S47.4: Reproductive control in cooperatively- and polygynously-breeding Acrocephalus species
Jan Komdeur
Zoological Laboratory, University of Groningen, PO Box 14, 9750 AA Haren, The Netherlands, fax 31 50-3635205, e-mail j.komdeur@biol.rug.nl
Komdeur, J. 1999. Reproductive control in cooperatively- and polygynously-breeding Acrocephalus species. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2910-2921. Johannesburg: BirdLife South Africa.
In biological terms a family exists when offspring continue to interact, into adulthood, with their parents. In nuclear families only a single male and female group member breeds (reproductive skew=1), while in extended families, two or more group members of one or both sexes reproduce (reproductive skew<1). Within cooperative societies families typically form via the retention of young with their parents. The result is a tight kin group with dominance asymmetry. Mating systems within and among group breeding Acrocephalus species range from nuclear families (with singular breeding) to extended groups (with plural breeding), comprising individuals of various degrees of relatedness. The formation and advantages of groups can be understood by integrating three overlapping areas of behavioral ecology: the ecological constraints and kin selection theories specifying the mechanisms that shape the differing forms of complex family structures, and the reproductive skew theory stressing the advantages of reproductive sharing, leading to extended group structures. Using a cross-taxonomic comparison of Acrocephalus species, I show the plasticity of reproductive control, and the variation in control mechanisms used by individuals to optimise their reproduction.
INTRODUCTION
Cooperatively breeding societies contain, at any one time, behaviourally distinct groups, with individuals of one group specialising in reproduction and individuals of the other group or groups specialising in helping individuals of the more reproductive group to reproduce. All individuals, of all groups, are omnipotent, and can thus switch between behavioral groups after reproductive maturity. Cooperatively breeding vertebrates have usually been divided into two categories, those with 'helpers-at-the-nest' (or den), which have a single reproductive pair or individual, and those with 'communal breeders', which have more than one reproductive of one or both sexes (Emlen 1991). It is very important to recognise that many species may express similar forms and variation of sociality. Variation in sociality among cooperatively breeding societies can be described along the quantitative axis developed by Vehrencamp (1979; see Emlen 1991), which permits estimation of the relative importance of inclusive fitness effects and personal fitness effects, and the degree to which helping is mutualistic (two or more individuals gain an immediate fitness advantage by cooperation) or altruistic (one individual gain fitness at a cost to the altruist’s own fitness).
Over the past 30 years studies have been initiated with the aim to understand why cooperative breeding occurs, and by 1991, enough cooperative breeding species have been studied in detail to test (Emlen 1991) and subsequently refine theory (Emlen 1997). The original paradox of cooperative breeding has largely disappeared with the knowledge that offspring assess the relative fitness profitabilities of staying home and helping versus dispersing and breeding. By helping individuals frequently improve their chances of becoming breeders outside their natal territories, and, by helping kin, gain indirect benefits. For example, inducing offspring to remain in their natal territories is positively associated with the proportion of territorial vacancies of lower quality than the natal territory, and with the shortage of both territories and mates. Studies on the Acorn woodpeckers Melanerpes formicivorus (Emlen 1984; Stacey & Ligon 1987), the Superb fairy-wrens Malurus cyaneus (Pruett-Jones & Lewis 1990) and Seychelles warbler Acrocephalus sechellensis (Komdeur 1992) have shown that larger families form when constraints on independent reproduction were larger and that family stability was greatest on the best quality territories. In some avian and mammalian co-operative breeders, a single female in each group produces all of the young, and subordinate fertile females do not breed until they attain a dominant position within the family (reproductive skew = 1). In other co-operative breeding systems a single female produces most of the offspring (skew < 1), or reproduction is equally distributed among all adult females (skew = 0). Similar contrasts occur in the distribution of male reproductive success in groups that include multiple males. If helpers increase their fitness by becoming reproductives themselves within families, a conflict is expected between the dominant and the subordinate of the same sex (Keller & Reeve 1994; Emlen 1995, 1997, 1999). So far, the ‘adaptive’ nature of helping has rarely been answered from the female or male parent’s point of view. As a consequence research on co-operative breeding has shifted to