber, and brood parasitic cuckoos Clamator spp.) is questionable. There is no other plausible explanation than climate for this remarkable dissimilarity in avifaunas.
Furthermore, the restricted number of species in WA in itself could be caused by climatic changes in historical times, eliminating tropical Palaeoafrican elements (Moreau 1966).
Zoogeography
If WA were not within the evolutionary refugium for a great number of species, massive colonisation from elsewhere would be necessary to build up the avifauna. The poverty of old elements in the forest suggests this may well be the case, at least during a certain period. Presence of some ‘young endemics’ seems to indicate its recolonisation before a more recent epoch of speciation (Moreau 1966). Finally, WA is possibly at present slowly filling with central African elements. The woodland and savanna fauna is more differentiated: it contains several endemic genera, some spilling over into EA, but younger elements may well have arrived recently from elsewhere.
If the carrying capacity of all non-montane tropical WA habitats would be equal to those in EA and SA, one would expect, resulting from a ‘communicating flow’ that their avifaunal composition would be very similar, if no barrier would obstruct exchange. For savanna birds exchange with EA, the ecological bottleneck of the dry region of South Sudan and Northeast Congo is a barrier; for exchange with SA, a (temporary) tolerance for forest vegetation would be necessary. The dissimilarity emphasises the efficiency of these obstacles.
Some birds with small ranges (philopatric species) are suspected to be slow colonisers (some Picidae, Capitonidae). But what about the others? Several birds are rarely recorded, possibly indicating either recent spread from the east or scarcity of their habitats. Would distance from the source be important? WA is outlying, but in the Sahel many species occur all the way to the west and apparently this distance is not a problem in highly seasonal and erratic systems (e.g. in the genus Serinus: the White-rumped Serin S. leucopygius, but not quite in the genus Anas: the Hottentot Teal A. hottentota did not yet spread more westwards than northernmost Nigeria). Especially in the mesic areas westward expansion seems to be a slow and still ongoing process. Why would colonisation speed differ among vegetation belts and would this relate to the seasonality or erratic nature of the system and the resulting adaptation of species for more opportunistic mobility (i.e. lower philopatry)? In the forest, I suspect slow colonisation in several species: e.g. the Speckled Mousebird Colius striatus is advancing progressively in Nigeria, the Afep Pigeon Columba unicincta was only fairly recently seen in Liberia and Nigeria (Colston & Curry-Lindahl 1986; Ash 1990). Data for the woodland species are insufficient, but a species such as the Red-capped Robin Cossypha natalensis is a candidate for recent expansion. As for the composition of the ‘localised species group’ in Table 2: they are more widespread in corresponding habitat in WA than elsewhere in Africa. No apparent common feature links them; localised species belong either to the forest, savanna or steppe habitats.
Other environmental factors
Topography in itself represents no obstacle for colonists from the rest of Africa. The extent of the montane areas is a measure for species numbers: virtually no endemic montane birds exist in Upper Guinea proper (Stattersfield et al. 1998) and the number in the Cameroon montane district is rather limited as well (Dowsett 1986). Fewer surfaces and less altitudinal gradient would give room for fewer niches and thus fewer species, also in non-montane cohorts. In fact, in EA and SA a good number of species perform local altitudinal migrations (Lewis & Pomeroy 1989; Harrison et al. 1997), a rare phenomenon in WA. This seasonal migration must be an important factor in order to avoid local extinction. On the other hand, in WA many species perform latitudinal migrations over quite long distances (Elgood et al. 1973).
The NDVI data (Fig. 4) let us presume that vegetation productivity could be higher in the Brachystegia (EA/SA) than in the Isoberlinia (WA) belt. This factor in combination with a specialisation in respectively highland and lowland environment may well be reasons for a fundamental distinction within certain groups between the regional African avifaunas.
WA’s avifauna is impoverished compared to other regions, although all families (except Promeropidae) are represented and some are equivalent in numbers, but quite different in taxonomical composition. It appears that the poverty in species is not evenly distributed over the groups. It is tempting to try to explain absence in WA of specific birds (and also localised distributions) either by absence of habitat or by zoogeographical causes.
