S49.4: Satellite tracking of White-chinned Petrels and comparison with other Procellariiformes
Antoine Catard & Henri Weimerskirch
CEBC-CNRS, 79360 Beauvoir, France, fax 33 5 49 09 65 26, e-mail henriw@cebc.cnrs.fr
Catard, A. & Weimerskirch, H. 1999. Satellite tracking of White-chinned Petrel and comparison with other Procellariiformes. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 3008-3023. Johannesburg: BirdLife South Africa.The foraging behaviour of 16 White-chinned Petrels Procellaria aecquinoctialis (mass 1-1.4 kg) breeding on the Crozet Islands was studied with miniaturised satellite transmitters taped to the back feathers of birds leaving the nest for a foraging trip. The results show that birds breeding in the sub-Antarctic forage in marine environments as diverse as the edge of pack-ice along Antarctica to the Benguela current off South Africa, 2342 and 3495 km from their nest, respectively. This is the longest distance from the nest that has been measured for a breeding seabird. The foraging pattern of these petrels are compared with those of several species of albatross, in terms of flight pattern and speeds, diurnal/nocturnal activity and the influence of wind. Albatrosses appear to be more specific in their foraging zones than White-chinned Petrels, which appear to range further from the breeding grounds because they move during the day as well as at night. Albatrosses and petrels are threatened by the development of fisheries in the Southern Ocean and a good knowledge of their foraging zones is essential for conservation management. Indeed, the White-chinned Petrel is the commonest species in the by-catch of long-liners and their extended foraging range overlaps with fisheries from Antarctic to tropical waters.
INTRODUCTION
The White-chinned Petrel Procellaria aequinoctialis, (WCP), is the largest burrowing petrel, with a mass of 1.2 kg and a wing span of 1.5 m. It is a widespread and abundant species throughout the Southern Ocean and therefore it plays an important role in this ecosystem. According to dietary information (Ridoux 1994, Croxall et al. 1995) and feeding frequencies (Pennycuick et al. 1984), the WCP was presumed to be a long-range pelagic feeder. Many of its life history traits (delayed maturity, long life expectancy, single-egg clutches, slow growth of the chick, long feeding trip duration and high wing loading) suggest that its life history strategy could be close to those of the larger Procellariiformes such as albatrosses. Satellite tracking transmitters are now small enough to be fitted on birds of the size of WCP and it is now possible to investigate the distribution of this petrel in relation to the marine environment. The aims of this paper are to provide information about the foraging strategy of WCP and to compare it with those of other species of Procellariiformes such as albatrosses which have already been studied with satellite tracking. A better understanding about the at sea behaviour of this species is of considerable interest for conservation purposes since WCPs are the primary seabird species to occur in the by-catch by longline fishing vessels (CCAMLR 1997).
METHODS
The field study was carried out on Possession Island (45°25’S, 50°45’E), Crozet Archipelago, in the south-western Indian Ocean. In a study colony, 62 burrows were fitted with trap doors to allow easy access to the nests. Since November 1995, 57 pairs were measured and banded with a metal ring on one foot and a darvic ring on the other. For sexing the adults, we considered that males are larger than females (Murphy 1936). For sexing birds whose mates had not been measured, we used a discriminant analysis. The inversion of metal and darvic rings within each pair was used to distinguish the partners from the trap door without handling the birds. Adult’s visits were revealed by disturbance of sticks placed across the entrance of the burrow. Throughout the breeding period, the rat population was trapped and poisoned regularly on the site. This is known to improve significantly the breeding success (Micol, pers. com.). At the beginning of the incubation, 29 pairs were available for the satellite tracking study. The foraging trips of WCPs were monitored using the Argos satellite tracking system (Fancy et al. 1988). The transmitters deployed (Microwave 100) weighed 30g, which corresponds to 1.6-2.9% of the bird body weight (range : 1.03-1.87kg). They were attached directly to the back feathers, using adhesive tape, reinforced with epoxy resin. The transmission interval was 90s. During two periods, 6 transmitters were deployed simultaneously on a total of 16 different birds of both sexes. Firstly, during the incubation period between 28 November and 31 December 1996, 10 foraging trips were obtained. Secondly during the chick rearing period, between 27 January and 22 February 1997, transmitters were fitted on 6 birds and recovered during a feeding visit after one, two or three foraging trips, with 14 trips tracked. No transmission failure and no loss of transmitters occurred during the whole study. Data have been analysed using ELSA software (CLS Argos, Toulouse, France) and home-made softwares. All classes of location accuracy provided by the Argos system were used. Only locations that were implausible (giving a flight speed > 90 km h-¹) were excluded. In order to test if the transmitter had an effect on the behaviour of the birds tracked we compared time spent at sea for birds carrying transmitters with that of others, not carrying transmitters. No significant differences between these two groups indicated that transmitters probably did not alter the behaviour of the WCPs (average 9.78 ± 3.64 d and 9.84 ± 5.86 d respectively, F1, 23= 0.0007, P>0.05). Information on direction and wind strength for every square of 1.5° in the western Indian Ocean was obtained from Météo France (Toulouse) for the incubation period.
RESULTS
WCPs display two different types of foraging trips, trips of long duration and short trips. During long trips,WCPs forage from Crozet in all directions and reach polar to sub-tropical latitudes (range between 31°S and 66°S). During short trips they remain on the Crozet shelf.