understanding the reasons for differences in reproductive skew within and between co-operative societies (Keller & Reeve 1994, Emlen 1995, 1997, 1999). Models of ‘optimal skew’ were produced (e.g. Emlen & Vehrencamp 1983; Emlen 1984; Reeve 1991; Reeve & Ratnieks 1993; Keller & Reeve 1994), which predicts the conditions under which dominant breeders should yield just enough reproduction to a subordinate to make it favourable for the subordinate to stay in the group and co-operate rather than to leave. The model is valid if: (1) presence of subordinates increases fitness of dominants; (2) subordinates are less likely to emigrate or to challenge the dominant if they are allowed a measure of reproduction; and (3) dominants can control the breeding attempts of subordinates without suffering a cost. Reproductive skew should be high when: (1) the chance of subordinates dispersing and successfully breeding is low; (2) subordinates are closely related to dominants; (3) the subordinate’s contribution to the productivity of the group is high (no need for compensation); and (4), when subordinates are unlikely to challenge the dominant successfully. Recently, this model has been subject of debate (Clutton-Brock 1998; Reeve et al. 1998). In particular, it has been questioned whether the frequency of subordinate breeding (and degree of skew) depends principally on the dominant’s capacity to control reproduction in subordinate. However, not only the dominant but also the subordinate should be able to manipulate control by the dominant. In this paper I will discuss whether the dominant and subordinate do regulate each other’s reproductive success, and if so what are the mechanisms used. Firstly, I will present data on reproductive skew and control by dominants in the Seychelles warbler. Seychelles warblers can breed in pairs and in extended families (co-operative breeding: helpers and plural breeding). Secondly, I will contrast control mechanisms used in cooperatively and polygynous breeding Acrocephalus species of known evolutionary relationships (Fig. 1; Leisler et al. 1997). Thirdly, I will and discuss mechanisms causing reproductive control success or failure.
I. Reproductive skew in the Seychelles warblerUntil 1988, the world population of the endemic Seychelles warbler was entirely confined to Cousin Island (29ha), where it has reached carrying capacity of c 320 birds (Komdeur 1992). The warbler is insectivorous, gleaning insect food from leaves, usually has a one-egg clutch once per year and has high annual adult survival (81.1%, 334 bird-years). The breeding pair remains in the same territory, sometimes for as long as nine years. Both territory quality, measured in terms of insect prey density, and the presence of helpers are important factors affecting the fitness of parents. Although warblers can breed successfully at 1 year of age, daughters often delay reproduction and function as helpers-at-the nest providing nourishment to their parents' offspring. Sons typically disperse. Seychelles warbler mothers adaptively modify the sex of their single egg toward daughters, the helping sex, when living on territories with rich resources where helpers increase parental reproductive success, but toward sons, the dispersing sex, when living on territories where resources are scarce and/or no helping benefits accrue (Komdeur et al. 1997). It seems that parents maximise their inclusive fitness by modifying egg sex ratio (Komdeur 1998). Because offspring can become breeders on their natal territories, it is unknown whether pairs in high-quality territories gain more inclusive fitness benefits from the production of daughters than from sons, and vice versa in low-quality territories. In this section I show experimentally the consequences of having (reproducing) subordinates on low- and high-quality natal territories, and whether dominants control the subordinate’s reproduction in less adaptive situations.
METHODSData collection
In 1995, 40 warbler territories were selected on Cousin comprising either a breeding pair (3 on high quality territory (hqt), 7 on low quality territory (lqt); for territory quality index, see below), a breeding pair with their adult daughter (11 on hqt, 8 on lqt), or a breeding pair with their adult son (5 on hqt; 6 on lqt). All birds were individually colour-ringed. During the breeding season (June-September) these territories were checked weekly for nests and eggs. During nest building and incubation period, nests were checked every day for presence of egg(s) or nestling(s), sometimes with the help of a mirror attached to a stick. For groups with two females the nests were checked for eggs half an hour before sunrise. Females lay their eggs very early in the morning and if only one female was on the nest during egg check and a new egg was recorded, the new egg was always laid by that particular female (Komdeur 1991). This was confirmed by DNA maternity analyses of bloodsamples taken from the putative egg laying females and bloodsamples of the resulting hatchlings from marked eggs (Kappe 1998). Eggs were marked with a waterproof marker immediately after laying.
