S48.5: Population Regulation and Social Behaviour in the Non-breeding Season
H. Ronald Pulliam & Juliet R. C. Pulliam
Institute of Ecology, University of Georgia, Athens, Ga., 30602 U.S.A.
Pulliam, H.R. & Pulliam, J.R.C. 1999. Population regulation and social behaviour in the non-breeding season. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2968-2982. Johannesburg: BirdLife South Africa.Although there have been numerous studies in both the breeding and non-breeding seasons of the factors that limit avian population size, most conceptual models of population regulation in birds have emphasised density-dependent factors during the breeding season alone. Starvation, predation, and disease also take their toll during the non-breeding season and density dependence on the wintering grounds may be as important to population regulation as density dependence on the breeding grounds. We develop a simple model of how factors on the wintering grounds might contribute to population regulation for birds that occupy heterogeneous landscapes where food supply and predation risk may differ among habitats. The assumptions of the model are mostly based on observations and studies of wintering sparrows and cardueline finches in the southern USA; however, the model may apply to other situations where predation risk is appreciable and food supply is fixed prior to the onset of winter. The model may help to clarify the conditions under which non-breeding territoriality evolves and how density-dependent mortality in the non-reproductive season and density-dependent reproductive success in the breeding season interact to determine overall population dynamics. The model may also help explain the tendency for some populations to exhibit periodic irruptions in population size. Finally, the model may be useful in understanding how the loss of habitat on the wintering grounds may contribute to population decline and increased probability of extinction in some species.
INTRODUCTION
Many bird species, especially those in temperate regions, exhibit marked seasonal variation in their social behaviours. For example, many species that defend territories during the breeding season are very social and form large feeding flocks with relatively little aggression during the non-breeding season. In other species, an individual may defend the same territory year around, or, in some migratory species, an individual may defend a breeding territory in one location and another territory in a different location during the winter. Although we have some understanding of when it pays to defend a breeding territory (Fretwell & Lucas 1970; Lack 1954) and we know something of the role breeding territoriality plays in population regulation (Arcese et al. 1992; Brown 1969; Ferrer & Donazar 1996; Holmes et al. 1996; Kluyver & Tinbergen 1953; Newton 1992; Rodenhouse et al. 1997), we know very little about the individual or population consequences of variation in social behaviour during the non-breeding season.
The sparrows (subfamily Emberizinae of the Emberizidae) and the cardueline finches (subfamily Carduelinae of the family Fringillidae) comprise two large groups of seed-eating birds with a wide variety of social behaviours and migratory habits. In temperate North America, most of the sparrows are insectivorous during the breeding season but most are granivorous during the winter months. Some cardueline finches are granivorous year round and others, similar to sparrows, are mostly insectivorous in the summer and granivorous during the winter. The northern temperate sparrows and finches are similar in that for most species, the winter diet consists of seeds that are produced the preceding summer or fall and the food supply typically declines from fall through winter until new seeds or insects are produced the following spring or summer. This declining winter food supply may be responsible for the fact that some sparrows and finches defend winter territories. Other species, however, are very social in the winter and, in some cases, form large roving flocks that move about during winter in search of food. Population sizes of both sparrows and finches fluctuate substantially from year to year and Bock & Lepthien (1976) have demonstrated a highly synchronous pattern of oscillating population sizes and irruptive southward migrations for a variety of cardueline species.
In this paper, we explore several questions related to the evolution and consequences various types of social behaviour in the non-breeding season. In particular, we develop a simple model based on a knowledge of sparrow and finch feeding and social behaviours in the non-breeding season to address the following interrelated questions:
1. Under what circumstances does non-breeding territoriality evolve?
2. Does social behaviour during the non-breeding season contribute to population oscillations and the tendency for periodic outbreaks in population size exhibited by some species?
3. How do density-dependent mortality in the non-reproductive season and density-dependent reproductive success in the breeding season interact to determine overall population dynamics?
4. How does habitat loss on the wintering grounds influence population dynamics and the probability of extinction?
The model relates the probability of over-winter survival to differences in social behaviour during the non-breeding season. The model is not meant to be an exact description of the behaviour or population dynamics of any one particular species but is designed to incorporate some important features of the winter behaviour and ecology of a large variety of species. In particular, the model is meant to capture some of the observations discussed below. The model by itself does not answer any of the questions presented above, but it does help focus the discussion and leads to a number of testable hypotheses which are presented later in the paper.
THE OBSERVATIONS
Field Sparrows Spizella pusilla in North Carolina.
Pulliam & Enders (1971) censused sparrows in old field habitats in North Carolina in a patchwork landscape of abandoned agricultural fields in different stages of old field succession from mid-November 1968 through late March 1969. For the first several weeks, Field Sparrows were found only in recently abandoned fields dominated by horseweed Leptilion sp. but by late December the Field Sparrows had abandoned the horseweed fields and were feeding only in somewhat older fields dominated by Aster sp. The Field Sparrows then fed exclusively in the Aster fields until mid January when they abandoned the Aster fields in favour of feeding in young pine stands. By early February, the Field Sparrows left the young pine stands and were found for the rest of the winter only in broomsedge Andropogon virginicus and other fields. This pattern of sequential use of habitats is not always found, and even in the same winter, Pulliam & Enders observed Field Sparrows only a few miles away from the first site that fed in Crabgrass fields Digitaria sp. through out the winter.