It is far from certain that all the available niches have been filled. Louette (1983) found in Cameroon that there were more species in the districts ‘forest’ and ‘sahel’ than in the intermediate ones belonging to woodland and savanna. A latitudinal gap between vicariants is apparent within some genera (example: the Northern Ant-eater Chat Myrmecocichla aethiops and the Sooty Chat M. nigra; Louette 1981). There is no plausible explanation for this phenomenon. If open niches would exist, could colonisation be more difficult in woodland than either in forest or in dry savanna? Do certain habitats buffer the expansion process more consistently than others?
All the regional studies point to the conclusion that the avifaunal composition of WA is in an unstable phase: the approximately 80 years of exploration did not suffice to detect with certainty the process of slowly expanding species. Certainly, disturbed forest and woodland areas receive rapidly immigrants typical for ‘savanna’ environment, a phenomenon visible in many coastal areas and cities of WA.
CONCLUSIONS
The factors investigated let us make the following conclusion for the peculiar composition of the WA avifauna:
Geography
Situated on a continent that has few species in general. Outlying location, no oceanic islands, no montane or temperate habitats. Size of region limits the number of species: WA forest district is only about one third of CEA. WA woodland and savanna districts only about half of those in SA and EA. Mostly lowland. An altitudinal gradient would yield more species.
Barriers
Limited by ocean, desert, forest (both habitat) barriers. Cameroon ridge acts as a barrier (bottleneck for forest birds) towards the coast. Small remnants of forest habitat in historical times, probably resulting in mass extinctions and little speciation. WA is open on one side only, which allows savanna birds exchange: near the ecological bottleneck in the dry region of South Sudan (refuges in EA would probably have had more possible contacts with one another, but SA seems equally isolated as WA).
Habitat
Parallel vegetation belts in WA versus mosaic vegetation structure elsewhere limits number of habitats and gives poor fragmentation chances in case of general climate change. NDVI data suggest vegetation productivity could be higher in the southern Brachystegia than in the WA Isoberlinia belt.
ACKNOWLEDGEMENTS
I am grateful to Marc Herremans for fruitful discussions on the manuscript, to Danny Meirte for the production of the relief and NDVI maps, and to Alain Reygel for the drawing of the other maps.
REFERENCES
Allport, G.A. & Fanshawe, J.R. 1994. Is the Thick-billed Cuckoo Pachycoccyx audeberti a forest dependent species in West Africa? Malimbus 16: 52-53.
Ash, J. 1990. Additions to the avifauna of Nigeria, with notes on distributional changes and breeding. Malimbus 11: 104-116.
Ash, J., Dowsett, R.J. & Dowsett-Lemaire, F. 1989. New ornithological distribution records from eastern Nigeria. Tauraco Research Reports 1: 13-27.
Colston, P.R. & Curry-Lindahl, K. 1986. The birds of Mount Nimba, Liberia. London; British Museum (Natural History).
Dowsett, R.J. 1986. Origins of the high-altitude avifaunas of tropical Africa. In: Vuilleumier, F. & Monasterio, M. (eds) High altitude tropical biogeography. Oxford; Oxford University Press.
Dowsett, R.J. & Dowsett-Lemaire, F. 1993. A contribution to the distribution and taxonomy of Afrotropical and Malagasy birds. Tauraco Research Reports 5: 1-389.
Elgood, J.H., Fry, C.H. & Dowsett, R.J. 1973. African migrants in Nigeria. Ibis 115: 1-45, 375-411.
Elgood, J.H., Heigham, J.B., Moore, A.M., Nason, A.M., Sharland, R.E. & Skinner, N.J. 1994. The birds of Nigeria. An annotated check-list. Tring; British Ornithologists’ Union.
Fjeldså, J. 1993. A comparison of African and South American avifaunas using molecular clocks. Proceedings of the Eighth Pan-African Ornithological Congress: 67-75.
Fry, H. 1980. An analysis of the avifauna of African northern tropical woodlands. Proceedings of the Fourth Pan-African ornithological Congress: 77-88.
Gaston, K.J. & Williams, P.H. 1996. Spatial patterns in taxonomic diversity. Oxford; Blackwell.
Germain, M. & Cornet, J.P. 1994. Oiseaux nouveaux pour la république centrafricaine ou dont les notifications de ce pays sont peu nombreuses. Malimbus 16: 30-51.