Incubation trips
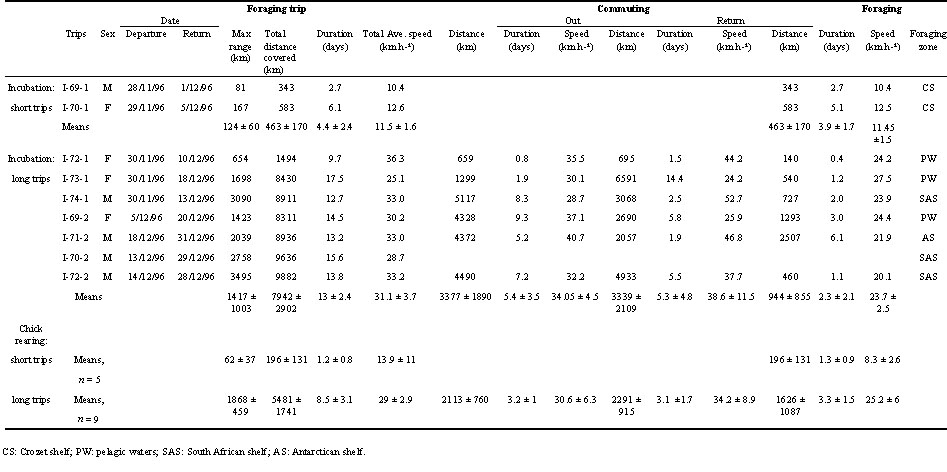
The 10 birds tracked during incubation covered on average 5653 km in 11.1 d for long trips, ranging as far as 3495 km from their colony. However, several types of foraging trips were recorded (Fig.1 & Fig. 2).Two birds remained in the inshore Crozet shelf (I-70-1; I-69-1). One bird made a simple small loop course to the north (I-72-1). Two birds made long complex courses over pelagic waters. One of those two foraged solely in sub-Antarctic waters. It flew directly to the west to a shoal (46°26’S, 42°31’E) before foraging to the south, making several loops with several stops (I-73-1). The other bird moved to the east and spent several days on the Kerguelen shelf before moving to sub-tropical latitudes (I-69-2). The three most spectacular foraging trips were large journeys to the South African shelf. One started to fly to the west reaching the Prince Edward Island shelf before turning towards the north (I-74-1). It spent 2 days foraging actively close to the south west South African coast, 50 km off Cape Town. This bird followed a straight track to return to Crozet, moving rapidly with an average speed of 52.7 km h-¹ over 3068 km. The longest foraging trip of the study was recorded for a bird making a large loop course, foraging along the South African shelf, moving to the north (up to 33°7’S, 16°11’), reaching the south Namibian coast (I-72-2). Although the maximum range was as far as 3495 km for a total duration of only 13.8 d, this bird did not return straight to Crozet but used a southern movement. When reaching the Benguela region, those two last birds followed closely the edge of the continental shelf. The third bird moved to the north to reach sub-tropical waters before turning to the west, following more or less the 34°S parallel to the South African shelf before returning to the colony (I-70-2). The last type of trip recorded during this session was made by a bird flying to the southern Antarctic waters. It spent two and a half days on a specific foraging area before following the ice pack edge closely, then turning north to return to Crozet (I-71-2).
Chick rearing
At this stage, individual birds alternated long and short trips. This alternation was confirmed by a three weeks continuous monitoring of the whole study colony. On average, birds were spending 64% of their foraging time on long trips and 36% on short trips. Tracked birds covered an average of 3593 km in 6 d for long trips ranging as far as 2420 km. One bird followed a small looping course to the north-west, intermediate in length between short and long typical trips (C-71-1). All long trips except two were similar to the trip to Antarctic waters of I-71-2 during incubation. Only one bird foraged in sub-tropical waters, making a looping course to the north east (C-74-1). None of the foraging trips recorded during the chick rearing period occurred in the South African shelf area. In addition five shorts trips within the Crozet shelf were recorded.
There were no significant differences between the incubation and the chick rearing long trips from a quantitative point of view. Velocity, time at sea and distance covered were similar (ANOVA, P>0.05).
The relationships between the time spent at sea and maximum foraging range or distance covered, during both incubation and chick rearing periods were significant (Fig. 3).
Influence of wind
The effect of wind on the movement of birds was analysed for the incubation period. We found no significant correlation between birds' velocity and wind speeds (r = 0.048, P = 0.2) However, with respect to the wind direction, birds flew preferentially with a particular angle between flight track and wind direction. They tended to fly leeward during the outward journey, whereas during the return trips they used lateral winds (Fig. 4). No significant differences in the average direction of the wind relative to the birds' flight path occurred between the outward and return journeys (X²4 = 7.078, P=0.132).