In contrast to the shifting habitat use described above for Field Sparrows, several other sparrow species studied by Pulliam & Enders showed little change in habitat use over the season. Song Sparrows Melospiza melodia and White-throated Sparrows Zonotrichia albicollis, for example, were found in a variety of habitats but were found in approximately the same numbers in each habitat type throughout the winter. Song Sparrows were mostly found alone or in pairs throughout the winter and appeared to be defending winter territories. One possible interpretation of these observations is that some species, like Song Sparrows, defend stable winter territories and consequently the population is distributed across a variety of habitat types with the more dominant individuals defending higher quality sites and the less dominant individuals occupying more marginal habitat. In other, more social species like the Field Sparrow, all individuals may crowd into the best available habitat at the beginning of winter and remain there as long as the food supply is sufficient. In some cases, this may result in staying in a single habitat for the entire winter and in other cases it may result in sequentially shifting to lower and lower quality habitats as the resources are sequentially depleted in each habitat occupied.
Chipping Sparrows Spizella passerina in Arizona.
At The Research Ranch in southeastern Arizona, grass seeds are produced in the fall following summer rains and seed production varies substantially from year to year (Pulliam & Parker 1979). In years of low to moderate seed production (less than 5 to 10 kg ha-1), sparrows consume upwards of 80 to 90% of all of the grass seeds produced and the over-wintering densities of sparrows in oak woodland habitat appears to be limited by the available food supply. For example, in the winter of 1973-74, grass seed production in oak woodland habitat at The Research Ranch was 1.08 kg ha-1 and the density of all sparrow species was 0.88 sparrows ha-1 (including 0.78 Chipping Sparrows ha-1). Pulliam & Parker estimated that an individual Chipping Sparrow consumes approximately 1.0 kg of grass seed for the September through March 'over-wintering' season. Thus, the sparrows in oak woodland at The Research Ranch in the winter of 1973-74 can be estimated to have consumed on the order of or 80% or more of the seeds available.
Years of higher summer rainfall at The Research Ranch are followed by higher seed production and higher over-wintering numbers of sparrows, especially in the oak woodland habitat favoured by Chipping Sparrows. For example, the summer rainfall in 1972-73 was higher than usual in southeastern Arizona and grass seed production was relatively high (4.63 kg ha-1), as was the sparrow density in the oak woodland (6.97 sparrows ha-1). These numbers suggest that there was not sufficient seed supply to last the entire winter based on an average seed consumption of 1 kg per bird for the entire winter. For most of this winter, sparrow numbers remained high in the oak woodland, but in late February the Chipping Sparrows suddenly abandoned the oak woodlands and began feeding in large flocks in open grasslands far from any tree or shrub cover.
Hawks are relatively abundant most winters at The Research Ranch, however, in most years, there is little evidence of successful predation on sparrows. The late winter of 1972-73 was an exception, as was one other winter when the Chipping Sparrow similarly abandoned the oak woodland in late winter. In the open grasslands, the sparrows formed unusually large flocks and these flocks exhibited a characteristic 'rolling' movement when birds at the back of the flock would fly over their flock mates to find a position at the front of the flock. In both years, numerous observations were made of hawks, especially American Kestrels ( Falco sparverius) chasing flocks of sparrows in the open grassland. The hawks would often fly low over the feeding flocks and when some of the sparrows would flush the hawks would pursue them. Often the flocks were several hundred meters from the nearest tree or shrub cover and on several occasion hawks were seen to successfully catch the sparrows on their way to the nearest trees.
Facultative migration in Chipping Sparrows.
Food supply varies substantially from year to year at The Research Ranch and fewer birds are found there in years of low seed production. Pulliam & Parker (1979) demonstrated that, on average, in years of low seed production, Chipping Sparrows migrated farther south than in years of high seed production. During the winter months, Chipping Sparrows are found from Central Arizona south through the highlands of northern Mexico. Pulliam & Parker surveyed sparrows in oak woodland and other habitats in a series of sites along a geographical gradient ranging from southern Arizona to southern Chihuahua. All of the sites where surveyed both in the winter of 1973-74 following an unusually dry summer in southern Arizona and in the winter of 1974-75 following an exceptionally wet summer. They found that migratory sparrows were almost 10 times as common in riparian and oak woodlands in Arizona in 1974-75 (following the wet summer) than they had been in 1973-74 (following the dry summer). The pattern in southern Chihuahua was almost exactly the reverse; there were approximately 5 times as many Chipping Sparrows and other migratory sparrows at the southern Chihuahuan site after the dry summer in Arizona than there were following the wet summer.
Facultative Territoriality in Yellow-eyed Junco Junco phaeontus.
During the winter months, Yellow-eyed Juncos in the Santa Catalina mountains near Tucson, Arizona, exhibit a highly variable social system (Moore 1972, Caraco 1979, Caraco & Pulliam 1978, Caraco et al. 1980a&b, Pulliam et al. 1974). On warm winter days, dominant individuals defend territories in areas near creek beds where seed density is highest. In most cases these winter territories are on the same sites as the summer breeding territories of the same individuals. On cold winter days, territories break down and large flocks congregate on the best feeding sites. Typically, when this happens, the former territory holder joins the flock and is the dominant individual in the flock. Consequently, the largest flocks form on the coldest days, smaller flocks are found on somewhat warmer days, and on particular warm winter days no flocks are formed at all and most of the birds are found defending territories either alone or in mated pairs.
Pulliam et al. (1974) and Caraco (1979) hypothesised that this facultative territorial defence was the result of dominant individuals having to spend so much time feeding on cold days (just to meet their energy requirements) that they had no time or energy available for territorial defence. Pulliam et al. attempted to test this hypothesis in the laboratory by keeping small flocks of birds in temperature-controlled chambers and recording feeding rates and aggressive encounters. In these experiments the rate of aggression was almost twice as high at 17-24oC than at 6-9oC. They also looked at temperature-dependent aggression in the field by placing two small seed dishes 35 cm apart and scoring the percent of the time there was an aggressive encounter when both dishes where simultaneously occupied. They found a strong relationship between temperature and aggression with aggressive conflict occurring only about 15% of the time when the temperature was below 0oC but occurring in excess of 80% of the time when the temperature exceeded 10oC. Caraco (1979) further tested the idea that time constraints prevented the juncos from defending territories on cold days by seed addition experiments in the field. He added large quantities of seed to some territories and found that, as expected, dominant individuals spent less time feeding and more time displacing intruders. He found that mean flock size declined for all temperatures and that the dominant birds were able to completely exclude all intruders at a cooler temperature when more food was available.