Goodwin, D. 1982. Estrildid finches of the world. London & Oxford; British Museum (Natural History) & Oxford University Press.
Hall, B.P Moreau, R.E. &. 1970. An atlas of speciation in African passerine birds. London; British Museum (Natural History).
Harrison, J.A., Allan, D.G., Underhill, L.G., Herremans, M., Tree, A.J., Parker, V. & Brown, C.J. 1997. The atlas of southern African birds (including Botswana, Lesotho, Namibia, South Africa, Swaziland and Zimbabwe). Johannesburg; Birdlife South Africa.
Karr, J.R. 1980. Geographical variation in the avifaunas of tropical forest undergrowth. Auk 97: 283-298.
Lack, P.C. 1986. Ecological correlates of migrants and residents in a tropical African savanna. Ardea 74: 111-120.
Leisler, B. 1992. Habitat selection and coexistence of migrants and Afrotropical residents. Ibis 134, supplement 1: 77-82.
Lewis, A. & Pomeroy, D. 1989. A bird atlas of Kenya. Rotterdam; Balkema.
Louette, M. 1981. The birds of Cameroon. An annotated check-list. Verhandelingen Koninklijke Academie Brussel (Klasse Wetenschappen) 43 (163): 1-295.
Louette, M. 1983. Bird species numbers in Cameroon vegetation districts. Malimbus 5: 45-49.
Louette, M. 1990. Distribution patterns in African lowland forest birds. In: Peters, G. & Hutterer, R. (eds) Vertebrates in the Tropics. Bonn; Museum Alexander Koenig: 237-247.
Louette, M. 1992. Barriers, contact zones and subspeciation in central equatorial Africa. Bulletin British Ornithologist’s Club Centenary Supplement 112A: 209-216.
Louette, M. in press. A redescription of African Goshawks Accipiter tachiro from Bioko and the adjacent mainland. Ostrich.
Louette, M., Bijnens, L., Upoki Agenonga, D. & Fotso, R.C. 1995. The utility of birds as bioindicators: case studies in equatorial Africa. Belgian Journal of Zoology 125: 157-165.
Louette, M. & Prigogine, A. 1982. An appreciation of the distribution of Dendropicos goertae and the description of a new race (Aves: Picidae). Revue de Zoologie Africaine 96: 461-492.
Moreau, R.E. 1966. The bird faunas of Africa and its islands. New York & London; Academic Press.
Morel, G.J. & Chappuis, C. 1992. Past and future taxonomic research in West Africa. Bulletin British Ornithologist’s Club Centenary Supplement 112A: 217-224.
Sibley, C.G. & Ahlquist, J.E. 1990. Phylogeny and classification of birds: a study in molecular evolution. New Haven; Yale University Press.
Snow, D.W. (ed.) 1978 An atlas of speciation in African non-passerine birds. London; British Museum (Natural History).
Snow, D.W. 1980. The affinities of African non-passerine birds to the Oriental and Palaearctic avifaunas. Proceedings of the Fourth Pan-African ornithological Congress: 71-76.
Stattersfield, A.J., Crosby, M J., Long, A.J. & Wege, D.C. 1998. Endemic bird areas of the world. Priorities for bird conservation. Birdlife Conservation Series Number 7. Cambridge; Birdlife International.
Tucker, J. 1997. NDVI map of Africa. NASA/GSFC GIMMS group. In: Pfirman, E. & Hogue, J. (eds) WINDISP3. FAO, Rome (electronic version).
Underhill, L.G., Herremans, M., Navarro, R.A. & Harrison J.A. 1998. Where do Palearctic migrant passerines concentrate in southern Africa during the austral summer? Proceedings of the European Ornithological Conference.
Table 1. Unique species from CEA (central equatorial Africa) with (supposedly) Oriental species as closest relatives, absent from WA (West Africa). (For brevity, no English names are given in the tables)

Table 2. Number of species within taxonomic groups of birds in WA (West Africa), compared to the other regions (forest district: F; woodland and savanna districts combined as S). This list makes a count of zoogeograpical (not necessarily ecological) vicariants; the net result is indicated. Species restricted to dry or temperate districts of SA (southern Africa), or to montane districts generally, are ignored as are the Viduinae (whose systematics still are experimental and whose ranges coincide with those of their estrildid hosts). For convenience, the sequence of species and family names follow Hall & Moreau (1970) and Snow (1978).
