S48.3: Impacts of nest depredation and brood parasitism on the productivity of North American passerines
Peter Arcese1 & James N.M. Smith2
1Department of Wildlife Ecology, University of Wisconsin, Madison, Wisconsin 53706, United States of America, e-mail arcese@calshp.cals.wisc.edu; 2Department of Zoology and Centre for Biodiversity Research, University of British Columbia, Vancouver, British Columbia, V6T 1Z4, Canada, e-mail smith@zoology.ubc.ca
Arcese, P. & Smith, J.N.M. 1999. Impacts of nest depredation and brood parasitism on the productivity of North American passerines. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2953-2966. Johannesburg: BirdLife South Africa.Nest depredation and brood parasitism may limit or regulate population size in many open nesting passerine birds, but brood parasitism probably has greater impacts on avian demography in North America than elsewhere. Brown-headed Cowbirds Molothrus ater, the commonest North American brood parasite, can parasitize a wide range of hosts at high intensities, and also can be abundant locally. By contrast, brood parasites in Europe and Asia are more specialised in their use of hosts, occur at lower densities and parasitize hosts at lower intensities. Even so, the cumulative impact of brood parasites on many hosts may be underestimated in descriptive as compared to experimental studies of populations. Results from a long-term study of Song Sparrows Melospiza melodia on Mandarte Is., Canada, and a comparative analysis of several studies of this species, show that observed rates of brood parasitism predict closely those of nest depredation. This pattern may occur because Brown-headed Cowbirds act as predators as part of their parasitic life history. This fact has the effect of making it hard to separate the impacts of nest predators on avian demography from those of brood parasites. Thus, experimental studies are needed to estimate reliably the independent impact of nest predators and brood parasites on the demography of passerines that are common hosts of brood parasites
INTRODUCTION
Avian population biology as a discipline commenced almost simultaneously in Europe and North America, but its development proceeded differently on the two continents. In Europe, most early work by von Haartman, Kluijver and Lack focused on small-bodied species that nest in cavities (Lack 1966), and many early studies stressed intraspecific competition for food as a key factor affecting population size (e.g., Perrins 1965). In contrast, the pioneering work of the amateur Margaret Nice (1937) on the open nesting Song Sparrow Melospiza melodia in North America did not lead immediately to a strong local tradition of bird population studies. In particular, although Nice noted that nest predators and brood parasites greatly affected reproduction in the Song Sparrow, relatively few studies of the ecological effects of nest depredation and brood parasitism were conducted over the next 50 years.
The biogeography of brood parasites in Europe and North American also has influenced the development of ideas about avian population limitation. North America has an abundant generalist brood parasite, the Brown-headed Cowbird Molothrus ater, that lays eggs in the nests of about one third of the continental avifauna (Friedmann & Kiff 1985), and that can parasitize over 25 hosts locally at appreciable levels (>10%; e.g., Cannings et al. 1987). In contrast, Northern Europe has a relatively rare and specialised brood parasite, the Common Cuckoo Cuculus canorus, that interacts strongly with only a few hosts in Britain (Chance 1940; Brooke & Davies 1987). Only one of these, the European Robin Erithracus rubecula, was the subject of early population studies (Lack 1954). Unlike the cowbird, the Common Cuckoo has relatively weak ecological effects on its host populations because of the small fraction of nests that typically are parasitised (Davies & Brooke 1989).
As a consequence of these historical matters, the ecological effects of nest predators and brood parasites received relatively little attention in bird population studies up to 1975 (but see Nice 1957; Mayfield 1963; Ricklefs 1969, 1973). Since the late nineteen seventies, however, work by Mayfield (1977), Gates & Gysel (1978), Nolan (1978), Brittingham & Temple (1983), Wilcove (1985), and May & Robinson (1985) have stimulated renewed interest in nest depredation and brood parasitism as factors that may regulate (cf Krebs 1970; Arcese et al. 1992; Smith & Arcese 1994) or limit population size (reviewed by Newton 1993, 1994). Subsequent concern about declines in North American passerine populations (Peterjohn et al. 1995; James & McCulloch 1995; Robinson et al. 1995a) also has focused interest on the effects of nest depredation and brood parasitism on seasonal reproductive success (SRS) (Martin 1995; Sherry & Holmes 1995). Regional declines in the number of large forest and grassland tracts, and increases in the number of nest predators and brood parasites in small tracts, are thought to have caused declines in SRS in several species (Terborgh 1989; Faaborg et al. 1995; Robinson et al. 1995a,b). Left unabated, we can assume that continued declines in SRS will lead some species into endangerment (e.g. Mayfield 1977; Grzybowski et al. 1986). Thus, identifying factors that reduce SRS, and that also can be ameliorated by management, is an important near-term goal (Martin & Finch 1995; Martin 1995; Robinson et al. 1995a,b).
In theory, one can identify the independent impacts of various deterministic causes of nest failure, such as those due to nest depredation and brood parasitism, and separate them statistically from the impacts of stochastic factors such as inclement weather. The rationale for doing so is that, once such deterministic causal factors are identified, we might design management plans to ameliorate their impacts on nesting success (Martin 1992; Robinson 1992; Robertson et al. 1994; Cote & Sutherland 1997); whereas we probably cannot expect to do so for stochastic factors. For example, faced with high rates of depredation by weasels Mustela nivalis on the nests of Great Tits Parus major, researchers at Marley Wood, Oxford, replaced wooden nest boxes with predator-proof boxes made of cement (McCleery & Perrins 1991). As a result, the rate at which clutches and broods were lost to nest predators declined from annual rates of about 35% from 1960-75 to near zero thereafter. A retrospective analysis of population counts before and after predator management suggested that a slightly declining population of tits increased after nest depredation was eliminated (McCleery & Perrins 1991).