Outward, return and foraging flight
We defined the foraging section of the trips as the part of the trip when birds are staying in a particular area, changing flight direction continuously. Outward and return tracks were considered as the section of the trip between the foraging area and the colony. For trips when birds are moving continuously, birds were considered as returning when the distance between the colony and the bird started to decrease. In both study periods, the velocity was significant higher for the return than for the outward journey. Average speeds are for outward tracks and for the return, respectively for the incubation period 31.4 ± 17.86 km h-¹ and 37.95 ± 19.87 km h-¹ and 28.07 ± 18.35 km h-¹ and 33.89 ± 20.24 km h-¹ respectively for the chick rearing period (ANOVA , F1, 336 = 9.97, P= 0.001 and, F1, 358 =7.9, P=0.005, for incubation and fledging respectively). The velocity was always lower in foraging zones than when birds were commuting (16.91 ± 15.21 km h-¹ and 33.01 ± 18.83 km h-¹ respectively for the incubation period, 22.71 ± 18.41 km h-¹ and 30.19 ± 19.30 km h-¹ respectively for the chick rearing period, ANOVA, , F1, 676 = 110.23, P< 0.001 and F1, 523 = 17.34, P< 0.001 respectively). During the incubation period, we found a lower mean velocity for birds flying over continental shelf (deep£ 1500 m) compared to oceanic waters (20.81 ± 20.50 km h-¹ and 31.34 ± 18.65 km h-¹ respectively, ANOVA, F1, 625 = 29.38, P<0.001).
Day and night time velocities
Daytime flight velocities were significantly higher than night time velocities during incubation but not during the chick rearing period (incubation: daytime velocity mean = 30.97 ± 21.14 km h-1, night time velocity mean = 25.64 ± 17.81 km h-¹; chick rearing: day time velocity mean = 28.13 ± 19.54 km h-¹ night time velocity mean =26.55 ± 19.89 km h-¹ ; ANOVA, F1, 676 = 8.55, P=0.0035 and, F1, 523 =1.98, P=0.15).
Moon phases effect
Flight speed was significantly lower during the full moon phase than for nights with half moon or no moon, for the chick rearing period but not for the incubation period. During the chick rearing period average speeds were 33.68 ± 20.45 km h-¹ for the new moon, 28.4 ± 16.9 km h-¹ for the quarter moon, 19.28 ± 15.59 km h-¹ for the full moon. During incubation average speeds were 25.21 ± 8.7 km h-¹ for the new moon, 28.68 ± 18.49 km h-¹ for the quarter moon and 27.68 ± 17.14 km h-¹ for the full moon (ANOVA, F2, 152 = 6.25, P=0.002 and F2, 114 = 0.34, P= 0.7 , respectively for the chick rearing and incubation period).
DISCUSSION
Foraging behaviour of White-chinned Petrel
Flying behaviour
The first notable result from this study is that WCPs have an extremely wide-ranging distribution during the breeding period. They forage from tropical to Antarctic waters, and are exploiting neritic as well as pelagic waters. Observation at sea of birds of unknown origin indicated that WCPs have a very wide latitudinal distribution (Woehler et al. 1990, Stahl et al. in press). Since it was difficult to envisage that breeding birds could forage at such distances from their breeding grounds, observations of birds far from breeding sites were usually thought to be of non-breeders. In particular the regular exploitation of remote resources from the Benguela region for the reproduction of a sub-Antarctic species was previously unsuspected, although it was known that WCPs winter there in large numbers (Rand 1962, 1963). Indeed, WCPs from Crozet have been recaptured there in winter (Weimerskirch et al. 1984). Seasonal geographic differences in distribution have been noted. WCPs are absent from Antarctic and sub-Antarctic waters in winter, with a total absence in July in the south west Indian Ocean waters (Woehler et al. 1990, Stahl et al. in press). During this period, however the numbers of WCPs in the Benguela region are at their highest (Jackson 1988).The South-west African coast appears therefore as a major feeding ground for WCPs in winter, but also for breeders during the spring. This area is a high productive place because of the presence of upwelling from the Benguela current (Ashmole 1971).
Apart from the extraordinarily wide-ranging capacity of breeding WCPs, the second major result of this study is that, especially during incubation, this species exploits very diverse marine environments through the use of contrasted foraging strategies. They exploit neritic waters of the Crozet, Kerguelen, Prince Edwards and other banks of the sub-Antarctic and Antarctic waters, as well as offshore waters of the Benguela area. Additionally they forage extensively in pelagic waters, at Frontal zones, along the Antarctic pack-ice or in oceanic waters without any particular hydrographic feature. To do this they undertake long or short-ranging looping courses over oceanic waters or neritic commuting trips (see Weimerskirch 1997 for definition) as well as more complex wide-ranging movements.
According to at sea survey results, WCP’s densities appear to change spatially throughout the year. Birds have a sub-tropical and sub-Antarctic distribution in the early stage of the breeding cycle whereas high densities occur in Antarctic waters from January to the end of March (Woehler et al. 1990, Stahl et al. in press). This movement from sub-tropical to Antarctic waters fits well with our results. Indeed, this study also revealed a striking change in the spatial distribution of birds between incubation and the chick rearing period, with most birds concentrating their foraging effort in Antarctic waters and over the Crozet shelf through the use of a two-fold strategy (Weimerskirch et al. 1994) during the latter period. We do not know the reason for this shift but it could be related to the changes in energy requirements related to the breeding stages, or to changes in food availability, for example some important resources in Antarctic waters becoming available only in January. Indeed, at this time most birds commute rapidly and directly to the same areas, indicating that prey availability or spatial distribution is highly predictable. It should be noted that the latitudes where birds forage correspond to the Antarctic divergence.