Observations on agonistic interactions between father, mother and their daughter or son were conducted during two periods: a 4-day period during which copulations take place (immediately after nest completion until egg laying), and a 4-day post-laying period which started after laying of the first egg. Agonistic behaviour was measured when the mother or the father and their son or daughter were simultaneously present within 2.5 m radius from the nest. During both periods, I observed the nest for 1.5 hours from 0600-0730 h. I decided on this period, because during this period egg laying takes place (Komdeur 1991). Agonistic interactions were classified into two categories: dominant (mother of father) chases subordinate (daughter or son), subordinate chases dominant. All eggs of groups with three birds present survived till nestling stage. At each nest, observations on feeding young were made in the second week after hatching for three hours, comprising three 1-hour segments equally spread over the day: 06h30-10h30, 10h30-14h30 and 14h30-18h30. For each half minute it was noted whether a bird was feeding the nestling, and if so, which bird.
Territory quality was expressed as mean insect prey available within a territory, because adult survival and reproductive success correlated positively with this. Territory quality was measured each month during the 1995 breeding season (for method, see Komdeur 1992), and the average territory quality was divided into three classes: low (0-1,500 insects present), medium (1,501-3,000 insects), and high quality (>3,000).
Experimental manipulation of joint-nestingTo determine the fitness consequences for breeding pairs having a joint-nesting daughter (laying her egg in her mother’s nest) on low- and on high-quality territories, I manipulated clutch sizes. In 1996, I added a marked egg to nests which contained one egg only and which were attended by a breeding pair and their helping daughter (5 on lqt, on 8 on lqt). Both eggs were of the same age to synchronise hatching. Warblers readily accept eggs and they provision unrelated nestling at the same rate as their own nestling (Komdeur 1998). Of the unmanipulated control groups (comprising a breeding pair with one helping daughter which all attended a nest with one egg) 5 were on low- and 6 were on high-quality territories. The nests were monitored for hatching success, and for number of nestlings reaching one year of age. Nestlings were individually colour-ringed.
Statistical analysesMeans are expressed with standard deviations. Statistical analyses consisted of non-parametric tests, probability values are two-tailed, and the null hypothesis was rejected at P < 0.05.
RESULTS
Experimental manipulation of clutch-size
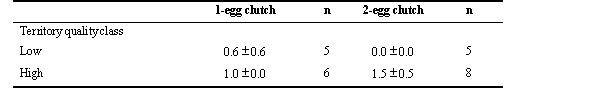
Of the groups which have raised one egg only, the average number of yearlings resulting from this clutch was twice as high for groups on high-quality territories than for groups on low-quality territories (Table 1). After addition of the conspecific egg, all conspecific eggs were accepted and incubated by the mother and daughter together with their single egg. All experimental clutches resulted in ‘twin’ nestling. On low-quality territories, the average number of yearlings resulting from 2-egg clutches was none, and less than that from 1-egg clutches (Table 1). However, on high-quality territories, the average number of yearlings resulting from 2-egg clutches was significantly higher than that from 1-egg clutches (Table 1). The difference between the changes in number of yearlings of the two groups was significant (F(Anova) = 10.93, df = 2, P = 0.004, n = 24).
Reproductive strategies
Of the 36 breeding groups monitored in 1995, 30 groups produced a nest with eggs. There was no bias in breeding failure between groups of different compositions (% breeding failure: breeding pairs: 20.0%, n = 10; breeding pairs with daughter: 10.5%, n = 19; breeding pairs with son: 9.1%, n = 11). Breeding pairs with a daughter present produced significantly larger clutches than a single breeding pair (Z = 2.98, n = 25, p = 0.003) or a breeding pair with a son present (Z = 3.24, n = 27, p = 0.001) (Table 2). This is because the mother and sometimes the daughter lay an egg in the same nest. The frequency of joint-nesting by the daughter on low- and on high-quality territories was similar (lqt: 57.1%, n=7; hqt: 60.0, n = 10), and clutch size produced by breeding pairs with a daughter was the same on low- and on high-quality territories (Table 2). Clutch sizes of breeding pairs and breeding pairs with a son helper were single-egg-clutches (Table 2). The dominant female did not lay a larger clutch when more helpers were present, thus adapting her clutch size to potential help with feeding of young. In both low and high quality territories the mother always laid an egg, and in some cases daughters produced an egg (Table 2). In groups with joint-nesting, in 6 cases the mother laid her egg a day before her daughter, and in 4 cases the daughter laid an egg before her mother. Parenthood analyses of nestlings produced in six groups with a mother, a father and their helping son showed that all nestlings were produced by the female and the father; the son never sires offspring with his mother (Kappe 1998). In 11 groups comprising a mother, a father and their ‘helping’ daughter the mother always produced a nestling, whereas in 45.5 % of cased her daughter produced an addition nestling in the same nest. The daughter’s offspring were always sired by the daughter’s father (n=5; Kappe 1998). Egg-dumping by females other than the group females did not occur (Komdeur 1991; Kappe 1998).