In North America, many researchers have suggested that predators, such as Raccoons Procyon lotor, or brood parasites, such as Brown-headed Cowbirds, impact a wide range of passerines negatively, especially near agricultural areas, where crop wastage, livestock or other factors facilitate their increase. If these causes have been attributed correctly, the negative impacts of specific predators or parasites might be ameliorated on a regional scale by manipulating habitat to reduce the frequency of contact between nesting passerines and their enemies (Robinson et al. 1995a,b). Or, where enemies pose an immediate threat to a particular species locally, managers might resort to the short-term solution of removing predators or parasites (Mayfield 1977; Grzybowski et al. 1986; Robertson et al. 1994; Cote & Sutherland 1997). To develop reliable management plans of this sort, we need to identify the causes of nest failure correctly, because an understanding of cause and effect is required for management plans to have their expected effects (Arcese & Sinclair 1997). Moreover, the public often has been resistant to the control of even introduced species for the purposes of conservation (Temple 1990, 1992). Thus, removal programs in particular need to focus on enemies that have demonstrable impacts. For these reasons, we review some current methods used to derive independent estimates of the effects of nest depredation and brood parasitism on SRS, and we present a qualitative analysis of these methods as the focus of this paper.
We conclude that researchers have the best chance of identifying the independent impacts of nest predators and brood parasites by comparing ecological models of populations in which predators or parasites are either naturally or experimentally present and absent. We show that estimates of the impacts of brood parasitism derived from observational studies of parasitised and non-parasitised nests in the same population are likely to confound the effects of nest predators and brood parasites, because these effects on SRS sometimes are functionally dependent (see also Pease & Grzybowski 1995). We illustrate this point by showing that nest depredation and brood parasitism are positively correlated in island and mainland populations of Song Sparrows, and that nest depredation is reduced in populations from which cowbirds are absent naturally or removed experimentally. Monitoring the SRS of individually marked females in populations that are managed experimentally to reduce the impacts of enemies at the nest should offer researchers the most precise and unbiased estimates for population modelling.
IMPACTS OF NEST PREDATORS ON PASSERINE PRODUCTIVITY
Nest depredation reduces the production of fledglings and limits SRS (Nice 1937; Ricklefs 1969; Newton 1993, 1994; Cote & Sutherland 1997). Thus, consistent increases in nest depredation can be expected to facilitate population declines, especially in species that typically breed only once each year (Newton 1993; Sherry & Holmes 1995). However, very few studies have used experimental or comparative methods to estimate the effect of specific predators on passerine nesting success or population size. As a result, reliable estimates of these impacts for passerines are unavailable (reviews in Newton 1993, 1994; Cote & Sutherland 1997).
Experimental methods include removing or reducing predators in some areas (Newton 1993, 1994) or excluding them from some nest sites (McCleery & Perrins 1991). Researchers might also compare statistically the rates of nest depredation in areas that are similar except for the natural presence and absence of a predator of interest, such as on adjacent islands. For example, Robertson et al. (1994) reduced populations of introduced ship rats Rattus rattus on Rarotonga, Cook Islands, to increase the nesting success and survival of an endemic flycatcher. Many researchers have removed predators to increase the nesting success or numbers of waterfowl, upland game birds and doves (Newton 1993, 1994; Cote & Sutherland 1997). Marcstrom et al. (1988) used a predator removal experiment on adjacent islands to show that predators limited the nesting success and population size of grouse. Informal predator exclusion experiments also have been tried by bluebird Sialia spp. enthusiasts in North America, who have designed nest boxes that are less attractive to European Starlings Sturnus vulgaris, House Sparrows Passer domesticus and wrens Troglodytes spp. and more impervious to raccoons (Radunzel et al. 1997). However, well-controlled removal, exclusion or comparative studies of specific nest predators of passerines have not been reported.
Instead, most researchers have attempted to estimate the impact of particular predators observationally, by assigning causes of failure based on the condition of nests and their contents at sequential visits (e.g., Pinkowski 1975; Martin & Hochachka 1997). This method is flawed, however, because it assumes that signs left at nests implicate particular predators reliably, and also that signs recorded at nests were made coincident with depredation rather than after abandonment. These assumptions have proved false in our experience, with the result that observational methods identified and estimated the impacts of individual predators inaccurately (see below). Newton (1993, 1994) also concluded that observational study designs were insufficient to test for a role of depredation in population limitation. James & McCulloch (1995) review a number of superior approaches for attributing cause and effect generally. We now describe our experience as an illustration.
Are mice predators of bird eggs?
For 16 years we interpreted the presence of Deer Mouse Peromyscus maniculatus faeces in the nests of Song Sparrows with eggs or young missing, or with shell fragments or parts of chewed nestlings, as evidence that deer mice caused most nest depredation on Mandarte Is., B.C. (Arcese et al. 1992). More recently, we noted that mouse faeces are common in nests from which all young had fledged successfully, and in nests with intact clutches of infertile eggs that were abandoned after an extended incubation (unpublished results). These observations suggested a new working hypothesis: mice visit nests regularly, are repelled by adult sparrows at active nests, but gain access to nests that fail or are abandoned for other reasons. This hypothesis implies that mice are scavengers at nests rather than predators.