WCPs always fly faster when they are returning to the colony than during the outward journey. This could be explained firstly by the hypothesis that when they have acquired enough energy they no longer search for food and /or try to return as quickly as possible to the nest ; as a result, their average speed increases. Indeed, the lower speed recorded over foraging areas was probably related to stops and flight deviations and is indicative of a high foraging activity. A second factor is that since most outward movements tended to be to the west, birds were therefore more likely to be favoured by the prevailing westerly winds on their return to the colony.
Comparison with predictions
The velocity of WCPs was calculated by Pennycuik (1982). He gave Vmp=34.9 km. h¹ as speed for minimum range and Vmr=57.6 km h¹ as speed for maximum range. Vmp was calculated for a bird minimising its effort per unit time. It is close to the average speed observed for WCPs tracked with satellite transmitters. Vmr is the speed calculated for a bird minimising its efforts per unit distance. It is faster than the average value recorded for satellite tracked birds, but not substantially different from the velocity of several birds returning to the colony. It should be noted that the values given by Pennycuick are for straight line short distance flights without wind whereas the values from satellite tracked birds are for longer distance straight line flight, i.e. encompassing a zig-zag flight.
Differences between sexes
Several species of Procellariformes are known to show sex specific foraging zones, noticeably Wandering Albatross Diomedea exulans (Weimerskirch & Jouventin, 1987, Weimerskirch et al. 1993) and the closely related Grey Petrels Procellaria cinerea (Bartle 1990). In both species, females and immatures forage in sub-tropical waters north of the convergence, whereas males are found further south. During incubation only male WCPs foraged on the South African shelf. Females appeared not to use commuting flights compared to males, flying more in looping or complex flight courses in sub-Antarctic or sub-tropical waters. During the chick rearing period, in Antarctic waters females appeared to forage in specific areas different from those of males, whereas different males commuted straightaway and foraged together at the same place. The smaller females could be at a disadvantage in competing with males at high density food patches such as behind vessels or in Antarctic waters. This was proposed to explain the difference in survivorship between the sex for the Westland Black Petrel Procellaria westlandica (Bartle, 1990). However, there is considerable overlap between the sexes in all morphometric measurements in WCPs (Hall, 1987), therefore sexual segregation may be interpreted cautiously for the WCP and further data are needed to determine whether sexual segregation in the foraging zones occurs in WCPs.
Comparison with Wandering Albatross
Although there are extensive differences in the size and life history traits of Wandering Albatrosses and WCPs, the foraging strategies of the two species show several similarities. One unexpected result is that WCPs are able to perform foraging trips of similar or even much longer range than the eight times larger Wandering Albatross. Both species during the chick rearing period use a two-fold strategy, alternating short trips on Crozet shelf edge and long trips to oceanic waters (Weimerskirch et al. 1993). However at this time the long commuting movements are more common in WCPs, whereas Wandering Albatrosses use commuting flights to the Kerguelen shelf to a much smaller extent, favouring looping movements.
The long looping courses of WCPs are very close to the pattern of Wandering Albatross long trips, especially those undertaken to the north. The specific zones used during the short commuting trips to the south-east edge of the Crozet shelf are very similar between the two species, suggesting that this is a highly productive zone.
Both species use the wind in a very similar way during their outward and return movements, favouring sideways or leeward winds to windward flight (Fig. 4), suggesting that wind direction plays a similar role in the flight of both species. However WCPs flew windward to a greater extent during the outward or return journey than Wandering Albatrosses, which tend to fly windward rarely. The absence of correlation between wind speed and birds' velocity suggest that, unlike albatrosses, WCPs are possibly able to increase their velocity by increasing their flapping flight, whereas this is probably not the case for Wandering Albatross. The closer examination of track directions and wind pattern suggests that rapid wind direction changes are less likely to influence the movement of WCPs compared to Wandering Albatross. However, similarly to Wandering Albatrosses (unpublished data), when moving to the north, WCPs follow an anticlockwise movement, probably to take advantage of wind directions. Indeed, easterly winds are the commonest in sub-Antarctic latitudes and thus more favourable for flying west, whereas westerly winds are dominant in the sub-Antarctic area.
Throughout the season, Wandering Albatross daylight velocity is about twice the night velocity. In WCPs the difference is not so extensive, being 1.11 times the night-time velocity (29 km h-¹ and 26 km h¹ respectively). This result suggests that WCPs may be still very active at night. Like other burrowing petrels, WCPs have a good nocturnal vision and are known to be one of the few Procellariiformes still active at night around fishing vessels (Barnes et al. 1997). Therefore, they may also active at night to feed on natural prey such as myctophids that vertically migrate at the surface at night (Holton 1969; Kozlov 1995). Their ability not only to feed but also to travel at night would also allow them to reduce overall commuting time.
Whereas Wandering Albatrosses are able to increase their foraging effort by flying at night during moonlight, we found the opposite results for the WCP during the chick rearing period and no differences appeared during incubation. Full moon was considered to influence positively WPC’s activity behind fishing vessels (Barnes et al, 1997).