Agonistic interactions
The prediction based on the results obtained from the clutch manipulation experiments is that on low-quality territories mothers should prevent their daughters from becoming joint-nesters, and hence be more aggressive toward potential helping daughters as to potential helping sons. However, no agonistic interactions were ever observed between the breeding pair and their offspring of either sex on low- and high quality territories during pre-laying and incubation period. On low- and high- quality territories when the mother or father, and their daughter were present within a radius of 2.5 m from the nest, I have never observed any agonistic interactions between either sex of the breeding pair and their daughter (number of observations (n) on low quality territory when mother - daughter were simultaneously near the nest: n = 320; father - daughter: n = 49; on high-quality territories: n = 440; father -daughter: n = 110). The same is true for breeding pairs and their ‘helping’ son (number of observations (n) on low quality territory when mother - son were simultaneously near the nest: n = 48; father - son: n = 18; on high-quality territories: mother-son: n = 58; father -son: n = 19).
Reproductive skew without suppression?
The advantage for a daughter staying with her parents is attaining breeding status, thereby increasing the benefits of philopatry by reducing the length of time that elapsed before she could reproduce. Both the clutch manipulation experiments and the data available showed that on high-quality territories both the mother and daughter gain fitness benefits of joint-nesting, whereas no benefits of joint nesting was accrued to the mother and daughter on low quality territories. Inbreeding avoidance does not occur and in general no difference in reproductive success was observed between incestuous breeding pairs and non-incestuous breeding pairs (Komdeur et al. 1998).
Compared with breeding in pairs, the dominant female breeder gained significantly higher inclusive fitness benefits from the activities of a same-sex subordinate in high-quality territories on the one hand (Z = 2.37, n = 13, p = 0.018), but not in low-quality territories (Z 2.27, n = 12, p = 0.024) (Table 2). On low-quality territories, the reproductive output of the group is smaller if the daughter stays than when she leaves. On low-quality territories the dominant gain no benefit by retaining her daughter and, therefore, the dominant female breeder does not need to concede some reproduction to the daughter as an incentive for the daughter to remain in the group. Given that the probability of daughters on both low and high-quality territories nest jointly with their mothers is equal, and that joint-nesting on low-quality is not adaptive, it is clear that in the Seychelles warbler mothers have no control over their daughters reproduction: (1) mothers do not prevent the daughter to lay; (2) mothers do not rejecting the egg laid by the daughter through nest desertion or egg ejection; the latter could have occurred in at least 4 cases during which the mother laid her egg in a nest containing her daughter’s egg; and (3), the mother does not anticipate reproduction by her daughter and does not respond by adjusting her own brood size, which could be adaptive on low-quality territories. If more female offspring are produced, one can expect that the probability of joint-nesting and intraspecific brood-parasitism will increase too, which is of disadvantage to the breeding pair on low-quality territories. Inbreeding avoidance has been considered to be a theoretical framework for understanding reproductive skew (Emlen 1996, Clutton-Brock 1998). In the Seychelles warbler inbreeding has no deleterious effect on offspring survival (Komdeur 1998), and hence the lack of inbreeding avoidance may explain the lack of direct reproductive suppression by the dominant. However, it seems that mothers ‘foresee’ and pre-control the negative effects of joint-nesting on low quality territories by producing male eggs (77.2%, n = 57), and by producing female eggs on high quality territories (87.5%, n = 32; Komdeur et al. 1997). Taking into account the adaptive egg sex modification by warbler females, it is clear that the reproductive strategies (breeding system and egg sex ratio) of the Seychelles warbler obeys the optimal skew theory (Emlen 1999): (1) dominant female on high-quality territories benefit from the activities of a female subordinate on high-quality territories and produce female eggs. (2) dominant females on low-quality territories benefit of a male subordinate and producing male eggs. In the next section I focus on the presence and aspects of reproductive control by dominant and subordinate birds in related co-operatively and polygynously breeding Acrocephalus species.