THE MODEL
The above observations suggest a continuum in the relationship between social behaviour and the use of space during the non-breeding season. Some species appear to defend winter territories where all or most intruders are excluded with the result that food disappears at a slower rate during the winter and the birds are able to remain resident throughout the winter. Other species defend food on a facultative basis, as permitted by the constraints on the time and energy available for defence. Presumably this results in a slower rate of food depletion and a greater likelihood that the territory will remain suitable all winter. Yet other species show little or no defence of winter food supplies and in these species individuals appear to crowd into whatever habitat is the best at the time and remain there until the food supply is so depleted that it pays to move into another habitat. In these species, depending on the level of food production and the initial bird population density, the initial habitat may be suitable all winter or for most of the winter, or the habitat may be suitable only for a few weeks. In the latter case, the birds may move to several distinct habitats over the course of the winter.
The consequences of variation in winter social behaviour are not clear. To clarify how social behaviour affects both individual survival probability and long-term population dynamics, we develop a simple model consistent with many of the observations discussed above. The purpose is not to model every detail of feeding and social behaviour but to identify a few important features that appear to have substantial consequences at both the individual and population levels.
Consider a landscape where there is a small amount of good habitat but abundant poor quality habitat. We assume that the predation rate is lower and therefore daily survival rate is higher in the good habitat than in the poor habitat. In the case of no winter territoriality, at the beginning of the non-breeding season all of the birds crowd into the good habitat where each individual consumes k kg of food per day. If there are G ha of good habitat and g kg of usable seeds per ha, how long will it be until the birds have consumed all of the available food and are forced to move into poor habitat?
If there were no winter mortality, the answer would be simple. The total amount of food available in the good habitat would be g kg ha-1 times G ha, and since each bird consumes k kg day, the food would last g G divided by k days. However, if in the good habitat, the mortality rate is s deaths per individual per day, then there will be
Nt = Nw exp(-st) (Equation 1)
birds left after t days if there were Nw birds at the start of winter. The amount of food consumed in this period of t days is found by integrating the product Nt, the number of birds at time t, times k, the amount of food consumed per bird per day, over the time interval of time, t, giving the result that k No (1 - e-st)/s kilograms of food are consumed in t days. Since this is the amount of food consumed by the bird population in any arbitrary time interval of t days, we can find how many days, T, the available food supply will last by setting k No (1 - e-st)/s equal to g G and solving for T. This gives the number of days that the food available in the good habitat will last as
T = -1/s ln( 1 - gGs/kNo). (Equation 2)
The utility of this equation can be illustrated with a simple example. Since many passerine species, including sparrows, have winter mortalities in the range of 30-70 %, let's assume that daily winter mortality in the good habitat is 0.002 deaths per individual per day (equivalent to about 33% mortality in a 200 day winter). In a typical year, grass seed production in the oak savanna habitat in southeastern Arizona is about 1 kg ha-1 and Chipping Sparrows consume about 0.01 kg per individual per day or about 1 kg each every 100 days (Pulliam & Parker 1979). If there are 100 ha of good habitat (oak savanna), how long does the food supply in the good habitat last and how many birds are remaining when the food in the good habitat is exhausted and the birds are forced to move into poor habitat? Obviously, if the number of birds at the start of winter is less than the number required to consume all of the food in the good habitat, they never need to abandon the good habitat. For example, if 50 birds are present at the beginning of winter, from equation 2 we see that the food supply in the good habitat will last for more than 200 days. Furthermore, setting t = 200 days in equation 1, we calculate that at the end of winter there are approximately 67 remaining birds.
What will the winter mortality be if more birds are present at the beginning of winter than can be accommodated in the good habitat? Under the assumption of no territorial defence, we assume that the birds will all feed in the good habitat until the food there is exhausted at which time they will move into the poor habitat where the mortality rate is higher. If, for example, 100 birds settle into the 100 ha of good habitat at the beginning of winter, using equation 2, we calculate that the food in the good habitat will last for 111.6 days and at that time there will be 80 birds left. If the winter lasts 200 days and the birds run out of food in the good habitat on day 111.6, then they must spend the remaining 88.4 days in the poor habitat where mortality rate is higher. Assuming a daily mortality of 0.004 in the poor habitat (twice the rate in good habitat), using equation 1 again, we calculate that if there are 80 birds on day 111.6, there will be only 56 birds left on day 200. In other words, in this example only 56 (or about 56%) of the 100 initial birds survive until the end of winter.