Comparison with other species
Foraging strategies
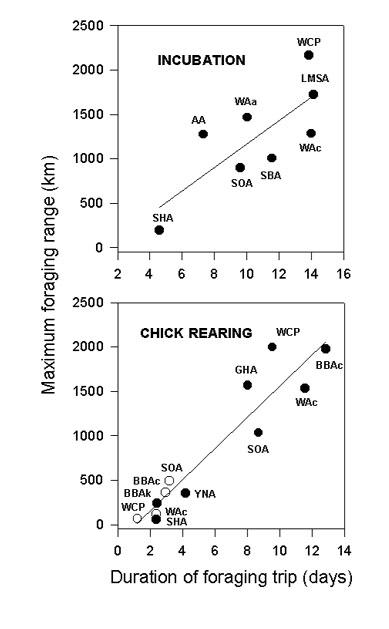
The foraging strategies described for six albatross species can be separated into two main patterns commuting flights on the one hand and searching flights on the other (Weimerskirch 1997). Commuting flights could be long or short whereas searching flights could be linear or in loop. WCPs use the whole range of strategies but are also able to make a large looping course to reach a specific area such as the South African shelf or the Antarctic divergence. Commuting trips to Antarctic waters are very similar to some recorded for Light-mantled Sooty Albatrosses Phoebetria palpebrata from Macquarie Island (Weimerskirch and Robertson 1993), or Crozet (Weimerskirch unpublished) and possibly for Short-tailed Shearwaters Puffinus tenuirostris breeding in Australia (Nicholls et al.1997). Waved Albatross Diomedea irrorata commute in a straight line to the Peruvian upwelling zone, 1200km from their nesting colony in the Galapagos archipelago ( Anderson et al, 1997). Southern Buller’s Albatrosses Diomedea bulleri mix two different patterns of movement during the incubation period, commuting trips to the mid Tasman sea and active searching in linear trips along New Zealand continental slope (Sagar and Weimerskirch, 1996). Whatever the favoured feeding areas of destination, we suggest that all commuting patterns could be related to the exploitation of particular marine areas of high productivities where most of the foraging activity probably takes place. These long commuting movements bring WCPs to the Benguela region, at a distance far in excess of that recorded for other seabirds commuting during the breeding season (Fig. 5).
Whereas WCPs are able to use a combination of the foraging strategies noted for albatrosses, none of the tracks recorded could be classified as a linear course. This behaviour was observed for Sooty Albatrosses Phoebetria fusca and Light-mantled Sooty Albatross over the oceanic waters, for Southern Buller’s Albatross (Sagar and Weimerskirch, 1996) and the Royal Albatross Diomedea epomophora along New Zealand shelf break (Nicholls et al. 1994).
Breeding adult Shy Albatrosses Diomedea cauta are relatively sedentary, travelling and feeding over the continental shelf within 300km of their Tasmanian breeding colonies (Brothers et al, 1998). In the same way, Royal Albatrosses breeding in New Zealand forage close to the coast over waters between 500 and 1000 m depth (Nicholls et al. 1994). It seems that these albatrosses are specialised for feeding over shelf areas, whereas the larger population of WCP at Crozet seems to be unable to find enough resources on the limited Crozet shelf throughout their breeding cycle, thus they travel further over oceanic zones.
Westland Petrels, the WPC's close relative, show a distinct foraging strategy. They tend to forage close to the colony but are able to range up to 800 km. Unlike WCPs, they forage only on the continental slope during the incubation period (Freeman et al, 1996). Shy Albatrosses, Royal Albatrosses Westland Petrels and to some degree the Southern Buller’s Albatrosses are less pelagic than WCPs or Wandering Albatrosses from Crozet Islands. This is probably related to the extensive size of the continental shelf areas, especially the slope areas and their related productivity, around Tasmania or New Zealand compared to Crozet. The foraging strategy of Crozet WCPs may therefore have evolved to optimise the patchiness and distance resources of their prey in the Indian Ocean.
Foraging flight features
Incubation period:
There is a good relationship between the duration of foraging trips and foraging range or distance covered for Procellariiformes tracked during the incubation period (Fig. 5). WCPs are found at a extreme position compared to all other species studied (Fig. 5), indicating that they are exploiting resources at longer range with greater average foraging trip length. We found no significant correlation between the mass of the birds and the maximum range reached (Spearman correlation test, P>0.1).
The flight features of WCPs and four species of albatross tracked during the incubation period are compared in Table 2. The outward mean speed is higher for the WCP than for any other species, while for the return track, WCP and the Light-mantled Sooty Albatrosses a significantly higher mean velocity than the three others. The foraging velocity is lower for all species compared to WCP. The high foraging velocity suggests that WCP's foraging could be more active, possibly related to higher manoeuvrability and lower energy requirement for the take-off. They appear to spend less time in a particular area and so relatively more time in commuting flights, probably being able to optimise commuting and foraging time better than albatrosses can.
Chick rearing period:
Several Procellariiformes use a two-fold foraging strategy as do WCPs, individuals foraging either nearby the colony or at long range distance (Weimerskirch et al.1994). These two groups of trips are clearly separated when trip duration and maximum range are plotted
(Fig. 5), with no species concentrating feeding activity at intermediate values. This correlation is more significant for the chick rearing period than for the incubation period (respectively P<0.01 and P= 0.05), which could be related to the higher energetic constraints during chick rearing when adults have to provision their chick frequently.