II. Variability of reproductive control within and among co-operatively and polygynously breeding Acrocephalus speciesOf the 27 Acrocephalus species of known evolutionary relationships (Leisler et al. 1997), five species are known to be facultative co-operative breeders and one species to be facultative polygynous.
Reproductive control in co-operatively breeding warblers
In a study on the moustached warbler Acrocephalus melanopogon (Fessl et al. 1996) intrusions at foreign nests are strongly-male biased. Some of these intruding males, which are unrelated to the pair male were observed incubating, feeding and defending the unrelated nestling. Helping behaviour occurred mostly in the presence of females (helping males can be ‘seen’) and aggressive encounters between intruders and pair females were not observed. This additional care was of positive effect on nestling success. In subsequent second broods, mate switching was more observed at nests with extra male care in contrast to nests where extra males did not help. I think that this case is an example of incomplete skew over a time frame: (1) the dominant male has no control over intrusive subordinate; and (2), dominant and subordinate male compete with one another to increase their respective reproductive shares.
In the black-browed reed warbler Acrocephalus bistrigiceps, which is highly related to the moustached warbler (Fig. 1), co-operative joint-nesting is not beneficial to the dominant female, but dominant females, however, allow subordinate females to lay in the same nest (Hamao & Ueda 1998). Because joint-nesting in this species is very uncommon, the selection pressure to control reproduction of subordinates has not been strong enough.
In the Henderson reed warbler Acrocephalus vaughani taiti, confined to eponymous Pacific island, a large fraction of the population breeds in trios (36%), consisting either two males and one female, or two females and one male. Trios contain unrelated birds and shared paternity and maternity is common. In contrast to the ecologically-similar Seychelles warbler, a young warble gain less from helping first order relatives than from dispersing and breeding elsewhere in either a pair or a trio. After joining a breeding pair reproductive control by dominants has not been observed. There does not seem to be a need for control, because the output of offspring per adult breeding in trios and in pairs is similar (Brooke & Hartley 1995).
On the basis of the Seychelles warbler data, I would expect converse control in co-operatively breeding warblers that exhibit female-biased dispersal and male philopatry, like the African reed warbler Acrocephalus baeticatus, with female biased dispersal and male biased helping (32% of nests; C. Eising, J. Komdeur, J. Buys & M. Reemer, unpublished manuscript). Because this is the only known Acrocephalus species with male helping, it would be worthwhile to further investigate the reproductive system, parenthood of nestlings, and the existence of reproductive control in the African reed warbler.
In the Australian warbler Acrocephalus australis joint-nesting by females is extremely rare (4.2%, n = 24; Berg 1998). In the case with joint-nesting the females were unrelated. Intra-specific brood parasitism through removal of the dominant’s egg and subsequent laying of the subordinate’s egg cause an initial expected reduction of 36% of the dominant’s clutch. Intra-specific brood parasitism through addition of an egg to the dominant’s clutch without removal reduced the hatching success of the dominant’s clutch, presumably due to inefficient incubation of the enlarged clutch. Experimental manipulation of clutches showed that dominants are able to avoid intra-specific brood parasitism through highly evolved egg discriminative abilities. Conspecific eggs placed into nests during egg laying were rejected in 52.6% of cases (n=19) (Welbergen et al. 1998). Egg-discrimination behaviour in the Australian warbler seems to have evolved to minimise the costs of parasitism. However, it is interesting that in the closely related Seychelles warbler dominant females breeding with joint-nesting daughters on low-quality territories have not evolved egg discrimination abilities.
