S47.3: Reproductive skew models and inter-species variation in adjustment of individual clutch sizes in joint-nesting birds
Ian G. Jamieson
Department of Zoology, University of Otago, PO Box 56, Dunedin, New Zealand, fax 64 3 479 7608, e-mail ian.jamieson@stonebow.otago.ac.nz
Jamieson, I.G. 1999. Reproductive skew models and inter-species variation in adjustment of individual clutch sizes in joint-nesting birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2894-2909. Johannesburg: BirdLife South Africa.In Part I of this paper I compare relative contributions to communal clutches by joint-nesting females, as a measure of reproductive skew, across four species / five populations and found support for both Optimal Skew Models (OSM) and Incomplete Control Models (ICM). This draws into question the generality of these two competing models. However, comparing skew values that do not differ significantly from each other and are from species that vary substantially in their egg-laying behaviour and ecology is dubious at best. Within species comparisons of reproductive skew values is a more sensible approach but quality data in this regard are lacking. In Part II, I focus on how skew is generated in the first place. I ask whether related co-breeding females are more sensitive than unrelated co-breeding females to the inclusive fitness costs of incubating an enlarged clutch and thus are more likely to adjust their clutch sizes down relative to the presumed optimal clutch size of single nesting females. Two advantages of this approach are that all joint female groups can be compared to single female groups within the same population, and that it focuses on the same mechanism (i.e. clutch adjustment) across a variety of communal species. Related co-nesting females do have a greater tendency to reduce the size of their individual clutches compared to unrelated co-nesters, suggesting related females are more affected by the inclusive fitness costs of having enlarged clutches/broods. However, other species-specific factors such as eggshell thickness may also be important in affecting the costs of enlarged joint clutches.
GENERAL INTRODUCTION
Vehrencamp (1980, 1983a, b) and Emlen (1982a) were the first to examine how direct reproduction is shared among members of cooperatively breeding groups of birds, what has been termed ‘reproductive skew’. Work on reproductive skew has expanded to include social insects (Keller & Vargo 1993; Reeve & Ratnieks 1993), and the resulting reproductive skew models have been proclaimed to provide a general framework for investigating factors shaping animal societies (Keller & Reeve 1994; Emlen 1995, 1996).
The first reproductive skew models attempted to explain the degree of skew within social groups based on the cost and benefits to the dominant breeder of a subordinate of the same sex breeding within its group. Thus the basic assumption of these optimal skew models (OSM) is that dominants control the reproduction of subordinates and low skew results from the dominant yielding a greater share of reproduction to an unrelated subordinate (Keller & Reeve 1994; Emlen 1995; Clutton-Brock 1998). Recently, incomplete control models (ICM) have been developed as alternatives to the original OSM. ICM assume that low skew and reproductive sharing are a consequence of the dominant's inability to control the reproduction of the subordinate who, in turn, competes directly with the dominant for a share of its group's reproductive output (Reeve et al. 1998; Emlen 1999). A review of the literature using both interspecific and intraspecific comparisons of cooperatively breeding birds showed more support for OSM than ICM although some data were inconclusive (i.e. were consistent with both OSM and ICM) and thus there was a plea for further studies (Reeve et al. 1998).
Unfortunately, there has been little attempt to compare a single measure of reproductive skew across species of cooperatively breeding birds. For example, in Reeve et al.'s nine pair-wise comparisons of reproductive skew and relatedness (their table 2), nine different measures of skew were used. For females, these ranged from quantitative measures (e.g. % of eggs laid by the dominant breeder; % of reproductive events in groups with > 1 female in which the dominant female is breeder), to qualitative measures (e.g. high versus low). If we wish to quantitatively test reproductive skew models, then a single measure needs to be agreed upon so researchers can go out and measure the same variables in the field.