The Short-tailed Shearwater is able to fly up to 1124 km in 22.32 hours (Nicholls et al, 1998). In a similar situation, one of our WCPs was recorded flying 1227 km per day, returning to the colony. This spread flight is the longest in a day of any seabird, giving an average speed of 51 km h¹. It indicates that medium-sized seabirds from the genus Procellaria and Puffinus have the capacity to fly faster over long distances than larger seabird species such as albatrosses, unlike previous predictions (Pennycuick et al. 1984 ; Pennycuick 1989).
Conservation implications
WCPs are one of the commonest seabirds following ships in the Southern Ocean (Bierman & Voos 1950). They scavenge discards or fisheries offal, but are also attracted by baits from longliners. WCPs are bold competitors for food, their ability to swallow large items of food and their tendency to scavenge behind fishing vessels could be interpreted as a previous adaptation to exploit the feeding activity of sea mammals (Summerhayes et al.1974). This, and their good diving capacity that allows them to take baits from submerged lines, make them the principal seabird in the by-catch in the Southern Ocean fisheries, especially in sub-Antarctic waters, but noticeably also in the Benguela area (Cherel et al. 1996 ; Barnes et al. 1997). WCP’s scavenging behaviour may be the result of an overlap between natural previous foraging zones and today’s fishing area, as suggested for albatrosses (Weimerskirch, 1997).
Through the results of this study, it is now possible to show to what extent WCPs are at risk from fisheries' activities during their foraging trips (Weimerskirch et al. in press). We can identify four kinds of fishing fleet on the area covered by WCPs. Firstly, a high level of fishing activity occurs in the southern Benguela region, which is a particularly attractive zone for WCPs throughout the year. In addition to trawling activities, in 1994 the longline fishery for Hake Merluccius capensis started in the shelf waters of South Africa, and was estimated to kill 8000 ± 6400 WCPs annually (Barnes et al, 1997). Secondly the rapid expansion of Patagonian Toothfish Dissostichus eleginoïdes above many sub-Antarctic island shelves is likely to cause high mortality of WCPs. Indeed, during the breeding period high densities of WCPs occur around the breeding grounds, due to central place foraging and long distance movements of birds travelling to and from colonies, which increases the chances of encounters between fishing vessels and birds. During the chick rearing period most of the foraging trips are short and take place within the inshore waters up to the shelf break, and overlap considerably with fishing zones. With a limited number of birds tracked, we can show already that Crozet WCPs are in contact during the breeding season with the fisheries operating around Prince Edward's, Crozet, Kerguelen, the Ob and Lena shelves as well as other smaller banks. Because many fleets are not under legal control around South Indian Ocean islands, by-catch mortality is probably very high, with tentative figures of 4000 to 8000 WCPs killed per year in the Prince Edward's and Crozet Islands fishing area alone (CCAMLR 1997). The two last major zones of fishing activities occur in sub-tropical waters, where WCPs forage mainly during the incubation period. The Japanese longline fishery for Southern Bluefin Tuna Thunnus maccoyi off South Africa and other Tuna fisheries for tropical Tuna in the Indian Ocean are known to cause high mortality of albatrosses (Brothers 1991), but little data are available for these fisheries in oceanic waters outside of Economic Exclusive Zones.
WCPs have only recently become a species for conservation concern, with major concerns being raised for albatross species whose populations have been closely monitored. No long-term population data are available for WCP, but several fisheries pose a threat to its populations throughout the year in the Indian Ocean. The multinational nature of the conservation management of this species, and the contribution of clandestine fishing activities make it difficult to develop a conservation programme to preserve a seabird population over such a wide area.
ACKNOWLEDGEMENTS
The studies were funded by IFRTP, programme n°109 directed by P. Jouventin. We thank the members of the 33th and 34th mission on Crozet Islands involved in the studies in the field, and particularly D. Aures and P. Lys for field assistance. We are also grateful to S. M. Waugh for helpful comments and improving the English.
REFERENCES
Anderson, D.J. 1997. Foraging range of Waved Albatross in the Eastern Tropical Pacific. In: Robertson, G. & Gales, R. (eds) The albatross biology and conservation. Chipping Norton; Surrey Beatty & Sons.
Ashmole, N.P. 1971. Seabird ecology and the marine environment. In: Farner, D.S., King, J.R. & Parkes, K.C. (eds) Avian Biology. Vol. 1: 223-286. New York; Academy Press.
Barnes, K.N., Ryan, P.G. & Boix-Hinzen, C. 1997. The impact of the Hake Merluccius ssp. longline fishery off South Africa on Procellariiform seabirds. Biol. Conserv. 82 : 227-234.
Bartle, J.A. 1990. Sexual segregation of foraging zones in Procellariiform birds: implications of accidental capture on commercial fishery longlines of Grey Petrels (Procellaria cinerea). Notornis 37: 146-149.
Bierman, W.H. & Voos, K.H. 1950. Birds observed and collected during the whaling expeditions of the "Willem Barentsz" in the Antarctic 1946-1947 and 1947-1948. Ardea 37 : 1-123.
Brothers, N. 1991. Albatross mortality and associated bait loss in the Japanese longline fishery in the Southern Ocean. Biol. Conserv. 55 :255-268.