To test if mice are egg predators, we placed intact nests in sites typically used by sparrows, each containing a painted, plasticine egg and a House Sparrow egg (6 trials, nnests=65; B. T. Martinez et al., unpublished manuscript). House and Song Sparrow eggs are similar in size, appearance and shell thickness. We found that mice visited 52% of nests within 48 hrs, as indicated by the presence of faeces in nests and incisor marks on plasticine eggs. Two real eggs were cracked but unopened. In two other nests both eggs were missing and no faeces were present. Five nests had faeces present and the plasticine egg missing. These results allowed us to conclude that Deer Mice on Mandarte Is. find and enter sparrow nests readily, bite plasticine eggs when they encounter them, but seldom depredate real eggs. Although mice on Mandarte Is. may occasionally kill nestlings, and perhaps even brooding adults (unpublished results), our current results show that sign at nests can be an unreliable clue to the identity of specific nest predators.
Using cameras, reflective powder or observing depredation directly, may improve indirect estimates of predator impacts. Infrared-illumination and miniature cameras also promise to make records of predator visits to nests more accurate and reliable. However, because of the high cost of such methods, and the methodological problems associated with documenting depredation at a large and unbiased sample of nests, experimental and comparative methods offer an attractive alternative for estimating the impact of particular predators. We now discuss some impacts of brood parasites on SRS of Song Sparrows and suggest that nest depredation and brood parasitism is linked in populations of Song Sparrows and, perhaps, other species.
IMPACTS OF BROWN-HEADED COWBIRDS ON HOST PRODUCTIVITY
Most researchers have estimated the impact of brood parasitism by Brown-headed Cowbirds, the commonest brood parasite in North America, by extrapolating from observations of the parasitised and non-parasitised nests of their hosts (May & Robinson 1985; Smith & Arcese 1994; Pease & Gryzbowski 1995; Payne & Payne 1998). In doing so, researchers have assumed that cowbirds affect hosts by: (a) removing a host egg prior to laying their own, (b) usurping parental care to the detriment of host young, and (c) reducing the survival of host young. These direct impacts of parasitism on the SRS of hosts have been reviewed extensively by Robinson et al. (1995a,b) and Payne (1998). Therefore, we focus instead on some potential indirect effects of parasitism on the SRS of hosts.
It is likely for many reasons that parameter estimates that are derived observationally, as described above, underestimate the cumulative impact of cowbirds on SRS. This is because cowbirds also cause nest depredation in some populations (Scott & McKinney 1994; Arcese et al. 1996) and may remove eggs or cause the abandonment of nests that they visit but do not parasitize subsequently (see below). DuBois (1956) relates a typical case of nest depredation by a cowbird at a song sparrow nest in Minnesota. He notes, 'the three [cowbirds] went into long grass…and seemed to be searching for something. As I knew of a Song Sparrow’s nest I watched closely. Suddenly one of the female cowbirds made a run and a lunge for the hidden nest, seemed to strike its contents, and, as I started forward, flew off carrying a very young nestling in her bill.' Upon observation of the nest DuBois found that the 'three callow young Song Sparrows that remained were packed together in a small pocket of grass about eight inches from the empty nest. One of them was bloody ab out the head but apparently not disabled. I replaced them in their nest, and for a few days they were cared for by their parents; but on May 25 [4 days later] the nest was empty.' DuBois’ account matches others reported in the literature and several observations of our own on Mandarte Is. and elsewhere (Arcese et al. 1996; Hatch 1996; A. B. Marr, personal communication).
Where cowbirds cause or facilitate nest depredation, or increase the rate of nest abandonment, estimates of cowbird impacts based on observations of parasitised and non-parasitised nests will underestimate the cumulative impact of cowbirds on host SRS. In the remainder of this section, therefore, we provide further evidence to suggest that cowbirds reduce the SRS of Song Sparrows by reducing the average clutch size of unparasitized nests, by increasing the rate of abandonment of unparasitized nests, and by causing or facilitating nest failure. We conclude the section by discussing evidence from other species for and against the hypothesis that cowbirds affect the SRS of hosts by means other than via the direct effects of parasitism.
Impact of cowbirds on host clutch size
Consider estimates of the fecundity of host females that tend parasitised and non-parasitised nests in one population. Because cowbirds often remove eggs from nests that they subsequently parasitize, host clutches in parasitised nests are smaller than clutches that are not parasitised (Smith 1981; Smith & Arcese 1994; Robinson et al. 1995b; Payne 1998). By reducing host clutch size, cowbirds also reduce the realised fecundity and SRS of hosts. Suppose, however, that cowbirds also remove eggs from a fraction of nests that are not parasitised subsequently, or that a fraction of hosts that are parasitised eject cowbird eggs from their nests. Because researchers recognise parasitism by the presence of parasite eggs (e.g., Sealy 1992; Payne & Payne 1998), these events in observational studies will cause the impact of cowbirds on the SRS of hosts to be underestimated. In effect, researchers using these methods will assign incorrectly an unknown fraction of nests to the ‘unparasitized’ group.
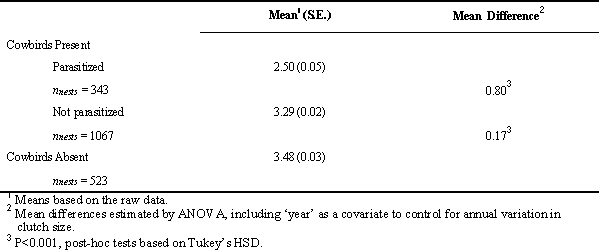
To estimate the size of biases that may result from assigning the status of nests incorrectly, we compared the clutch size of Song Sparrow nests scored as parasitised or not parasitised in 15 years when cowbirds were common and 7 years when cowbirds were absent from Mandarte Is. We controlled statistically for other factors that may have varied among years and affected clutch size by including ‘year’ as a covariate in the analysis. We found that the size of non-parasitised clutches in years when cowbirds were present was 0.80-0.97 eggs larger than clutches with cowbird eggs recorded in them (raw and adjusted mean differences, respectively; P < 0.001, Table 1). In years without cowbirds, however, clutch size also was 0.17-0.19 eggs larger than unparasitized clutches in years with cowbirds present (raw and adjusted means, respectively; P < 0.001; Table 1). Thus, the presence of cowbirds was associated with a reduction in the size of unparasitized clutches that was about 20% as large as the reduction estimated by comparing parasitised and non-parasitised clutches in the same year. In other words, our comparison of parasitised and non-parasitised clutches suggests that cowbirds remove about one egg from every nest they parasitize. In contrast, our comparison of unparasitized nests in years with cowbirds present and absent suggests that cowbirds also remove about one egg from every five nests that they do not parasitize subsequently. This result reinforces our contention that simple comparisons of parasitised and non-parasitised nests in a single study population will not estimate reliably the impacts of cowbirds on host clutch size.