For a variety of reasons, the best data for testing reproductive skew models in birds will come from plural breeding species (see Emlen 1999; Magrath 1999). Furthermore, measuring reproductive skew is inherently easier in co-breeding females that lay eggs in a single nest than co-breeding males that copulate to fertilise the eggs of one or more females. In addition, a subordinate male's ability to participate directly in reproduction is not only influenced by the dominant male but by receptivity of the breeding female(s) (see Magrath 1999). Obtaining access to the opposite sex is, in theory, less of a problem for the subordinate female and therefore her reproductive output is more likely to be affected by competition with the dominant female for access to a nest. For these reasons I confine my analysis below to joint nesting species and I use the proportion of eggs that two females contribute to an incubated joint clutch as a measure of reproductive skew. The proportion of eggs that two females lay, as opposed to the proportion of eggs that hatch or the proportion of offspring that fledge, is assumed to be a more direct measure of reproductive skew in joint nesting females because it is less likely to be affected by factors outside the females' immediate control (e.g. infertility, accidental breakage, predation, starvation, etc.).
In this paper, I focus on interspecific variation in reproductive skew in joint nesting birds from two different approaches. In Part I of the paper, I examine the predictions derived from Optimal Skew versus Incomplete Control Models with respect to genetic relatedness of co-breeding females. In Part II, I focus on how skew in reproduction between two females sharing the same nest is generated in the first place and ask whether females adjust their clutch sizes in directions predicted by inclusive fitness models.
PART 1: OPTIMAL SKEW VERSUS INCOMPLETE CONTROL MODELS IN JOINT NESTING BIRDS
INTRODUCTION
Two of the main parameters that are said to affect skew are ecological constraints on independent breeding and relatedness between the dominant and subordinate breeder (Keller & Reeve 1994; Reeve et al. 1998). In OSM, reproductive skew is predicted to be high between related co-breeders because a dominant can monopolise a greater share of breeding by taking advantage of strong ecological constraints on natal dispersal and asserting its reproductive dominance over younger kin-subordinates. Subordinates tolerate this situation because of gains in inclusive fitness. On the other hand, skew is expected to be low among unrelated co-breeders because the dominant breeders needs to yield a greater share of reproduction to an unrelated subordinate to obtain its assistance when ecological constraints on independent breeding are weak. This assumes there is some overall advantage to group breeding under these conditions (Vehrencamp 1983b; Emlen 1996).
In ICM, skew is also predicted to be low when co-breeders are unrelated because both dominants and subordinates should compete equally for a share of reproduction (Reeve et al. 1998). When co-breeders are related and relatedness is symmetrical (i.e. each co-breeder is equally related to each other's offspring such as is the case for siblings), then skew is expected to remain low since both the dominant and subordinate will exert decreasing effort in aggressive competition to enhance their share of reproduction. However, if relatedness is asymmetrical (i.e. co-breeders are not equally related to each other's offspring as when a mother and a daughter co-breed), then skew is expected to be high because the dominant should exert more competitive effort than the subordinate. In the latter case, ICM makes the same prediction as OSM (Reeve et al. 1998).
The strongest tests of reproductive skew models will come from intraspecific comparisons of skew. However, as I will show, the number of joint nesting species that vary intraspecifically with respect to the parameters of the model is few. Therefore the main objective of Part I of this paper is to examine the predictions of OSM versus ICM by comparing a single measure of female reproductive skew across several species of joint nesting birds.
Study species and methods of analysis
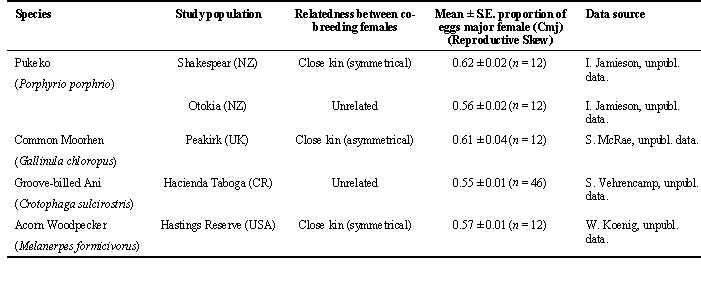
There are four species of cooperative breeders; Pukeko or Purple Swamphen Porphyrio porphyrio, Common Moorhen Gallinula chloropus, Groove-billed Ani Crotophaga sulcirostris and Acorn Woodpeckers Melanerpes formicivorus, in which joint-nesting by females is not uncommon and which are sufficiently well studied to provide data on clutch sizes of individual females laying in joint nests (as an estimate of reproductive skew). This part of the paper analyses reproductive skew data across five populations of these four species of joint-nesting birds that differ in terms of whether or joint females are related (see Table 1).