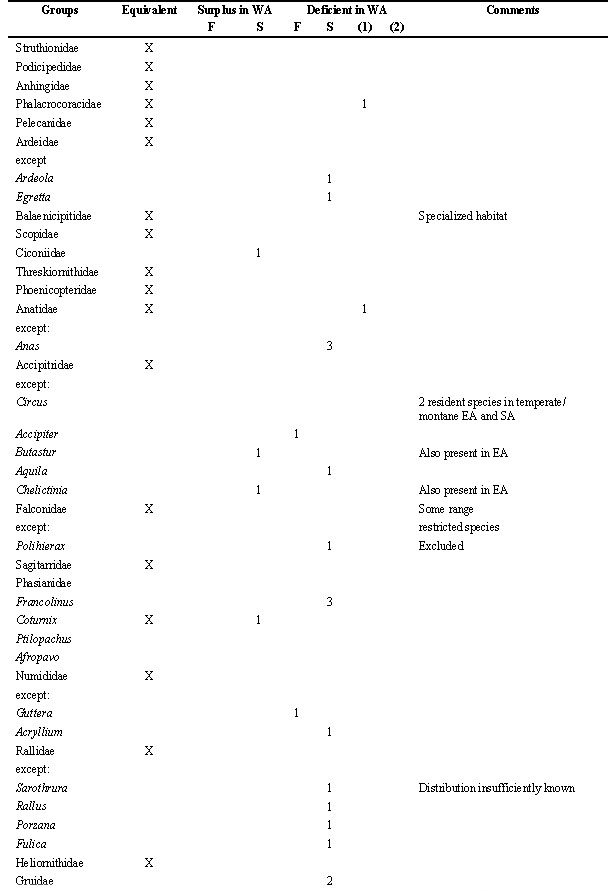
Table 2. continued
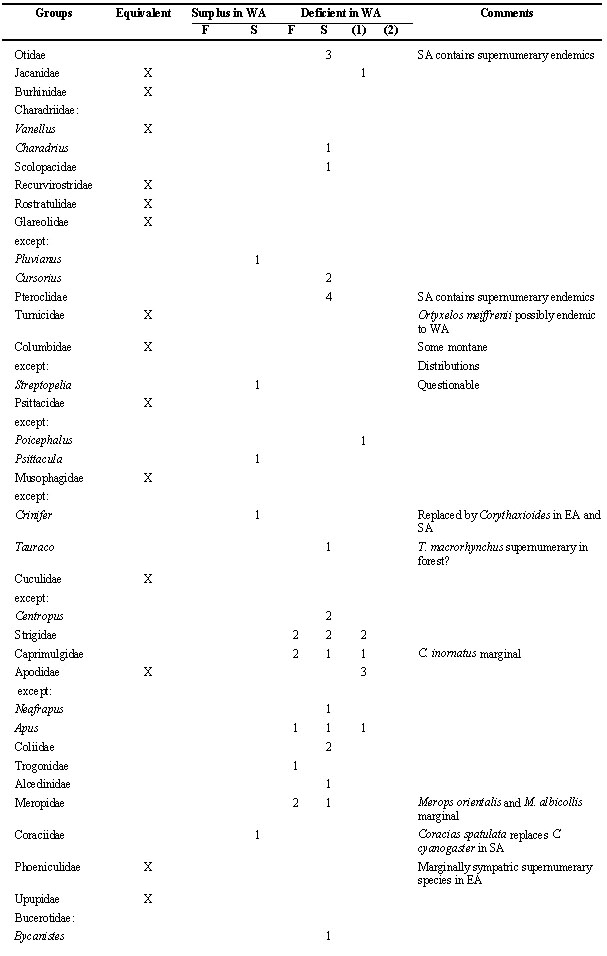
Table 2. continued
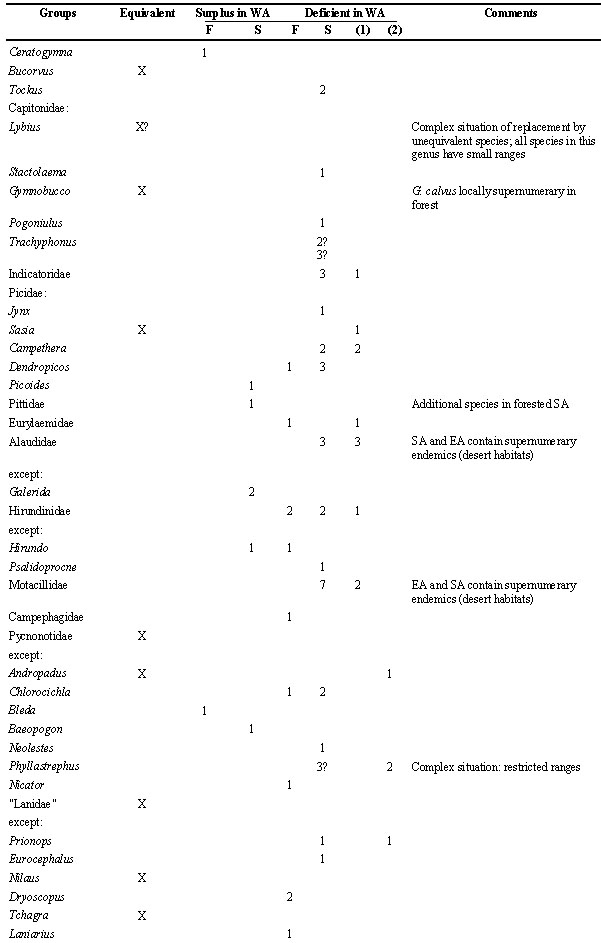
Table 2. continued