Impact of cowbirds on nest abandonment
Observational studies estimate the impact of cowbirds on nest abandonment in the same way that they estimate cowbird impacts on host clutch size (e.g., Pease & Grzybowski 1995). Abandoned nests with cowbird eggs are recorded as having failed due to parasitism. Nests without cowbird eggs are assigned as having been abandoned, usually due to unidentified causes. Many kinds of disturbance near nests may cause abandonment in cowbird hosts, especially early in the nesting cycle, and many researchers avoid approaching early nests for this reason (Payne & Payne 1998; our personal observations). It is possible, therefore, that one unrecognised cause of abandonment involves interactions between hosts and cowbirds that occur prior to egg-laying by cowbirds. In these instances, if cowbirds avoid laying in abandoned nests on subsequent visits, traditional methods of assigning cause to nest abandonment will result in underestimates of the cumulative impacts of cowbirds on host SRS.
Song Sparrows are not known to abandon nests as a consequence of cowbird parasitism (Nice 1937; Smith 1981), but they do abandon about 3% of all clutches on Mandarte Is. (Hatch 1996). Moreover, our prior work suggests that sparrows recognise cowbirds as enemies at the nest (Smith et al. 1984), and it is possible that nest abandonment is a consequence of interactions between sparrows and cowbirds at the time that a cowbird first visits the nest to remove a host egg. Therefore, we asked if abandonment by Song Sparrows was more common in years with cowbirds present than absent to test if interactions with cowbirds might cause abandonment even in the absence of parasitism.
Hatch (1996) compiled all causes of nest failure from 1982 to 1995 for Song Sparrows on Mandarte Is., including the fraction due to the abandonment of completed clutches. Using these data, we found that the annual rate of abandonment averaged 0.8% (S. E. = 0.48) of all nests in four years with cowbirds absent but was 4.3% (S. E. = 0.84) in 10 years with cowbirds present. This difference represents a five-fold increase in the annual rate of abandonment in the presence of cowbirds (Two-sample t-test: t11.8=3.23, P < 0.01). These results support further our general hypothesis that cowbirds have impacts on nests that are not parasitised, and they suggest that observational studies will underestimate cowbird impacts by attributing abandonment to cowbird activity only when abandoned nests contain cowbird eggs.
Impacts of cowbirds as nest predators
It is well documented that, in addition to ejecting individual eggs, cowbirds sometimes cause entire nests to fail by destroying whole clutches or broods (see above; Scott & McKinney 1994; Arcese et al. 1996). We have suggested elsewhere that cowbirds do this regularly on Mandarte Is. to facilitate re-laying by hosts whose nests are discovered too late in the nesting cycle to be parasitised successfully (Arcese et al. 1992, 1996). We also have presented much evidence to show that nest failure is more common among Song Sparrows on Mandarte Is. when cowbirds are present than when they are absent, both within breeding seasons and across years (Arcese et al. 1996; Smith & Arcese 1994). However, it also is possible that cowbirds simply facilitate nest depredation by their activities near host nests that are associated with parasitic behaviour generally. For example, cowbirds regularly drop ejected eggs in the vicinity of host nests (Scott et al. 1992; Arcese et al. 1996). As expected if cowbirds facilitate nest failure, Payne & Payne (1998) found that the parasitised nests of indigo buntings failed more often than non-parasitised nests in Michigan (see also Robinson et al. 1995b).
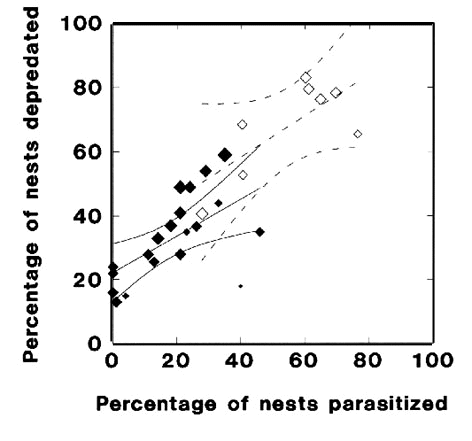
To test further our general hypothesis that nest depredation is either caused or facilitated by cowbirds, we explored the relationship between nest depredation and brood parasitism in 10 populations in which Song Sparrows were studied for from two to 22 years. This included 6 small islands near Mandarte Is. (2-3 yrs; Smith et al.1996), Mandarte itself (22 yrs; Arcese et al. 1996), San Miguel Is., CA (Sogge & van Ripper 1988) and mainland sites in Ohio (Nice 1937), San Francisco (Johnson 1956) and British Columbia (Rogers et al. 1997; Smith & Taitt 1998). We also took advantage of the fact that on Mandarte Is. cowbirds were rare or absent in 7 of 22 years (see Table 1), and that at one mainland study site cowbirds were removed to reduce the rate of parasitism in two of 7 years. This gave us 12 paired estimates of nest depredation and parasitism at the population level. Because sample size varied markedly between sites and treatments (nnests = 5-1520), we weighted pairs of points by square-root of n, after transforming it to its z-score, following Gilbert (1973). Weighting and transforming the data improved the fit of the general model but had no effect on the level of significance of the analysis.