The first egg laid in joint nests in Common Moorhen is known to sometimes disappear and no further eggs are laid until 6 days later, on average, when both females lay in a new nest, usually on the same day (McRae 1996). Only the number of eggs laid in these second nests are analysed in Table 1. However, Groove-billed Anis and Acorn Woodpecker females that lay second in a joint nest are known to regularly remove eggs belonging to the first-laying female before they initiate laying themselves in the same nest. First-laying females are also known to lay extra eggs to compensate for those that are removed (Vehrencamp et al. 1986; Koenig et al. 1995). For these two species, the estimate of reproductive skew is based on the final incubated clutch only (as it is in the other joint-nesting species), since the size of the incubated clutch directly relates to the number of offspring that each female will produce. The effect of egg removal on the fitness of both the actor and the recipient in these species is discussed in detail elsewhere (Vehrencamp et al. 1986; Koenig et al. 1995).
The researchers associated with each of the above species provided unpublished data on female clutch sizes. Groups consisting of two laying females only are analysed. Completed clutches and first clutches of the season are used unless the first clutch was incomplete. If data for more than one season for a single female or a pair of co-breeding females were available, then the largest of the clutches is used to give maximum estimates. This rule was followed except for co-breeding Acorn Woodpeckers where data were analysed by group-years because the order in which females laid and the pattern of egg removal varied from year to year (Koenig et al. 1995).
Eggs in communal clutches were assigned to females by a variety of methods (see Vehrencamp et al. 1986; Koenig et al. 1995; McRae 1996; Jamieson 1997). For the purposes of analysing reproductive skew, each female in a co-breeding unit was placed into one of two categories. Pukeko in the Shakespear population are the only communal breeder where dominance status of females is relatively easy to assess and co-breeders were categorised as alpha or beta females (Jamieson & Craig 1987). For joint nesting female Pukeko at Otokia, dominance relationships between some females were known in some groups but which eggs came from which females was less certain. Therefore eggs in communal clutches, which were marked as they were laid, were divided into two groups based on differences in background colour, spot pattern, size and shape (Jamieson 1997). The two groups were categorised as either major (clutch with larger number of eggs) or minor (clutch with fewer or equal number of eggs) based on relative differences in clutch size. Joint-nesting females in Common Moorhens are normally mother - daughter combinations but they do not display dominance interactions; females were thus categorised as senior or junior based on age (McRae 1996). Co-breeding female Grooved-billed Anis and Acorn Woodpeckers were categorised as first- or second-laying females based on the order of egg laying in the communal clutch (Vehrencamp et al. 1986; Koenig et al. 1995). For simplicity, females that were expected to have the greater proportion of eggs in a joint clutch (i.e. alpha female for Pukeko; senior female for Common Moorhens; second-laying female for Grooved-billed Anis and Acorn Woodpeckers) are referred to as 'major' (Cmj) and females expected to have the smaller proportion of eggs referred to as 'minor' (Cmn).
RESULTS AND DISCUSSION
The most effective means of testing reproductive skew models is to restrict comparisons of skew to within species, as in the case of Pukeko (see also Jamieson 1997). Reproductive skew, as measured by the average proportion of eggs in the incubated communal clutch belonging to the major female, was significantly higher in the population where co-breeding females were related (i.e. Shakespear) than where females were unrelated (i.e. Otokia) (Mann Whitney test, W = 193.0, P < 0.05; Table 1). Related major and minor females consist of a range of combinations including mother-daughter, siblings and half-sibling pairings which mate promiscuously with closely related males (Craig & Jamieson 1988, 1990), and thus relatedness is likely to be symmetrical (see Reeve et al. 1998). Therefore the increase in skew with the increase in relatedness between the two populations supports the OSM and not the ICM. Further support for OSM is indicated by the observation of greater levels of aggression and dominance assertion between related co-breeders (Jamieson 1997).