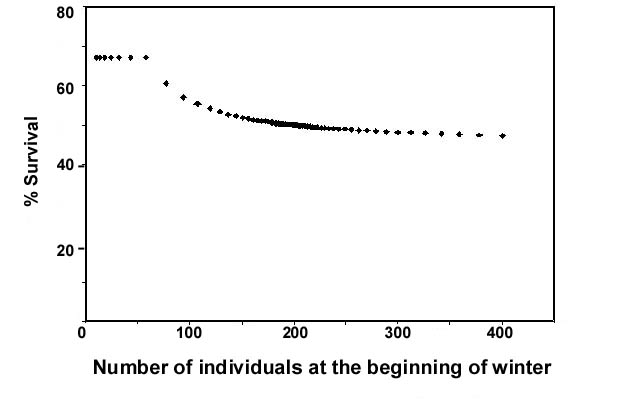
Figure 1 shows the probability of surviving winter as a function of the initial density (birds per 100 ha) in the good habitat at the start of winter if food production is 1.0 kg ha-1, food consumption is 0.01 kg per individual per day, and the mortality rate is 0.002 deaths per individual per day in the good habitat and 0.004 in the poor habitat. Using these parameters, so long as fewer than 60 birds arrive per 100 ha of good habitat, approximately 67% survive and winter survival is independent of population density. However, above 60 birds per 100 ha, the winter survival declines so that if 400 birds arrive at the beginning of winter only 189 birds (47.3 %) will be alive at the end of winter.
Figure 2A shows the population consequence of the assumptions made so far concerning winter survival probability if we make the additional assumption that for every bird alive at the end of winter there will be 2 birds alive at the beginning of the next winter. (This assumes that reproductive success (b) is density-independent and later in this paper we consider the consequence of relaxing this assumption.) Each panel in Figure 2 was drawn by starting with 10 birds at the beginning of the first winter and then calculating over-winter survival as explained above and multiplying by 2 to get the number of birds at the beginning of the second winter. This procedure was repeated over and over to get the population size in future years. Each panel of Figure 2 shows two curves, one for population size at the beginning of winter (Nw) and one for population size after the winter at the beginning of the breeding season (NB). Notice that in Figure 2A, the population growth curve is very similar to that of the familiar logistic growth equation.
The above calculations assume that there is no territorial defence of winter resources; consequently all birds crowd into the best habitat until resources there are exhausted at which time they move into the next best habitat. At the other end of the social behaviour spectrum, we can assume that dominant individuals defend winter territories in the good habitat from which they exclude all intruders from the start of winter. In the simplest case, we assume that all individuals who fail to secure a territory in the good habitat are forced to move into the poor habitat from the start of winter and that resources in the poor habitat are not defensible so no territories are established there. (In this simple version of the model, we make no distinction between juveniles who are in their first winter and adults who have survived one or more previous winters. The model, however, does not change if we make the more reasonable assumption that the adult birds have a higher chance of securing a territory in good habitat and, therefore, have a higher probability of survival than do juvenile birds.)
Presumably there is a cost to territorial defence and this cost can be expressed as additional mortality associated with the time and energy spent defending the winter territory. If the daily mortality rate in the good habitat with no territoriality is sG and the daily rate of mortality in the poor habitat is sP, then the daily mortality for a territorial bird (st ) in the good habitat must be somewhere between sG and sP. This is because there is some cost to territorial defence and if this cost results in a daily mortality greater than sP there would be no benefit to territoriality.
To see how non-breeding territoriality influences over-winter survival probability, assume a territory size of 2 ha and a mortality rate of territory holders of 0.0025 deaths per individual per day (slightly higher than sG to account for the cost of territorial defence). Using these values and keeping all other parameters as before, we use equation 1 to calculate that 60.7% of territory holders survive winter and 44.9% of non-territory holders survive. Thus, if 100 birds are present at the start of winter and 50 of these set up territories on the 100 ha of good habitat and the other 50 do not secure territories, there will be approximately 53 birds still alive at the end of winter. Similarly, if 400 birds are present at the beginning of winter, there will be about 30 territory holders at the end of winter and about 157 non-territorial birds at the end of winter for a total population of about 187. These calculations make the simplifying assumption that territory holders that die during the winter are not replaced from the pool of non-territorial individuals. Changing this assumption results in more birds surviving winter but does not change any of the qualitative conclusions discussed below.
Figure 2B shows population size over time for a territorial population assuming, as before, that for every birds alive at the end of the winter there will be two birds alive at the beginning of the next winter. Comparing Figure 2A and Figure 2B, one sees relatively little difference between population dynamics for a territorial population and a non-territorial population. This is decidedly not the case if we make winter mortality in the poor habitat higher but leave all other parameters as before. In this case, over-winter survival of a social population declines precipitously with increasing initial population at the start of winter if the survival in the poor habitat (sP = 0.0014 in the example) is substantially larger than that in the good habitat. The population consequence of this change is illustrated in Figure 2C where it is seen that population size does not reach an asymptote as for logistic growth but rather oscillates between high and low population sizes.
The reason for the population oscillation seen in Figure 2C, is that the social birds crowd into the better habitat at the beginning of winter and if the population size is large they quickly exhaust the food there and are forced into the poor habitat where survival is very low. Consequently, there is a relatively small population at the start of the next winter and the birds are able to spend the entire winter in the good habitat where survival is high resulting in a large initial population the following winter. This cycle is repeated over and over with the population oscillating between a high and a low population. Under these conditions of a very poor alternative habitat, winter territoriality makes a big difference as shown in Figure 2D. Regardless of the initial population size, about the same number of birds survive winter in the good habitat where territories are defended but virtually no birds survive in the poor habitat. The result is a stabilisation of over-winter survival and a stable population size with no oscillations for the territorial population.