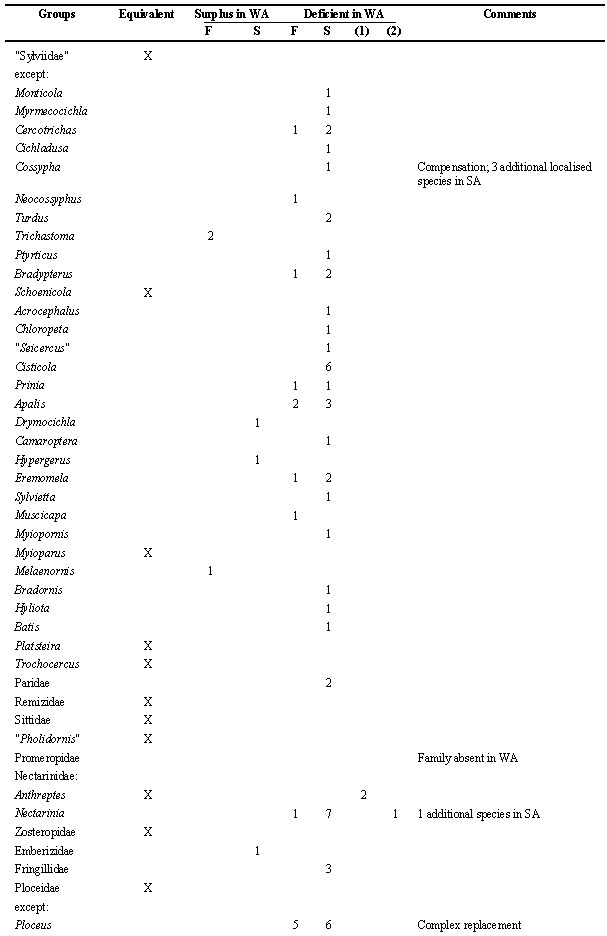
Table 2. continued
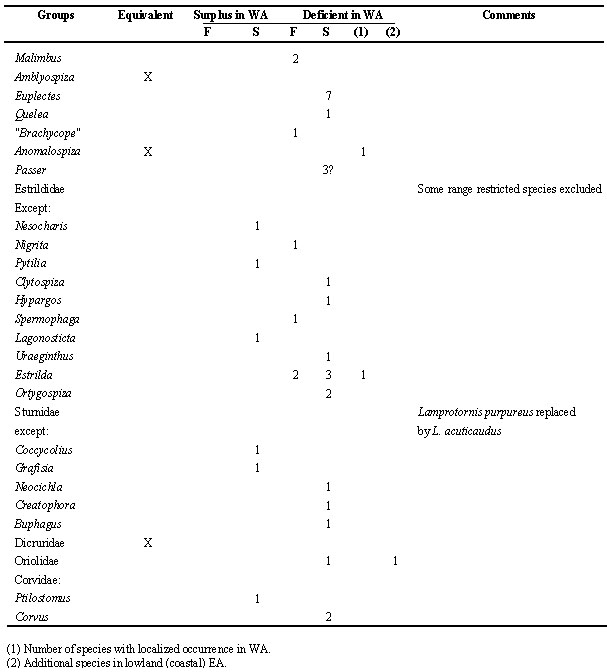
Table 3. Forest dependent species in WA (West Africa), more widespread in other regions.