However, major females in the Otokia population do not appear to benefit from the presence of unrelated co-breeding females and therefore a basic premise of the OSM is violated. Comparisons of lifetime fitness where the number of care-giving males within a group are controlled for indicate that monogamous females produce more young than polygynous females and polyandrous females produce more young than polygynandrous females on a per capita basis (Jamieson & Quinn, unpubl. MS). Although total reproductive output is slightly higher in groups with two breeding females than in groups with one female (i.e. k > 1) and the minor female may be better off co-breeding than breeding singly on a low quality territory or not breeding at all, there is no evidence that the major female benefits from the association. Joint female nests suffer from higher egg loss through accidental breakage than single female nests, and since males do a substantial part of the incubation and chick care, a major female is always better off to have an additional male join her group than an additional (unrelated) female. Resident females attempt to prevent non-resident females from settling on their territory, but once the latter gains access to the nest and lays her eggs all aggression ceases. These observations suggest that the initiation of nest sharing between females result from the inability of the major female to prevent the minor female from laying in her nest. Once the minor female establishes herself, the major may make reproductive concessions so that the minor will remain and help raise the joint clutch. However, there is no behavioural evidence that females can control the reproductive output of the other female, and thus it is difficult to envisage that any 'concession' could potentially occur (Jamieson & Quinn, unpubl. MS).
Even when a species such as the Pukeko nicely fits the criteria for distinguishing OSM from ICM as outlined by Emlen (1999), strong support for one model over the other is not forthcoming. Furthermore, similar data on intraspecific variation in reproductive skew with respect to relatedness is generally lacking in other joint nesting species. Reeve et al. (1998) citing McRae (1996) reported that in Common Moorhens, related female co-breeders had significantly higher skew than unrelated female co-breeders. However, McRae (1996) cautioned that the sample size for unrelated females in the population was small (n = 3) and that one of the unrelated females was adopted and may have been treated as a relative by the major female. For these reasons I did not include the groups with unrelated co-breeders in a separate analysis. It would be interesting to examine a Common Moorhen population where ecological constraints were low (i.e. natal dispersal was high) and compare the degree of skew between unrelated joint-nesting females. The next best comparison would be between Common Moorhens and Dusky Moorhens (Gallinula tenebrosa) which are similar in size and breeding habits but co-breeding females in Dusky Moorhens are thought to be unrelated (Garrett 1980). A pilot study of a small population in Brisbane, Australia found that groups comprised 2 to 3 co-breeding females, joint clutches were large (15 - 18 eggs), and skew relatively low (nest 1 = 8 + 7 eggs; nest 2 = 7 + 6 + 5 eggs; nest 3 = 7 + 6 + 3 eggs). Egg removal before laying was also detected (Jamieson, unpubl. data).
Acorn Woodpeckers are one of the few cooperative breeders other than Pukeko to show inter-population variation in relatedness of co-breeders. However, in the population where group members are generally unrelated, only males co-breed (Koenig & Stacey 1990). Co-breeding groups consisting of unrelated females are rare (two cases) in the population where co-breeders are normally related (usually sisters) but there was no significant difference between unrelated and related females in any reproductive parameters, including the number of eggs laid and the number removed (Koenig et al. 1995). Similar intraspecific variation in relatedness in Grooved-billed Anis has not been observed although co-breeding females are not normally related (Vehrencamp 1983b).