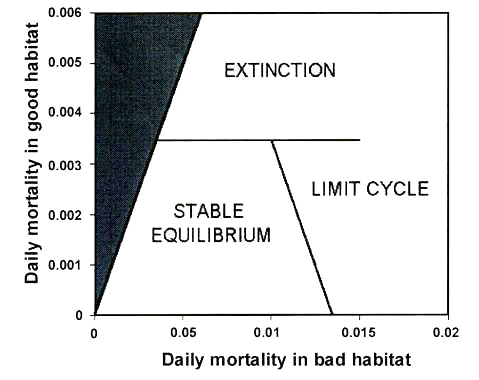
The results for a non-territorial population are summarised in Figure 3. This figure shows the combination of values of daily mortality in the good habitat (sG) and the poor habitat (sP) for which limit cycles do and do not occur. Limit cycle is a general term and can apply to two point oscillations between high and low populations or to more complex cycles with many population sizes. Notice that limit cycles are more likely to occur when mortality rates, particularly the mortality in the poor habitat, are very high. Also, notice that if daily mortality in the good habitat is sufficiently high, the population always goes extinct. Figure 3 is drawn for the conditions of 1 kg ha-1 seed and 0.01 kg seed consumed per bird per day, so that a single bird consumes 2 kg of seed over the entire winter and every hectare supports 100 bird days of seed consumption. Obviously, if the seed production were higher or the feeding rate lower, the value of sG for which the population goes extinct, or for that matter the precise location of the boundary between the region of a single equilibrium point and limit cycles, would be different from that shown in Figure 3; however, the qualitative results would be similar as would the general shapes of the regions shown in Figure 3.
CONCLUSIONS AND TESTABLE PREDICTIONS
There are a number of simplifying assumptions in the model developed above that limit its generality. For example, the model assumes that as long as there is sufficient food to meet daily requirements, individual birds choose habitats on the basis of minimising predation risk. Habitat use by birds in the non-breeding season is influenced by many factors, including food availability and predation risk. For example, Schluter (1982) found that the abundances of ground finches in different habitats in the Galapagos Islands are roughly proportional to the abundance of food in those habitats. Similarly, Pulliam & Parker (1979) found strong correlations between food abundance and sparrow abundance in oak woodland habitats in southeastern Arizona; however, a number of investigators including Pulliam & Parker (1979), Repasky & Schluter (1994 and 1996), and Wiens & Rotenberry (1981) have found little or no correlation between sparrow abundance and food. A possible common theme in these observations is that the correlation between food supply and bird abundance is greater in habitats where predation risk is relatively low, such as islands and woodlands, and poorer in habitats, such as open grasslands and shrublands, where predation risk is high.
The model developed in this paper above incorporates both differences in predation risk and food supply associated with different habitats. The basic assumption of the model is that sparrows choose to feed where their risk of predation is lowest as long as they can find sufficient food. As a result all mortality, in the model, is due to predation and none is due to starvation, because birds are assumed to move to another habitat, where predation risk is higher, before they run out of food. In the woodlands and grasslands of southeastern Arizona, food appears to be limiting in the woodlands but is always plentiful in the open grasslands where predation risk is higher; however, this situation may not be general, and, in many landscapes, there may be no alternative habitats with plentiful food available. Furthermore, although food may not be limiting most of the time, starvation may be significant during occasional extreme weather events (Grant, 1986; Smith et al.1996). An interesting variation on the model developed here would be to have limited food in all habitats and thus forcing the birds to choose simultaneously between predation risk and starvation risk. A useful starting point for such a modelling exercise would be Gilliam's (1982) rule of minimising the ratio of predation risk to feeding rate (Werner & Gilliam 1984).
Another assumption of the model that limits its generality is that the birds utilise a declining food supply throughout the winter with no new food is produced during the winter. This assumption holds in the arid grasslands of southern Arizona where most seeds eaten by sparrows are produced in the late summer and early fall following the summer rains. There is a second peak of seed production in these grasslands following the winter rains; however, these seeds are not available until early spring when most of the winter migrants have left (Pulliam & Brand 1975). The logic of the model developed here could be adapted to a situation where appreciable food is produced during the non-breeding season, but the analysis would be more complicated and the birds might be expected to move back and forth between habitats as relative habitat suitability changed through the season.
Under what circumstances does non-breeding territoriality evolve?
We assume that territorial behaviour in the non-breeding season should evolve so long as the survival cost of defending a territory is less than the survival benefits gained from having a longer-lasting food supply. The lack of overt signs of territorial behaviour in winter suggests that this condition is not met for some species of sparrows and cardueline finches. We suggest several reasons why this should be so. First, winter food resources may be very difficult to defend. This may be especially true for cardueline finches that feed on seeds in widely scattered trees. The fact that some species of sparrows do defend resources shows, however, that winter food defence is feasible. Why would winter food be easier for some sparrow species to defend than for others? The example of facultative territorial defence in Yellow-eyed Juncos discussed earlier provides one plausible answer to this question. In this species, territories are defended when food levels are high and/or energy demands are low. Several lines of experimental and observational evidence already discussed indicate that juncos are not territorial whenever they have to spend so much time feeding that they do not have sufficient time and energy to defend their food resources against intruders.
The observations on Yellow-eyed Juncos suggest what we call the Territoriality Continuum Hypothesis. The hypothesis is that larger species of sparrows are more likely to defend winter territories because they do not need to spend as much time feeding as do smaller species due to the allometric relationships among body size, energy requirement, average seed size eaten, and food availability. Among the sparrows, social behaviour in the non-reproductive season does often vary in a consistent fashion with body size (Pulliam & Mills 1977). For example, among the common wintering sparrows found in or near woodland habitat at The Research Ranch in Arizona, the Chipping Sparrow (12.3 g) is the smallest species but is found in the largest flocks (typically 15 to 50 individuals). Intermediate-sized species like Savannah Sparrow Passerculus sandwichensis (20.1 g), White-crowned Sparrow Zonotrichia leucophrys (29.4 g) and the Vesper Sparrow Pooecetes gramineus (25.7 g) are typically found in small flocks of 5 to 10 individuals. The largest sparrow species at The Research Ranch, the Brown Towhee Pipilo fuscus (44.4 g), is almost always found alone or in pairs during the winter months. A very similar pattern holds in the southeastern United States with Field Sparrows (12.5 g) and Chipping Sparrows being found in large flocks, Dark-eyed Juncos Junco hyemalis (19.6 g) and White-throated Sparrows Zonotrichia albicollis (25.9 g) being in intermediate-sized flocks, and Rufous-sided Towhees Pipilo erythrophalmus (40.0 g) typically being found alone.