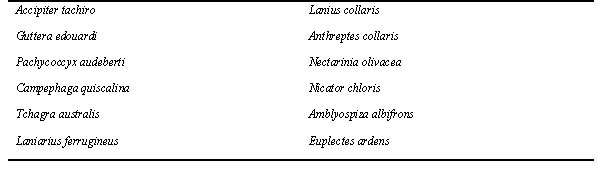
Fig. 1. Delimitation of West Africa with vegetation districts.
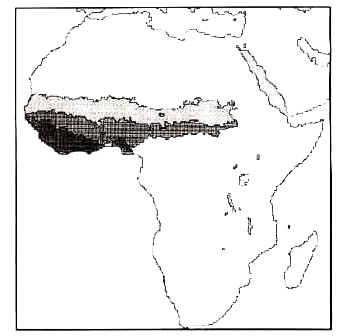
Fig. 2. The relief of Africa in steps of 300 m. Areas above 1500 m in black.
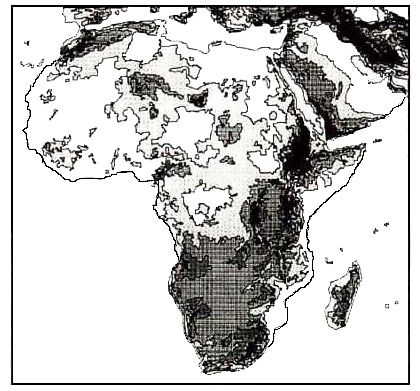
Fig. 3. African vegetation districts (after Louette 1981).
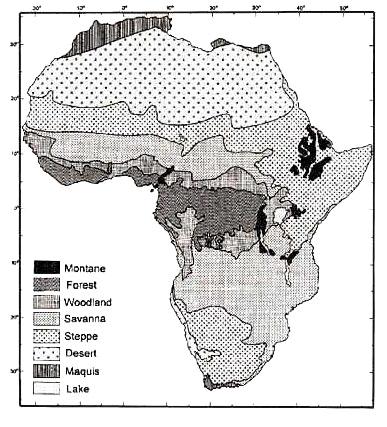
Fig. 4. The Normalised Difference Vegetation Index for Africa, averaged for 10 years and grouped in ‘soil’ and 4 different vegetation classes (degree of greenness indicated by density of shading).
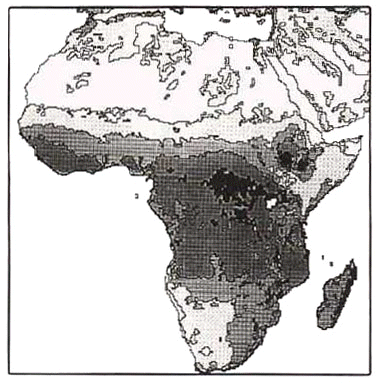
Fig. 5. Distribution of taxa in the superspecies Dendropicos (Mesopicos) goertae (after Louette & Prigogine 1982). Squares: goertae; triangles: spodocephalus; circles: griseocephalus. Forest district in oblique barring.
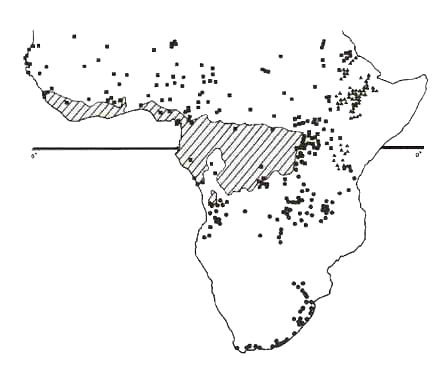
Fig. 6. Distribution of subspecies of the African Goshawk Accipiter tachiro.