Can we draw any conclusions about the validity of OSM versus ICM using interspecific comparisons? Reproductive skew, as measured by the average proportion of eggs in the incubated communal clutch belonging to the major female, ranged from 0.55 to 0.62 across the five species/populations (Table 1). The mean estimates of skew varied significantly among the five groups (Kruskal-Wallis test, H = 14.5, P < 0.01; see Table 1 for sample sizes), but only the ani and the Pukeko population at Shakespear differed significantly from each other in pair-wise comparisons (Non-parametric multiple comparisons test, Q = 3.8, P < 0.05), although the difference between the two Pukeko populations verged on significance (Q = 2.5, P
@ 0.05). Skew values for individual groups ranged from 0.43 to 0.88, but it is clear that the means for all species are closer to the low end of the kew scale (i.e. 0.50) despite co-breeding females being both related and unrelated (Table 1).Skew for related groups would have been much higher if all potential female breeders including non-breeding mature helpers had been used in the estimate of skew. This is because mature helpers in the form of retained offspring are common in cooperative kin groups whereas they are either absent or rare in groups in which unrelated co-breeders form groups (Vehrencamp 1983b; Jamieson 1997). Therefore the differences in reproductive skew between related and unrelated co-breeding females could be accounted for primarily by the presence or absence of helpers which in itself can be readily explained by traditional ecological constraint models (Emlen 1982b; Brown 1987; Koenig et al. 1992). The strongest tests of reproductive skew models will come from comparisons of social groups that differ in terms of relatedness but have similar numbers of effective breeders. That being said, absolute differences in skew between related and unrelated co-breeding females did not vary substantially across cooperative species (Table 1).
Focusing on relative differences in average skew across species indicates support for both OSM and ICM. Related Common Moorhens have relatively high levels of skew similar to related groups of female Pukeko (Table 1). Minor female moorhens are generally daughters of the dominant female and mate incestuously with their father in the group (McRae 1996), indicating that relatedness is likely to be asymmetrical and thus high skew is consistent with the predictions of both models (see Reeve et al. 1998).
Reeve et al. (1998) concluded that several examples of cooperative species (including anis) which have unrelated co-breeders and low reproductive skew support OSM because if ICM were correct, one would also find low skew among co-breeding kin that were symmetrically related such as in the case of co-breeding siblings. Co-breeding Acorn Woodpeckers are generally sisters (Koenig et al. 1995) and their reproductive skew is low relative to related co-breeding female Pukeko and Common Moorhens but similar to unrelated co-breeding Pukeko and Groove-billed Ani (Table 1). Therefore the low skew between co-breeding female woodpeckers tends to support ICM and not OSM. The low skew between unrelated co-breeding anis is consistent with both models and therefore is inconclusive by itself.
Among cooperatively breeding birds including joint-nesters, Reeve et al. (1998) found greater support for the OSM when examining the predictions of skew and genetic relatedness. Our analysis of joint-nesting birds found support for one or the other models, even within the same species, or the data were inconclusive. However, comparing reproductive skew values that do not differ significantly from each other and are from species that vary substantially in their behaviour, ecology and phylogenetic history is dubious at best. Comparisons within rather than across species are a more sensible approach but quality data in this regard is lacking (see above).
An alternative approach to understanding the affects of genetic relatedness on reproductive competition in joint-nesting birds is to hypothesise that related co-breeding females should be more sensitive to the inclusive fitness costs of incubating an enlarged joint clutch than unrelated co-breeding females (as outlined by Cant (1998)). This hypothesis predicts that related co-breeders would be more likely than unrelated co-breeders to adjust their individual clutch sizes down relative to the optimal clutch size of single-nesting females for the species in question. Two clear advantages of this approach are that all joint female groups can be compared to single female groups within the same population, and that it focuses on the same potential mechanism (i.e. clutch adjustment) across a variety of communal species. The second part of the paper examines how exactly the level of skew in clutch ownership comes about in joint nesting species.