Reproductive control in polygynously breeding great reed warblers
In the polygynously breeding great reed warbler Acrocephalus arundinaceus in Sweden and Japan polygynous males feed their young produced by their second females, less frequent than their young produced by their dominant female (Bensch 1995, and Urano 1990, respectively). In both populations there is a significant difference in reproductive control by females. In the Swedish population females that chose mated males were far less successful than primary females or monogamous females, because of little or no male assistance. Both primary and monogamous females were assisted by their males equally in raising their nestlings and hence have similar reproductive success (Bensch 1995). Secondary females that initially chose mated males but which achieved a primary position after failure of the originally primary female’s nest enjoyed an increased fledging success, because of more male assistance (Bensch 1996). Based on these findings there is no need for the primary female to control reproduction of the secondary female, but a need for the secondary female to control reproduction of the primary female. The secondary female has developed two strategies to produce successfully. Firstly, in order to gain breeding status, secondary females often settle when primary females were in the laying or incubation stage rather than in the pre-laying stage. Aggressions between an already-settled female and a new female were frequently observed during a period just after the settlement of the new female, but eventually aggressions subdued because the primary female’s nest attendance (Bensch 1996). Secondly, in order to gain male parental care and higher reproductive success, secondary females often destroy eggs of primary females (Hansson et al. 1997). A possible strategy to reduce infanticide by secondary females could be the production of more sons by primary females, in order to lower the chance of becoming a polygynously breeding dominant female in the next year. However, the sex of nestlings produced by primary females did not differ from unity (Westerdahl et al. 1997). It is unknown whether maternal feeding frequency is biased towards daughters as compared to sons.
In the Japanese great reed warbler population the lowered paternal feeding frequency to broods of secondary females did not increase the mortality by starvation. This was because the warm climate emancipated secondary females from brooding and enabled them to compensate for the deficiency of feeding by males. Secondary females do not need to control paternal and infanticide of primary female’s eggs by secondary females has never been observed (Urano 1990).
III. Conclusions and future work
It is clear that within and among related co-operatively and polygynously breeding Acrocephalus species there is a substantial variation in the dominant’s control of reproductive success of the subordinate, and vise versa. In the Seychelles warbler dominant females are unable to discriminating her own eggs from that produced by subordinates whereas in the highly related Australian warbler dominant females regulate joint-nesting by the subordinate females by rejecting the subordinate’s eggs. Even between populations of the same species there is great variation in the presence of reproductive control. If secondary females of the great reed warbler need paternal care, infanticide of primary female’s egg occurs, whereas if parental is not required, then reproductive control by the subordinate is absent.
In addition, if reproductive control occurs, the mechanisms used by individuals to regulate reproductive success of the other group individual of same sex varies. We have seen that in Acrocephalus species, control can occur through egg recognition and rejection in joint-nesting species, through infanticide of eggs in polygynously breeding species, through settling within a group when the primary female is on eggs and has to attend her nest rather than challenging the subordinate, and through adaptive modification of egg sex ratios by dominant females. One has to be careful in not concluding to quickly that reproductive control in a certain species is absent. For example at first glance Seychelles warbler females do not show aggression toward competing joint-nesting subordinate females and thus did not seem to control subordinate’s reproduction. However, a long-term study revealed that dominant females produce male eggs, in order to avoid having joint-nesting daughters in the future.
As explained by Emlen (1996, 1999) nuclear family (singular breeding) species are poor candidates for testing skew theory. Tests of the different skew models are easier in plural breeding (extended family) species (Emlen 1999), and in non co-operative, polygynously or polyandrously breeding species. The potential for variation in skew in such species is large, because there often is more than one breeder of one or both sexes, and there is enlarged variation in relatedness between individuals (Emlen 1999). Although I have analysed the reproductive skew present in one population of the Seychelles warbler, it is worth to examine variation in skew values among different populations. After translocation of warblers to Aride island, not co-operative breeding but polygynously breeding and two-egg clutches produced by single females were the norm. Polygynously breeding and two-egg clutches produced by a single female on Cousin island have rarely been observed. Given the high survival on Aride, group structures are extremely complex and ideal to study skew theory.
Given that females control fertilisation of her eggs, I expect that for a dominant female it is easier to control reproductive success of subordinate males than that of subordinate females (joint-nesting). Hence, the reproductive skew model could be an explanation for the fact that in most co-operative breeding species most males stay and help and females disperse. Worthwhile would be to compare control mechanisms in hole-nesting and open-nesting cooperatively breeding species. I expect that for hole-nesting species egg discrimination by visual cues would be more difficult than for open-nesting species. If control is required, I expect relatively more male helpers in hole-nesting than in open-nesting species.
In general I expect that birds are more likely to violate the assumptions of the model than mammals. Mammals seem to have a larger scope to regulate the subordinate’s reproductive success than birds. For example as compared with birds, mammals have long gestation periods, a noticeable pregnancy, a defined timing of conception, and lactation. It would be worthwhile to compare control mechanisms between mammals and birds.
REFERENCES
Bensch, S. 1995. Annual variation in the cost of polygyny: a ten year study of great reed warblers Acrocephalus arundinaceus, Japanese Journal of Ornithology 44: 143-155.