A regression of the percent of nests depredated versus those parasitised across populations indicated that 91% of all variation in depredation rate was accounted for statistically by the rate of brood parasitism (F1,11 = 144.7, R2 = 0.91, P < 0.001; Fig. 1). The slope of the relationship suggested that depredation increased directly with nest parasitism (b = 0.93, S. E. = 0.09, t = 11.7, P < 0.001). An analysis of the unweighted data gave a slightly shallower slope (b = 0.79, S. E. = 0.15) and smaller fraction of variance accounted for (R2 = 0.71), but the statistical significance of the analysis was unchanged. Overall, these results indicate that the frequency of nest depredation in Song Sparrows depends strongly on the level of brood parasitism in a wide variety of habitats.
To test whether predators other than cowbirds might also affect nest depredation, we tallied the number of species of vertebrates resident in the populations under study and suspected by researchers to have been responsible for events of nest depredation. Because some islands in the study were small and isolated, they contained no resident predators. Mainland sites were recorded as containing up to 9 species of predators, because we made the conservative decision to count more than one co-occurring species of snake or murine rodent as a single species. The true number of predators at mainland sites was therefore underestimated. With predator richness tallied, we re-ran our analysis and found that the fraction of variation in nest depredation accounted by our model increased to 98% (Fig. 1). However, we found no significant interaction between parasitism and predator richness, suggesting that the impacts of cowbirds and other nest predators were additive and statistically independent.
Independent effects of cowbirds as predators
It is possible that variation in predator richness is primarily responsible for the strong correlation between parasitism and nest depredation across island and mainland populations (Fig. 1). We therefore tested for the independent effect of changes in the rate of parasitism on nest depredation by comparing these rates simultaneously on Mandarte Is. and Mainland, B.C., where cowbirds were either naturally present or absent or were reduced in abundance via a removal experiment. We found nearly identical relationships in each population (Fig. 2). Because predator richness did not vary noticeably within study sites over the study period, this suggests that changes in the rate of parasitism were the primary cause of changes in nest depredation.
Impacts of brood parasites on hosts other than Song Sparrows
Researchers have reported a correspondence between high rates of parasitism and nest depredation at regional levels, especially in the upper mid-west U.S. (Robinson et al. 1995a; Donovan et al. 1997). This result is expected if cowbird and predator abundance each are correlated positively with habitat features at the landscape level, such as the degree of forest fragmentation. However, this also is the predicted result if cowbirds cause or facilitate nest depredation, as we have suggested here and elsewhere (Arcese et al. 1992, 1996). Small-scale removal experiments with suitable controls could help to identify the independent impacts of cowbirds and predators on host nesting success in many areas where nest depredation and brood parasitism are known to be positively correlated.
Where cowbird removals have been tried, hosts generally have shown marked improvement in SRS and some populations protected from cowbirds have increased in size (Probst 1986; review in Robinson et al. 1995b). However, data for species other than the Song Sparrow are sparse and have yet to support strongly the idea that cowbird removal reduces nest depredation, as opposed to parasitism, markedly. For example, Walkinshaw (1983:table 42) showed that cowbird removals reduced the rate of nest parasitism from 69% of 52 nests of Kirtland’s Warblers Dendroica kirtlandii in 1966-71 to 6% of 230 nests in 1972-77. Over the same periods, the number of fledglings per nest increased from 0.81 to 2.67 (Walkinshaw 1983). However, cowbird removal was coincident with only a modest 18% decline in the rate of egg depredation (18.5% of 119 eggs before removal versus 15.1% of 971 eggs after; two-tailed log-likelihood test: G1 = 3.51, P = 0.06; Walkinshaw 1983:table 37). Further experiments of this type would be improved if they: a) included control sites where cowbirds were not removed, and b) monitored the rates of total nest failure attributable to depredation and other causes. Such experiments would be especially practical in species such as Northern Cardinals Cardinalis cardinalis, Indigo Buntings Passerina cyanea or Yellow Warblers Dendroica petechia that are parasitised commonly by cowbirds but are abundant locally.
In Spain, recent work has supported earlier speculation by Chance (1940) and Zahavi (1979) that cuckoos also cause host nests to fail as part of their parasitic life history (Soler et al. 1995). Experiments designed to estimate the impacts of brood parasitism by the Great Spotted Cuckoo Clamator glandularis on the SRS of Magpies Pica pica suggest that cuckoos have their greatest negative impact on hosts via egg destruction (Soler et al. 1996). Thus, although cuckoos are more specialised in their use of hosts, parasitize hosts at lower intensities and usually are less abundant than the Brown-headed cowbird, it is possible that some species of cuckoos have marked impacts on particular hosts. Comparisons of the rate of nest depredation in populations of preferred hosts with cuckoos present and absent may help to estimate the cumulative impacts of parasitism by cuckoos on the host SRS.
CONCLUSIONS
For open-nesting passerine birds, depredation of eggs and young is now widely recognised to be a frequent factor that limits (Martin 1992; Newton 1993, 1994; Holmes et al. 1992; Sherry & Holmes 1995), and sometimes regulates (Krebs 1970; Arcese et al. 1992, 1996; Smith & Arcese 1994), population size. Brood parasitism probably limits populations less often, except perhaps in the Americas, where brood parasitic species of cowbirds sometimes reach high densities relative to their hosts (Friedmann & Kiff 1985). In particular, where generalist brood parasites like Brown-headed and Shiny Cowbirds Molothrus bonariensis are abundant, brood parasitism and the depredation of eggs and young by cowbirds and other predators may affect passerine populations in complex ways that, as yet, are largely unexplored (Grzybowski & Pease 1995, see above).