PART II: EVIDENCE OF CLUTCH ADJUSTMENT IN JOINT-NESTING BIRDS
INTRODUCTION
Cant (1998) noted an important distinction in reproductive competition between males and females in joint nesting birds. Whereas the net benefits to a subordinate male increase as a linear function of the level of paternity share, the net benefits of breeding for a subordinate female reach an intermediate optimum level (because the direct costs of breeding increase with larger brood size). In attempting to explain the level of reproductive skew that will result when two females lay eggs in the same nest, Cant's (1998) model emphasises the effect that increased brood size in joint nesting females has on inclusive fitness when a dominant cannot prevent a subordinate from laying in its nest. Because of the latter condition, Cant termed this an 'incomplete control model'. This terminology has now been challenged and Cant's model is not thought to be an ICM as defined above but an OSM specifically tailored to joint nesting females (see Emlen 1999). However, Cant's model does provide some novel predictions with respect to reproductive skew and, more importantly, focuses more on the process that produces skew rather than the outcome per se. It is these processes that I want to examine and elaborate on in this section of the paper.
Cant's (1998) model predicts that skew should increase with increasing relatedness between dominant and subordinate breeders but for reasons different than those given in classical OSM. It assumes the dominant 'moves' first and produces its own optimum number of eggs and then the subordinate chooses its best option given the dominant's decision. By adding eggs to a communal clutch, the subordinate not only reduces the per capita fitness of the dominant when the total number of eggs goes over the optimal clutch size (because of over crowding), she also reduces her overall inclusive fitness if the dominant is related. The net benefits of producing additional eggs are lower for related versus unrelated subordinates and thus the former should be selected to add fewer eggs to a dominant's nest in related groups, resulting in a higher skew between the two females.
Cant's model is based on the premise that both subordinates and dominants control their own reproductive effort by adjusting the number of eggs they lay with respect to each other. If two females laid in the same nest and there was no egg tossing, two general outcomes are possible. Both dominant and subordinate could lay a clutch the size they normally would have laid if they had nested as a single female (Cs), what is assumed to be the optimum clutch size for the species in Cant's model. Thus the two females produce a joint clutch of size 2Cs indicating that no adjusting of clutch size is occurring; this might be considered as a null hypothesis. Alternatively, one or both joint-nesting females could adjust their individual clutches by either laying fewer eggs than Cs, perhaps to reduce the overall costs of incubating a communal clutch that is over the optimum size (as discussed in Cant's model), or by laying more eggs than Cs, perhaps as a consequence of each trying to out-lay the other to gain a greater proportion of eggs in a communal clutch (Jamieson & Quinn, unpubl. MS).
A second means by which females can adjust communal clutches other than by laying larger or smaller clutches than Cs is by removing the other female's eggs before laying their own. Removal of another female's eggs is thought to have two benefits for the egg remover; (1) it synchronises egg laying between the females and (2) lowers reproductive skew (Vehrencamp 1977, 1983b; Mumme et al. 1988; Koenig et al. 1995). It is interesting to note that the two communal species which regularly remove eggs, Groove-billed Anis and Acorn Woodpeckers, involve unrelated and related co-nesting females, respectively. Both show relatively low reproductive skew, as predicted by both OSM and ICM. Yet Cant's (1998) model further predicts that related co-breeding Acorn Woodpecker females should be more sensitive to the costs of enlarged joint clutches and thus should be more likely to lay a smaller number of eggs once egg ejection has ceased than unrelated co-breeding anis. Thus the objectives of Part II of this paper are to determine individual females in joint-nesting species adjust their clutches with respect to the inclusive fitness costs of incubating a clutch over the optimum size.
Study species and methods of analysis
The same four species that were compared in Part I are also examined for evidence of clutch adjustment. I added a fifth species, Tasmanian Native Hen Gallinula mortierii to the analysis. Although eggs of individual females cannot be distinguished in communal nests of Tasmanian Native Hens (Goldizen et al. unpubl. MS), a comparison of the clutch size distribution of joint nests to those of other joint nesting species might suggest whether clutch adjustment is occurring (see below). The treatment of unpublished data and the categorisation of co-breeding female clutches into major (Cmj) and minor (Cmn) are the same as outlined in Part I. For the two egg-removing species, clutch adjustment is examined with respect to the size of the final incubated clutch and not the total number of eggs laid.