Social behaviour in the non-breeding season may vary consistently with body size among various sparrow species because of the relationship between body size and the time required to find sufficient food. Although larger sparrows require more total food, they also eat substantially larger seeds and tend to be found in habitats where food is more plentiful. Furthermore, larger species of sparrows can consume a much greater range of seed sizes than smaller species (Pulliam 1985). Detailed analysis of the energetics of foraging (Pulliam 1980 & 1985 and Benkman & Pulliam 1988) suggest that the larger sparrows do not need to find and consume as many seeds per day and do not need to feed as long per day in order to meet their daily food requirements. If this is so, larger sparrows may have more time to devote to territorial defence and, since they only occur in patches of high seed density, they may have more defensible food supplies. Of course, large body size per se may also make it easier for larger species to defend resources against intruders of other smaller species. This argument does not imply that other factors such as availability of cover do not also influence winter sociality (see Pulliam & Mills 1977 for example), but it does help explain why body size is correlated to non-breeding social behaviour in sparrow species.
Does social behaviour during the non-breeding season contribute to population oscillations and the tendency for periodic outbreaks in population size exhibited by some finch species?
The model developed above suggests that winter territoriality stabilises population fluctuations and therefore that species that are more social during the winter are more likely to exhibit periodic oscillations in population size than are territorial species. It is interesting to note in this regard that most carduelines do not defend winter territories and that they are especially subject to regular population oscillations and outbreaks. The model suggests that oscillations are to be expected in species that do not defend winter territories and that highly periodic oscillations do not require any external forcing functions such as fluctuations in weather or cone crop.
There has been substantial disagreement about both the degree of synchrony and the causes of population irruptions of boreal seed-eating birds. Lack (1954), for example, analyzed data on Paleartic species and concluded that food supply did play a role in periodic irruptions, but that the irruptions did not occur at regular intervals as had been suggested by some earlier workers. Lack also argued that different species 'irrupt independently of each other' and that the same species may irrupt in one part of its range but not in another. Ulfstrand (1963) found only a weak relationship between irruptions and seed cone crop failure, and Newton (1970) argued that irruptions occur when population levels are high and are only slightly modified by the size of the cone crop. Bock & Lepthien (1976), on the other hand, document highly periodic, synchronous irruptions of a number of North American boreal seed-eating cardueline finches including Evening Grosbeak Hesperiphona vespertina, Purple Finch Carpodacus purpureus, Pine Grosbeak Pinicola enucleator, Common Redpoll Acanthis flammea, Pine Siskin Spinus pinus, Red Crossbill Loxia curvirostra, and White-winged Crossbill L. leucoptera. Bock and Lepthien also argue that population oscillations and irruptions are the consequence of synchronised seed crop fluctuations of high-altitude tree species and even suggest alternating years of high and low precipitation are responsible for alternating years of high and low seed crop production and irruptions of boreal seed-eating birds.
Whereas we find Bock and Lepthien's data on regular, almost alternate-year, irruptions of some North American seed-eating finches quite convincing, we find little support for the idea that the pattern is driven by climate fluctuations and highly synchronous seed crops. Seed cone crops do fluctuate but probably not in the highly synchronous, alternate-year manner, suggested by Bock and Lepthien. The model presented above suggests that even in the absence of cone crop failures, seed-eating birds that have little or no defence of winter food supplies will show highly periodic fluctuations and irruptions. These fluctuations may result in alternate year irruptions that are occasionally interrupted by unusually good or unusually bad seed crops. A year of very high rainfall may delay an irruption and a year of unusually low rainfall may result in two irruption years in a row. Spatial variation in rainfall may also result in partial irruptions of the subpopulation in one region but not that in another region and may result in different species irrupting independently of one another. This argument does not rule out that there has been evolution of some degree of synchrony among the tree species with regard to the production of mast seed crops, but it does suggest that this synchrony is the result of the bird irruptions rather than the cause of it.
Ironically, fluctuations in seed crops may actually decrease the likelihood of periodic irruptions rather than cause them. The regular oscillations produced in the model developed above are the result of a constant food supply coupled with high rate of natural population increase on the part of the birds. A constant food supply ensures that when the bird population level reaches a certain threshold there will be a population decline because the food supply is insufficient for all of the birds to remain in good habitat for all of the winter. As already mentioned, an unusually high or low seed crop interrupts the regular oscillation of population size by sometimes allowing for continued population growth following very good seed crops or preventing population growth when food production is very low even if bird population size is also low. This may be why we have detected no evidence of regular oscillations in the numbers of sparrows of the southwestern United States despite the fact that many of the species show no signs of winter territoriality. Summer rainfall in southern Arizona is extremely erratic and seed production may vary in response to rainfall as much as two orders of magnitude (see Pulliam & Parker 1979). As already discussed, in years of low rainfall and seed production sparrow numbers in Arizona are low because the sparrows facultatively migrate farther south. The high variance in rainfall results in high fluctuations in the number over-wintering birds, but these fluctuations are not in the pattern of periodic oscillations.
How do density-dependent mortality in the non-reproductive season and density-dependent reproductive success in the breeding season interact to determine overall population dynamics?