Analytical (Pulliam, this symposium), simulation (Pease & Grzybowski 1995, Rodenhouse et al., this symposium) and empirical statistical models all can be used to advance understanding of the factors that limit and regulate populations of open nesting passerines. We have stressed the usefulness of predictive statistical models for estimating the independent impacts of nest depredation and brood parasitism on SRS. Such models may facilitate the development of well designed management plans for individual species or communities by focusing management on ameliorating the effects of the most detrimental enemies at the nest (see also Martin 1995). However, the reliability of predictive models depends heavily on the quality of the parameter estimates used. We suggest that researchers derive estimates of the effects of nest predators and brood parasites by comparing populations with and without the enemy of interest present, before drawing conclusions about their impacts on SRS. Where the removal of predators or parasites is not possible, studies of island populations of passerines provide good opportunities to study species in the natural presence and absence of enemies.
ACKNOWLEDGEMENTS
We thank the many people that have worked on the Song Sparrow populations on Mandarte and its surrounding islands and the mainland. Most recently, these people have included L. Keller, A. Marr, M. Hatch, T. Sullivan, M. Taitt, B. Martinez and G. Jongejan. We are especially grateful to B. Martinez for allowing us to cite her unpublished manuscript on egg predators of sparrows. The Tsawout and Tsyecum Bands of Saanich generously allow us to work on Mandarte. Our work has been funded primarily by continuing grants from the Natural Sciences Research Council of Canada to J.N.M.S., and, more recently, Young Investigator Award IBN 9458122 from the National Science Foundation (U. S.) to P.A.
REFERENCES
Arcese, P. & Sinclair, A. R. E. 1997. The role of protected areas as ecological baselines. Journal of Wildlife Management, 61:587-602.
Arcese, P., Smith, J.N.M & Hatch, M.I. 1996. Nest predation by cowbirds and its consequences for passerine demography. Proceedings of the National Academy of Sciences, 93: 4608-4611.
Arcese, P., Smith, J.N.M., Hochachka, W.M., Rodgers, C.M. & Ludwig, D. 1992. Stability, regulation and the determination of abundance in an insular Song Sparrow population. Ecology 73: 805-822.
Brittingham, M. C. & Temple, S.A. 1983. Have cowbirds caused forest songbirds to decline? BioScience 33:31-35.
Brooke, M. de L. & Davies, N.B. 1987. Recent changes in host use by cuckoos, Cuculus canorus, in Britain. Journal of Animal Ecology 56:873-883.
Cannings, R.A., Cannings, R.J. & Cannings, S.J. 1987. Birds of the Okanagan Valley, British Columbia. Victoria; Royal British Columbia Museum: 420pp.
Chance, E. 1940. The Truth About the Cuckoo. London; Country Life: 400pp.
Cote, I.M. & Sutherland, W.J. 1997. The effectiveness of removing predators to protect bird populations. Conservation Biology 11: 395-405.
Davies, N.B. & Brooke, M.deL. 1989. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. Journal of Animal Ecology 58:225-236.
Donovan, T.M., Jones, P.W., Annand, E.M. & Thompson, F.R. III. 1997. Variation in local scale edge effects on cowbird distribution and nest predation. Ecology 78:2064-2075.
DuBois, A. D. 1956. A cowbird incident. Auk 73:286.
Faaborg, J, Brittingham, M., Donovan, T. & Blake, J. 1995. Habitat fragmentation in the Temperate Zone. In: T.E. Martin & Finch, D.M. (eds) Ecology and Management of Neotropical Migratory Birds: A Synthesis and Review of Critical Issues. New York; Oxford University Press: 357-380.
Friedmann, H. & Kiff, L.F. 1985. The parasitic cowbirds and their hosts. Proceedings of the Western Foundation of Zoology 2:226-304.
Gates, J. E. & Gysel, L.W. 1978. Avian nest dispersion and fledging success in field-forest ecotones. Ecology 59:871-883.
Gilbert, N. 1973. Biometrical Interpretation. London; Oxford University Press: 179pp.
Gryzbowski, J.A., Clapp, R.B. & Marshall, J.T. 1986. History and population status of the Black-capped Vireo in Oklahoma. American Birds 40:1151-1161.
Hatch, M. I. 1996. Individual variation in the avoidance of nest predation in Song Sparrows. M.Sc. thesis, University of Wisconsin-Madison.
Holmes, R.T., Sherry, T.W., Marra, P.P. & Petit, K.E. 1992. Multiple brooding and productivity of a neotropical migrant, the Black-throated Blue Warbler (Dendroica caerulescens), in an unfragmented temperate forest. Auk 109: 321-333.
James, F. C. & McCulloch, C.E. 1995. The strength of inferences about causes of trends in populations. In: T.E. Martin & D.M. Finch (eds) Ecology and Management of Neotropical Migratory Birds: A Synthesis and Review of Critical Issues. London; Oxford University Press: 40-51.
Johnson, R.F. 1956. Population structure in salt marsh Song Sparrows. Part II: density, age structure and maintenance. Condor 58: 254-272.
Krebs, J.R. 1970. Regulation of numbers in the Great Tit (Aves: Passeriformes). Journal of Zoology (London) 162:317-333.
Lack, D. 1954. Two robin populations. Bird Study 1:14-17.