Brothers, N., Gales, R. Hedd, A. & Robertson, G. 1997. Foraging movements of the Shy Albatross Diomedea cauta breeding in Australia; implications for interactions with longline fisheries. Ibis. 140: 95-106.
CCAMLR. 1997. Report of the Fifteenth Meeting of the Commission. CCAMLR, Hobart. CCAMLR: Annex 5: 85-114.
Cherel, Y., Weimerskirch, H. & Duhamel, G. 1996. Interactions between longline vessels and seabirds in Kerguelen waters and a method to reduce mortality. Biol. Conserv. 75 : 63-70.
Croxall, J.P., Hall, A.J., Hill, H.J., North, A.W. & Rodhouse, P.G. 1995. The food and feeding ecology of the White-chinned Petrel Procellaria aequinoctialis at South Georgia. J. Zool., Lond. 237 : 133-150.
Freeman, A.N.D., Nicholls, D.G., Wilson, K-J. & Bartle, J.A. In press. The use of satellite tracking and radio tracking methods with Westland Petrels (Procellaria westlandica). Mar. Ornithol.
Hall, A.J. 1987. The breeding biology of the White-Chinned Petrel Procellaria aequinoctialis at South Georgia. J. Zool. Lond. 212: 605-617.
Holton, A.A. 1969. Feeding behaviour of a vertically migrating Lanternfish. Pac. Sci. 23 :325-331.
Jackson, S. 1988. Diets of the White-Chinned Petrel and Sooty Shearwater in the Southern Benguela region, South Africa. The Condor 90 : 20-28.
Jouventin, P. & Weimerskirch, H. 1990. Satellite tracking of Wandering Albatrosses. Nature 343: 746-748.
Kozlov, A.N. 1995. A review of the trophic role of mesopelagic fish of the family Myctophidae in the Southern Indian Ocean. CCAMLR Science. 2 :71-78.
Murphy, R.C. 1936. Oceanic birds of South America. New York; Mac Millan.
Nicholls, D.G., Murray, M.D., & Robertson, C.J.R. 1994. Oceanic flights of the Northern Royal Albatross. Corella. 18(2): 50-52.
Nicholls, D.G., Stampton, P., Klomp, N.I. & Schultz M. 1998. Post-breeding flight to Antarctic waters by a Short-Tailed Shearwater Puffinus tenuirostris. Emu. 98: 79-82.
Pennycuick, C.J. 1982. The flight of Petrels and Albatrosses (Procellariiformes), observed in South Georgia and its vicinity. Phil. Trans. R. Soc. Lond. 300 : 75-106.
Pennycuick, C.J. 1989. Bird flight performance : A practical calculation manual. Oxford; Oxford University Press: 153pp.
Pennycuick, C.J., Croxall, J.P. & Prince, P.A. 1984. Scaling of foraging radius and growth rate in Petrels and Albatrosses (Procellariiformes). Ornis Scand. 15: 145-154.
Rand, R.W. 1962. Seabirds south of Madagascar. Ostrich 33: 48-51.
Rand, R.W. 1963. Seabirds in the southern Indian Ocean. Ostrich 34: 121-128.
Ridoux, V. 1994. The diets and dietary segregation of seabirds at the sub-Antarctic Crozet Islands. Mar.Ornithol. 22: 76-82.
Sagar, P.M. & Weimerskirch, H. 1996. Satellite tracking of Southern Buller’s Albatrosses from the Snares, New Zealand. The Condor. 98: 649-652.
Stahl, J.C., Bartle, J.A., Jouventin, P., Roux, J.P. & Weimerskirch, H. in press. Atlas of seabird distribution in the south-west Indian Ocean. Mar. Ornithol.
Summerhayes,C.P., Hofmeyr, P.K. & Rioux, H. 1974. Seabirds off the southern coast of South Africa. Ostrich 45 :88-109.
Weimerskirch, H. 1997. Foraging strategies of Indian Ocean Albatrosses and their relationships with fisheries. In: Robertson, G. & Gales, R. (eds) The Albatross biology and conservation. Chipping Norton; Surrey Beatty & Sons: 168-179.
Weimerskirch, H., & Jouventin, P . 1987. Population dynamics of the Wandering Albatross of the Crozet Islands : causes and consequences of the population decline. Oikos 64 : 464-473.
Weimerskirch H. & Robertson, G. 1994. Satellite tracking of Light-Mantled Sooty Albatross. Polar. Biol. 14: 123-126.
Weimerskirch, H., Jouventin, P., Mougin, J.L., Stalh, J.C. & Van Beveren, M. 1984. Banding recoveries and the dispersal of seabirds breeding in French Austral and Antarctic territories. The Emu 85 : 22-32.
Weimerskirch, H., Salamolard, M. Sarrazin, F. & Jouventin, P. 1993. Foraging strategy of Wandering Albatrosses through the breeding season: a study using satellite telemetry. The Auk 110: 325-342.
Weimerskirch, H., Chastel, O., Ackermann, L., Chaurand, T., Cuenot-Chaillet, F., Hindermeyer, X. & Judas, J . 1994. Alternate long and short foraging trips in pelagic seabirds parents. Anim. Behav. 47 : 472-476.
Weimerskirch, H., Catard, A., Prince, P.A., Chérel, Y. & Croxall, J.P. In press. Foraging White-chinned Petrels Procellaria aequinoctialis at risk : from the tropics to Antarctica. Biol. Conserv.