Bensch, S. 1996. Female mating status and reproductive success in the great reed warbler: is there a potential cost of polygyny that requires compensation? Journal of Animal Ecology 65: 283-296.
Berg, M.L. 1998. Offspring sex and parental care in the Australian warbler. BSc thesis. Melbourne University, Melbourne, Australia.
Brooke, M. de & Hartley, I.R. 1995. Nestling Henderson reed warbler (Acrocephalus vaughani taiti) studied by DNA fingerprinting: unrelated coalitions in a stable habitat? Auk 112: 77-86.
Clutton-Brock, T.H. 1998. Reproductive skew, concessions and limited control. Trends in Ecology and Evolution 13: 288-292.
Emlen, S.T. 1984. Co-operative breeding in birds and mammals. In: Krebs, J.R. & Davies, N.B. (eds) Behavioural ecology: an evolutionary approach, 2nd ed.: 305-335. Oxford; Blackwell.
Emlen, S.T. 1991. The evolution of co-operative breeding in birds and mammals. In: Krebs, J.R. & Davies, N.B. (eds) Behavioural ecology: an evolutionary approach, 3rd ed.: 301-337. Oxford; Blackwell.
Emlen, S.T. 1995. An evolutionary theory of the family. Proceedings National Academic Sciences 92: 8092-8099.
Emlen, S.T. 1996. Reproductive sharing in different types of kin associations. American Naturalist 148: 756-763.
Emlen, S.T. 1997. Predicting family dynamics in social vertebrates. In: Krebs, J.R. & Davies, N.B. (eds) Behavioural ecology: an evolutionary approach, 4th ed.: 228-253. Oxford; Blackwell.
Emlen, S.T. 1999. Reproductive skew in cooperatively breeding birds: an overview of issues. In: Adams, N. & Slotow, R. (eds) Proc. 22 Int. Ornithol. Congr., Durban, University of Natal.
Emlen, S.T. & Vehrencamp, S.L. 1983. Co-operative breeding strategies among birds. In: Brush, A.H. & Clark, G.A. (eds) Perspectives in ornithology: 93-133. Cambridge; Cambridge University Press.
Fessl, B., Kleindorfer, S. & Hoi, H. 1996. Extra male parental behaviour: evidence for an alternative mating strategy in the moustached warbler Acrocephalus melanopogon. Journal of Avian Biology 27: 88-91.
Hamao, S. & Ueda, K. 1998. Nest sharing by polygynously mated females in the black-browed reed warbler Acrocephalus bistrigiceps. Ibis 140: 176-178.
Hansson, B., Bensch, S. & Hasselquist, D. 1997. Infanticide in great reed warblers: secondary females destroy eggs of primary females. Animal Behaviour 54: 297-304.
Kappe, A. 1998. Detecting genetic variation; application of molecular techniques in conservation biology. PhD Thesis, State university of Groningen, Groningen, The Netherlands.
Keller, L. & Reeve, H.K. 1994. Partitioning of reproduction in animal societies. Trends in Ecology and Evolution 9: 98-192.
Komdeur, J 1991. Co-operative breeding in the Seychelles warbler. PhD Thesis, Cambridge University, Cambridge, England.
Komdeur, J 1992. Importance of habitat saturation and territory quality for evolution of co-operative breeding in the Seychelles warbler. Nature 358: 493-495.
Komdeur, J. 1998. Long-term fitness benefits of egg sex modification by the Seychelles warbler. Ecology Letters (in press).
Komdeur, J., Daan, S., Tinbergen, J. & Mateman, C. 1997. Extreme adaptive modification in sex ratio of the Seychelles warbler’s eggs. Nature 385: 522-525.
Komdeur, J., Kappe, A. & Zande, L. van de 1998. Influence of population isolation on genetic variation and demography in Seychelles warblers; a field experiment. Animal Conservation (in press).
Leisler, B., Heidrich, P, Schulze-Hagen, K. & Wink, M. 1997. Taxonomy and phylogeny of reed warblers (genus Acrocephalus) based on mtDNA sequences and morphology. Journal of Ornithology 138: 469-496.
Pruett-Jones, S.G. & Lewis, M.J. 1990. Habitat limitation and sex ratio promotes delayed dispersal in superb fairy-wrens. Nature 348: 541-542.