Lack, D. 1966. Population studies of birds. Oxford; The Clarendon Press.
Marcstrom, V., Kenward, R.E. & Engren, E. 1988. The impact of predation on boreal tetraonids during the vole cycles: an experimental study. Journal of Animal Ecology 57: 859-872.
Martin, T.E. 1992. Breeding season productivity: what are the appropriate habitat features for management? In: Hagen, J.M. & Johnson, D.W. (eds) Ecology and Conservation of Neotropical Migrant Land Birds. Washington, DC.; Smithsonian Institution Press: 455-473.
Martin, T.E. 1995. Summary: model organisms for advancing understanding of ecology and land management. In: T.E. Martin & Finch, D.M. (eds) Ecology and Management of Neotropical Migratory Birds: A Synthesis and Review of Critical Issues. New York; Oxford University Press: 477-484.
Martin, T.E. & Finch, D.M. 1995. Introduction: importance of knowledge and its application in Neotropical migratory birds. In: T.E. Martin & D.M. Finch (eds) Ecology and Management of Neotropical Migratory Birds: A Synthesis and Review of Critical Issues. New York; Oxford University Press: xiii-xvi.
Martin, T.E. & Hochachka, W.M. 1997. Breeding biology research and monitoring database. Biological Resources Division, U.S.G.S. Montana Cooperative Wildlife Research Unit, University of Montana, http://pica.wru.umt.edu/BBIRD/ (18 August 1997).
May, R.M. & Robinson, S.K.
1995. Population dynamics of avian brood parasitism. American Naturalist 126: 475-494.Mayfield, H. 1963. The Kirtland’s Warbler. Bloomfield Hills, Michigan; Cranbrook Institute of Science: 242pp.
Mayfield, H.F. 1977. Brood parasitism: reducing interactions between Kirtland’s Warblers and Brown-headed Cowbirds. In: S.A. Temple (ed) Endangered Species: management techniques for preserving threatened species. Madison; University of Wisconsin Press: 85-91.
McCleery, R.H. & Perrins, C.M. 1991. Effects of predation on the numbers of Great Tits Parus major. In: Perrins, C.M., Lebreton, J.D. & Hirons, G.J.M. (eds) Bird Population Studies: Relevance to Conservation and Management. Oxford; Oxford University Press: 129-147.
Newton, I. 1993. Predation and limitation of bird numbers. Current Ornithology 11:143-198.
Newton, I. 1994. Experiments on the limitation of breeding bird densities: a review. Ibis 136:397-411.
Nice, M.M. 1937. Studies in the life of the Song Sparrow. I. A population study of the Song Sparrow. Transactions of the Linnean Society of New York 4:1-247.
Nice, M.M. 1957. Nesting success in altricial birds. Auk 74:305-321.
Nolan, V. Jr. 1978. The ecology and behavior of the Prairie Warbler Dendroica discolor. Ornithological Monographs 26:1-595.
Payne, R.B. 1998. Brood parasitism in birds: strangers in the nest. Bioscience 48:377-386.
Payne, R.B & Payne, L.L. 1998. Brood parasitism by Brown-headed Cowbirds: risks and costs on reproductive success and survival in indigo buntings. Behavioral Ecology 9:64-73.
Pease, C. M. & Grzybowski, J. A. 1995. Assessing the consequences of brood parasitism and nest predation on seasonal fecundity in passerine birds. Auk 112:343-363.
Perrins, C.M. 1965. Population fluctuations and clutch size in the Great Tit, Parus major. J. Animal Ecology 34:601-647.
Peterjohn, B.G., Sauer, J.R. & Robbins, C.S. 1995. In: T.E. Martin & Finch, D.M. (eds) Ecology and Management of Neotropical Migratory Birds: A Synthesis and Review of Critical Issues. New York; Oxford University Press: 3-39.
Pinkowski, B.C. 1975. A summary and key for determining the causes of nesting failures in Eastern Bluebirds using nest boxes. Inland Bird Banding News 47:179-186.
Probst, J.R. 1986. A review of factors limiting the Kirtland’s Warbler on its breeding grounds. American Midland Naturalist 116:87-100.
Radunzel, L.A., Muschitz, D.M., Bauldry, V.M. & Arcese, P. 1997. A long-term study of variation in the breeding success of Eastern Bluebirds by year and cavity type. Journal of Field Ornithology 68:7-18.
Ricklefs, R.E. 1969. An analysis of nesting mortality in birds. Smithsonian Contributions in Biology 9:1-48.
Ricklefs, R.E. 1973. Fecundity, mortality, and avian demography. In: Farner, D.S. (ed.) Breeding Biology of Birds. Washington, D.C,: National Academy of Sciences: 366-435.
Robertson, H.A., Hay, J.R., Saul, E.K. & McCormack, G.V. 1994. Recovery of the Kakerori: an endangered forest bird of the Cook Islands. Conservation Biology 8:1078-1086.
Robinson, S.K. 1992. Population dynamics of breeding Neotropical migrants in a fragmented Illinois landscape. In: Hagen, J.M. & Johnson, D.W. (eds) Ecology and Conservation of Neotropical Migrant Land Birds. Washington, DC.; Smithsonian Institution Press: 408-418.
Robinson, S. K., Thompson, F.R., Donovan, T.M., Whitehead, D.R. & Faaborg, J. 1995a. Regional forest fragmentation and the nesting success of migratory birds. Science 267: 1987-1990.
Robinson, S.K., Rothstein, S.I., Brittingham, M.C., Petit, L. J. & Grysbowski, J.A. 1995b. Ecology and behavior of cowbirds and their impact on host populations. In: Martin, T.E. & Finch, D.M. (eds), Ecology and Management of Neotropical Migratory Birds: A Synthesis and Review of Critical Issues. New York; Oxford University Press: 428-460.