Woehler, E.J., Hodges, C.L. & Watts D.J. 1990. ANARE research notes : An atlas of the pelagic distribution and abundance of seabirds in the southern Indian Ocean, 1981 to 1990. Antarctic Division.
Table 1. Information and comparison for 24 foraging trips of 16 White-chinned Petrels.

Table 2. Comparison of foraging trip characteristics between White-chinned Petrels and several species of albatross during the incubation period.
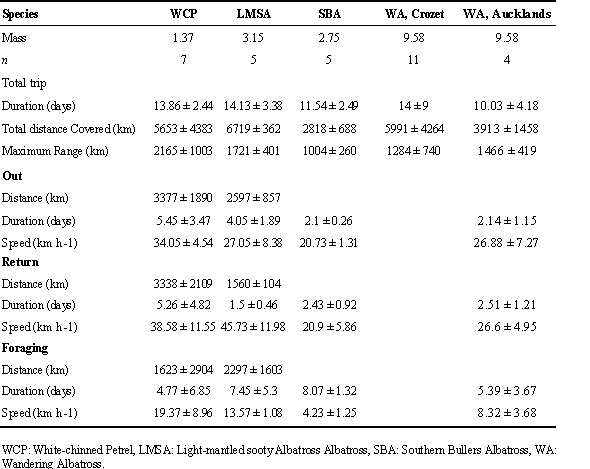
Fig. 1. Map of the south-western Indian Ocean showing the track of 3 incubating WCPs. Dotted lines indicate - 1500 m depth contours.
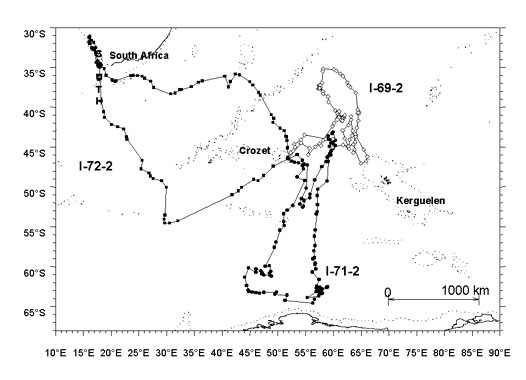
Fig. 2. Map of the south-western Indian Ocean showing the track of 3 incubating WCPs. Dotted lines indicate - 1500 m depth contours.
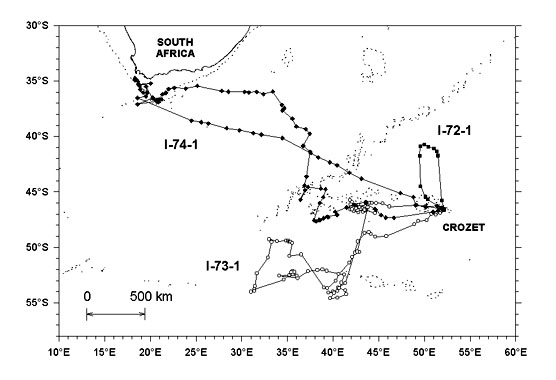
Fig. 3. Relationships between time spent at sea and maximum foraging range as well as distance covered during incubation (top) and chick rearing periods (bottom). For maximum range and duration of trips, Y= 147 X -13.2, r2= 0.54, P< 0.05 and Y= 201 X - 3.99, r2= 0.87, P< 0.01 for incubation and chick rearing respectively. For distance covered and duration of trips, Y= 624.2 X - 835.17, r2= 0.85, P< 0.02 and Y= 642 X - 322, r2= 0.94, P< 0.01 for incubation and chick rearing respectively.
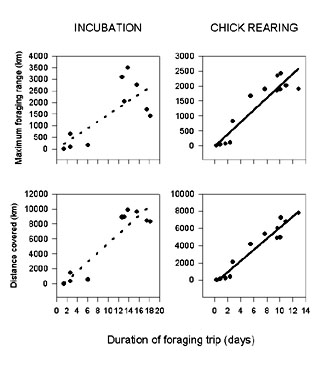
Fig. 4. Comparison between Wandering Albatross and WCP for the percentage of flight tracks to windward and leeward for outward journey (top) and return to the nest (bottom).
Fig. 5. Relationships between time spent at sea and maximum foraging range for several species of procellariiformes. (Y= 131.3 X -145.76, r2= 0.6, P= 0.05 and Y= 175.5 X - 201.7, r2= 0.9, P< 0.01 for incubation (top) and chick rearing (bottom) respectively). Species are : WCP : White-chinned petrel, LMSA : Light-mantled Sooty Albatross, WAc : Wandering Albatross from Crozet Island, WAa : Wandering Albatross from Auckland Island, SBA : Southern Buller’s Albatross, SOA : Sooty Albatross, AA : Amsterdam Albatross Diomedea amsterdamensis, SHA : Shy Albatross, BBAc : Black-browed Albatross from Campbell Island Diomedea melanophris, BBAk : Black-browed Albatross Albatross from Kerguelen Islands, GHA : Grey-headed Albatross Diomedea chrysostoma, YNA : Yellow-nosed Albatross Diomedea chlororhynchos. White circles are for short trips in species using short and long trips.