So far we have focused on population regulation in the non-reproductive season. This is perhaps a healthy antidote to years of focus in the avian literature on the role of breeding territories in population regulation with little or no mention of winter mortality (but see Goss-Custard 1994 and Sherry & Holmes 1996). In reality, events during the breeding season as well as those during the over-wintering season and during migrations contribute to population regulation, and we need to learn how the influences combine to determine overall population dynamics.
The sort of model developed above can, in theory, easily be extended to include the joint influence of events during both the breeding and non-breeding periods. In the model, it was assumed that for every bird alive at the end of the winter, a constant number (b) birds were alive at the beginning if the next winter. Thus, reproductive success and survival in the breeding season were assumed to be density-independent and constant. Changing the mean reproductive success, but still keeping it density independent, has a rather simple effect of the model outcome: increasing b results in a greater overshoot of the food supply and therefore in more pronounced population oscillations whereas smaller b results in less oscillation in population size.
In concept, it is quite easy to add density-dependent breeding success to the model as long as there is sufficient data. If one knows how average breeding success changes with breeding population size, this can be used to make reproductive success a function of population size in the model. Although the technical analysis of the model extended to include density dependence in the breeding season is beyond the scope of the present paper, the general result will be to dampen population oscillations provided that the breeding season density dependence begins to take effect at a sufficiently low population size. For example, if the breeding season density dependence is sufficiently strong, a population may be regulated to such a low population size that over-wintering populations never run out of food in the best habitat and therefore show no density dependence on the wintering grounds. In this case, social behaviour during the winter becomes irrelevant to population regulation and accordingly, the lack of defence of winter territories does not contribute to population oscillations. At the other extreme, density dependence during the breeding season may be so weak or may not be exhibited until such high population levels that it becomes irrelevant, in which case what happens during the non-breeding season becomes extremely important to population regulation. In general, knowledge of what happens in both seasons will contribute to our understanding of population dynamics.
How does habitat loss on the wintering grounds influence population dynamics and the probability of extinction?
Habitat loss appears to be an important cause of population decline in many species and severe habitat loss may increase the likelihood of extinction. Is habitat loss on the wintering grounds more or less important than habitat loss on the breeding grounds? Of course, there is no general answer to this question and the relative importance of density dependence in the breeding and non-breeding seasons must both be considered as discussed above. However, one case that is particularly interesting and easy to analyse occurs when a species that is normally regulated by events on breeding grounds, and is highly social in the non-breeding season, suddenly faces a massive loss of wintering habitat although it has had no evolutionary history with winter food or habitat limitation.
Some species may have never experienced any density-dependent mortality on their wintering grounds. Since there is some cost to resource defence, we would expect to find little or no defence of winter food supplies in these species. Sudden severe loss of over-wintering habitat may result in a population experiencing winter food shortages or being forced into habitat where predation rate is very high for the first time in its evolutionary history. Unlike in a species with strong resource defence in which at least some of the more territorial individuals would be expected to survive, in a species with no tendency to defend resources, all individuals are in the same boat and this could lead to catastrophic population decline. Ironically, the larger the population size, the more likely such a decline becomes. Thus, a population that experiences a number of years of particularly good conditions on the breeding grounds just at a time when winter habitat is being lost may be especially prone to a severe population decline.
Human activities are having an unprecedented impact on the habitat of many bird species. In some cases, human activities result in dramatically less habitat being available and in other cases human activities may actually increase the availability of habitat. For some species, human activities may be increasing the amount of breeding habitat available while at the same time decreasing the amount of wintering habitat available. Furthermore, for those species that have traditionally been limited by the availability of breeding habitat, a sudden shift in the relative availability of breeding and non-breeding habitat may result in increased likelihood of population declines and increased vulnerability to extinction. A detailed consideration of the breeding and non-breeding season habitat requirements and social behaviour of migratory bird species in relationship to large-scale land-use trends may be a useful way of predicting which species are most vulnerable to extinction.
REFERENCES
Arcese, P., Smith, J.N.M., Hochachka, W.M., Rogers, C.M. & Ludwig, D. 1992. Stability, population regulation, and the determination of abundance in an insular Song Sparrow population. Ecology 73: 805-822.
Benkman, C.W & Pulliam, H.R. 1988. Comparative feeding ecology of North American sparrows and finches. Ecology 69: 1195-1188.
Bock, C.E. & Lepthien, L. W. 1976. Synchronus eruptions of boreal seed-eating birds. American Naturalist 110: 559-571.
Brown, J.L. 1969. Territorial behavior and population regulation in birds. Wilson Bulletin 81: 293-329.
Caraco, T. 1979. Time budgeting and group size: a test of theory. Ecology 60: 618-627.
Caraco, T. & Pulliam, H.R. 1978. Time budgets and flocking dynamics. Proc. of the 17th Int. Ornithol. Congr.: 807-812.
Caraco, T., Martindale, S.M. & Pulliam, H.R. 1980a. Avian flocking in the presence of a predator. Nature 285: 400-401.
Caraco, T., Martindale, S.M. & Pulliam, H.R. 1980b. Avian time budgets and distance to cover. Auk 97: 872-875.
Ferrer, M. & J.A. Donazar, J.A. 1996. Density-dependent fecundity by habitat heterogeneity in an increasing population of Spanish Imperial Eagles. Ecology 77: 69-74.
Fretwell, S.D. & Lucas, H.L. Jr. 1970. On territorial behavior and other factors influencing habitat distribution in birds. I. Theoretical development. Acta Biotheoretica 19: 16-36.
Gilliam, J. 1982. Habitat use and competitive bottlenecks in size-structured fish populations. Ph.D. thesis. Michigan State University, East Lansing, MI.
Goss-Custard, J.D., Caldow, R.W.G., Clarke, R.G., Durell, S.E.A. le V. dit, Urfi, J. & West, A.D. 1994. Consequences of habitat loss and change to populations of wintering migratory birds: predicting the local and global effects of studies of individuals. Ibis 137: S56-S66.