Rogers, C.M., Taitt, M.J., Smith, J.N.M. & Jongejan, G. 1997. Nest predation and cowbird parasitism create a demographic sink in wetland-breeding Song Sparrows. Condor 99:622-633.
Scott, D.M., Weatherhead, P.J. & Ankney, D. 1992. Egg-eating by female Brown-headed Cowbirds. Condor 94:579-584.
Scott, P.S. & McKinney, B.R. 1994. Brown-headed Cowbird removes Blue-gray Gnatcatcher nestlings. Journal of Field Ornithology 65:363-364.
Sealy, S.G. 1992. Removal of Yellow Warbler eggs in association with cowbird parastism. Condor 94:40-54.
Sherry, T.W. & Holmes, R.T. 1995. Summer versus winter limitation of populations: what are the issues and what is the evidence? In: Martin, T.E. & Finch, D.M. (eds) Ecology and Management of Neotropical Migratory Birds: A Synthesis and Review of Critical Issues. New York; Oxford University Press: 85-120.
Smith, J.N.M. 1981. Cowbird parasitism, host fitness and age of the host female in an island Song Sparrow population. Condor 83:152-161.
Smith, J.N.M. & Arcese, P. 1994. Brown-headed Cowbirds and an island population of Song Sparrows: a 16-year study. Condor 96:916-934.
Smith, J.N.M., Arcese, P. & McLean, I.G. 1984. Age, experience, and enemy recognition by wild Song Sparrows. Behavioral Ecology and Sociobiology 14:101-106.
Smith, J.N.M., Taitt, M.J., Rogers, C.M., Arcese, P., Keller, L.F., Cassidy, A.L.E.V. & Hochacka, W.M. 1996. A metapopulation approach to the population biology of Song Sparrows. Ibis 138:120-128.
Smith, J.N.M. & Taitt, M.J. 1998. The Song Sparrow and the Brown-headed Cowbird: enemies and friends. North American Ornithological Conference, 6-12 April, 1988, St. Louis, MO.
Sogge, M.K. & van Riper, C., III. 1988. Breeding biology of the San Miguel Island Song Sparrow (Melospiza melodia miconyx). Technical Report No. 26. Cooperative National Park Resources Studies Unit. Davis; University of California: 115pp.
Soler, M., Soler, J.J., Martinez, J.G. & Moller, A.P. 1995. Magpie host manipulation by Great Spotted Cuckoos: evidence for an avian mafia? Evolution 49:770-775.
Soler, M., Soler, J.G. & Soler, J.J. 1996. Effects of brood parasitism by the Great Spotted Cuckoo on the breeding success of the magpie host: an experimental study. Ardeola 43:87-96.
Temple, S.A. 1990. The nasty necessity: eradicating exotics. Conservation Biology 4:113-115.
Temple, S.A. 1992. Exotics birds: a growing problem with no easy solution. Auk 109:395-397.
Terborgh, J. 1989. Where Have all the Birds Gone? Essays in the Biology and Conservation of Birds that Migrate to the American Tropics. Princeton, NJ; Princeton University Press: 207pp.
Walkinshaw, L.H. 1983. Kirtland’s Warbler: the natural history of an endangered species. Bloomfield Hills, Michigan; Cranbrook Institute of Science:pp.
Wilcove, D.S. 1985. Nest predation in forest tracts and the decline of migratory songbirds. Ecology 66:1211-1214.
Zahavi, A. 1979. Parasitism and nest predation in parasitic cuckoos. American Naturalist 113:157-158.
Table 1. Mean (S. E.) size of clutches with and without cowbird eggs in 15 yrs when cowbirds were active on Mandarte Is. and 7 yrs when cowbirds were absent or laid only one egg (2 yrs).

Fig. 1. Percentage of nests depredated versus parasitised in 10 Song Sparrow populations studied for 2 or more years. Point size weighted by the square-root of predator richness at each site (see text). ‘Present’ and ‘Absent’ denote estimates from the sub-set of years in which cowbirds were present on Mandarte Is. versus rare or absent. ‘Removal’ and ‘Control’ denote estimates from mainland sites in British Columbia where parasitism was reduced experimentally in two years by trapping cowbirds. Parasitism (t = 4.79, P < 0.001) and predator richness (t = 6.05, P < 0.001) together accounted for 98% of variation in nest depredation but the interaction between these variables was not significant (t = 0.77, P = 0.46).
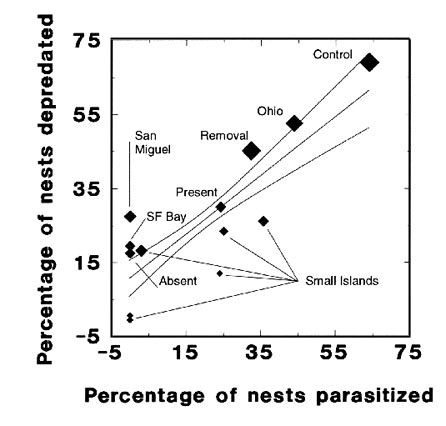
Fig. 2. Annual percentage of nests depredated versus parasitised on Mandarte Is (closed diamonds) and at two sites on the British Columbia mainland (open diamonds). Wide variation in the rate of parasitism occurred on Mandarte Is. because cowbirds were absent from in several years and on the mainland because cowbirds were removed in two years. Parasitism and nest depredation were correlated strongly (F1,27 = 20.7, P < 0.001), but the effect of site was only marginally significant (F1,27 = 4.1, P = 0.053).