Reeve, H.K. 1991. Polistes. In: Ross, K.G. & Matthews, R.W. (eds) The social biology of wasps: 99-148. Ithaca, New York; Cornell University Press.
Reeve, H.K. & Ratnieks, F.L.W. 1993. Queen-queen conflicts in polygynous societies: mutual tolerance and reproductive skew. In: Keller, L. (ed.) Queen number and sociality in insects: 45-85. Oxford: Oxford University Press.
Reeve, H.K., Emlen, S.T. & Keller, L. 1998. Reproductive sharing in animal societies: reproductive incentives or incomplete control by dominant breeders? Behavioural Ecology 9: 267-278.
Stacey, P. & Ligon, J.D. 1987. Territory quality and dispersal options in the acorn woodpecker, and a challenge to the habitat-saturation model of co-operative breeding. American Naturalist 130: 654-676.
Urano, E. 1990. Inta-sexual relationships among polygynously mated female great reed warblers Acrocephalus arundinaceus. Japanese Journal of Ornithology 38: 109-118.
Vehrencamp, S.L. 1979. The roles of individual, kin, and group selection in the evolution of sociality. In: Merler, P. & Vandenbergh, J.H. (eds) Handbook of behavioural neurobiology. Vol. 3. Social behavior and communication: 351-394. New York; Plenum Press.
Welbergen, J. 1998. Intricate adaptations against intra- and interspecific brood parasitism in the Australian warbler, Acrocephalus australis. BSc thesis. Melbourne University, Melbourne, Australia.
Westerdahl, H., Bensch, S., Hansson, B., Hasselquist, D. & Von Schantz, T. 1997. Sex ratio variation among broods of great reed warbler Acrocephalus arundinaceus. Molecular Ecology 6: 543-548.
Table 1. Mean annual number of yearlings (± S.D.) resulting from 1-egg clutches and 2-egg clutches (experimentally enlarged 1-egg clutches through egg addition) on low- and high-quality territories (1996-1997). All nests were tended by Seychelles warbler groups comprising a breeding pair and their daughter. n = number of groups. The difference between the changes in number of yearlings of the two groups was significant (F(Anova) = 10.93, df = 2, P = 0.004, n = 24).

Table 2. Mean annual number of yearlings (± S.D.) produced by Seychelles warbler breeding pairs, breeding pairs with their helping son, and breeding pairs with their ‘helping’ daugher on low- and high-quality territories (1995-1996). n = number of groups; r = coefficient of relatedness between offspring and breeding pair, inclusive fitness mother = fitness mother + 0.5 * fitness of her daughter.
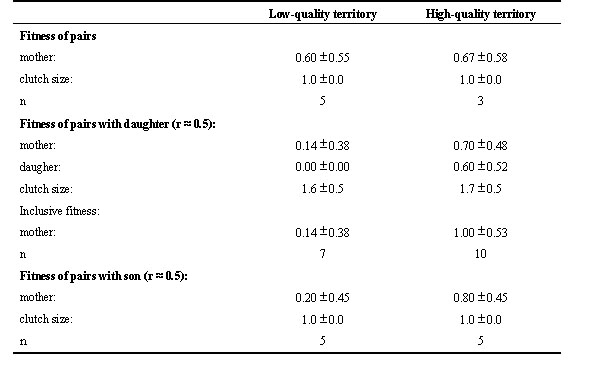
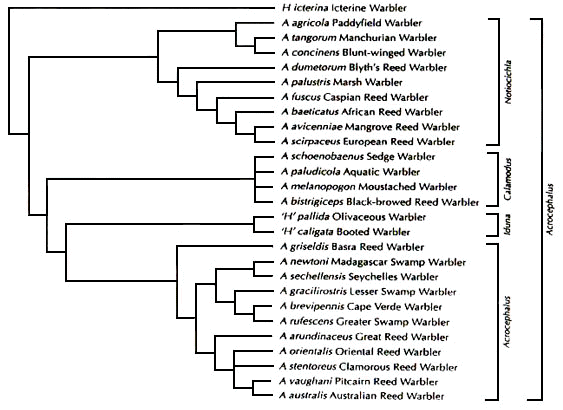
Fig. 1. Evolutionary relationships among several Acrocephalus warblers as inferred from DNA-sequences. Groupings on the right indicate broad and narrow genus limits (adapted from Leisler et al. 1997).