Grant, P.R. 1986. Ecology and Evolution of Darwin's Finches. Princeton Univ. Press, Princeton, N.J.
Holmes, R.T., Marra, P.P. & Sherry, T.W. 1996. Habitat-specific demography of Black-throated Blue Warblers. (Dendroica caerulescens): implications for population dynamics. Journal of Animal Ecology 65: 183-195.
Kluyver, H.N. & Tinbergen, L. 1953. Territory and regulation of density in titmice. Archives Neerlandaises de Zoologie 10: 265-289.
Lack, D. 1954. The natural regulation of animal numbers. Clarendon Press, Oxford.
Moore, N.J. 1972. The ethology of the Mexican Junco (Junco phaeontus palliatus). Ph.D. Dissertation, University of Arizona, Tucson, Az.
Newton, I. 1970. Irruptions of crossbills in Europe. In Watson, A. (ed) Animal populations in relation to their food resources. Oxford; Blackwell Press: 337-357.
Newton, I. 1992. Experiments on limitation of bird numbers by territorial behavior. Biological Reviews 67: 129-173.
Pulliam, H.R. 1980. Do Chipping Sparrows forage optimally? Ardea 68: 72-82.
Pulliam, H.R. 1985. Foraging efficiency, resource partitioning and the coexistence of sparrow species. Ecology 66: 1829-1836.
Pulliam, H.R. & Enders, F.W. 1971. The feeding ecology of five sympatric finch species. Ecology 52: 557-566.
Pulliam, H.R. & Brand, M.R. 1975. The production and utilization of seeds in a desert grassland. Ecology 56: 1158-1166.
Pulliam, H.R. & Parker, T.H. 1979. Population regulation of sparrows. Fortschritte der Zoologie 25: 137-147.
Pulliam, H.R., Anderson, K.A., Misztal, A. & Moore, N. 1974. Temperature-dependent social behaviour in juncos. Ibis 116: 360-364.
Pulliam, H.R. & Mills, G.S. 1977. The use of space by sparrows. Ecology 58: 1393-1399.
Repasky, R.R. & Schluter, D. 1994. Habitat distributions of wintering sparrows along an elevational gradient: test of food, predation and microhabitat structure hypotheses. Journal of Animal Ecology 63: 569-582.
Repasky, R.R. & Schluter, D. 1996. Habitat distributions of wintering sparrows: foraging success in a transplant experiment. Ecology 77: 452-460.
Rodenhouse, N.L., Sherry, T.W. & Holmes, R.T. 1997. Site-dependent regulation of population size: a new synthesis. Ecology 78: 2025-2042.
Schluter, D. 1982 Distributions of Galapagos ground finches along an altitudinal gradient: the importance of food supply. Ecology 63: 1504-1517.
Sherry, T. W. & Holmes, R.T. 1996. Winter habitat quality, winter limitation, and conservation of neotropical migrant birds. Ecology 77: 36-48.
Smith, J.N.M. , Taitt, M.J., Rogers, C.M., Arcese, P., Keller, L.F., Cassidy, A.E.V. & Hochachka, W.M. 1996. A metapopulation approach to the population biology of the Song Sparrow Melospiza melodia. Ibis 138: 120-128.
Ulfstrand, S. 1963. Ecological aspects of irruptive bird migration in northwestern Europe. Proc. of the 13th Int. Ornithol. Congr.: 780-794.
Werner, E.E. & J.F. Gilliam. 1984. The ontogenetic niche and species interactions in size-structured populations. Annual Review of Ecology and Systematics 15: 393-425.
Wiens, J.A. & Rotenberry, J.T. (1981). Habitat associations and community structure of birds in shrub-steppe environments. Ecological Monographs 51: 21-41.
Fig. 1. Probability of surviving winter declines as the density of birds at the start of winter increases. Seed production is set at 1 kg ha-1 and seed consumption is 0.01 kg per individual per day. Daily mortality (deaths per individual per day) in the good habitat is set at 0.002 and daily mortality in the poor habitat is set at 0.004.

Fig. 2. Population size increases with time for non-territorial and territorial populations. Reproductive success (b) is set at 2.0 and seed consumption and seed production are as in Fig. 1. Daily mortality (deaths per individual per day) in the good habitat is set at 0.002 for a non-territorial population and 0.0025 for a territorial population. Daily mortality in the poor habitat is set at 0.004 for panels A and B and at 0.0014 for panels C and D. See text for further details.
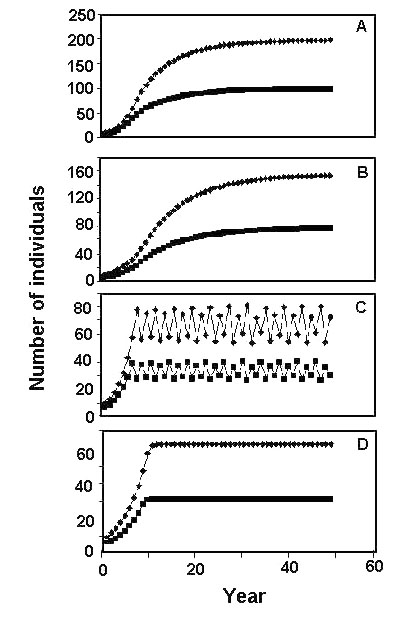
Fig. 3. Different combinations of daily mortality in the good (
sG) and poor (sP) habitat result in different patterns of population dynamics. For lower values of sG and sP, population size increases gradually and eventually reaches a single stable equilibrium. For higher values of sG and sP population sizes increase and finally reach a limit cycle of two or more equilibrium points.