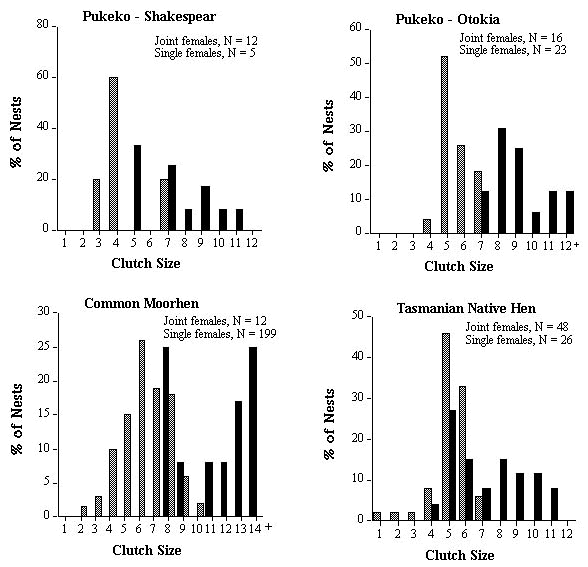
The optimum clutch size for a species could be estimated by the modal clutch size of single nesting females. In practice though, the majority of clutches of single nesting females range over two or more clutch sizes rather than a single modal value. Therefore the clutch size or range of clutch sizes within which at least 60% of the single female clutches fell was termed the 'modal clutch range' (MCRs). I present the percentage of major (Cmj) and minor (Cmn) clutches that fell below and above the expected MCRs (see Table 2). I compare statistically the observed frequency distribution of clutch sizes for Cmj and Cmn to expected frequency distributions, generated from the observed distribution of clutch sizes for Cs (see Fig. 1), using Kolmogorov-Smirnov one sample test (Siegel & Castellan 1988). MCRs was also used to generate an expected modal clutch range for joint clutches (or MCRj). For example, if 60% of females lay a clutch of 4 eggs and 40% lay 5 eggs, we would expect 100% of communal clutches to fall within the range of 8 to 10 eggs if females were not adjusting their clutch sizes when they nested jointly. I present the percentage of joint clutches (Cj) that fell below and above the expected MCRj (Table 2).
RESULTS & DISCUSSION
Reproductive skew is higher in the Shakespear population of Pukeko, where joint nesting females are related, than in the Otokia population where females are unrelated (Table 1). In Shakespear, mean size of joint clutches (Cj) was only 1.7 times as large as mean size of single female clutches (Cs) with the majority of joint clutches (58%) smaller than would be expected if females were not adjusting their individual clutch sizes (Table 2). The frequency distribution of clutch sizes of minor females was significantly different from that of single-nesting females (Fig. 1; Kolmogorov-Smirnov one sample test, Dmax = 0.53, P < 0.01), with 83% of minor females laying clutches smaller than modal clutch size of single females (Table 2). This is the pattern predicted by Cant's model for when the subordinate or minor female is related to the dominant or major female.
In the Otokia population, although mean joint clutches (Cj) were larger than at Shakespear, mean clutch size of single-nesting females (Cs) was also larger and thus the increase in size of joint clutches relative to single female clutches was the same in both populations (1.7, Table 2). As with Shakespear, the frequency distribution of clutch sizes of minor females at Otokia was also significantly different from that of single females (Fig. 1; Kolmogorov-Smirnov one sample test, Dmax = 0.63, P < 0.01), with 66% of minor females laying clutches smaller than the modal clutch size of single females (Table 2). There was no significant difference in the frequency distribution of clutch sizes between major and single females in either populations (Fig. 1; Kolmogorov-Smirnov one sample test, Dmax = 0.22 and 0.29 respectively, both P values > 0.10), suggesting that most major females were not increasing their clutch size relative to optimum clutch size of single females.
However, there are subtle differences between the two populations of Pukeko. For example, a small proportion of minor females (17%) at Otokia laid clutches larger than the MCRs where as no minor females did at Shakespear (Table 2). This can be seen best in the scatter plots shown in Figure 2. Points falling on the line indicate zero skew between the two females. We can see that more points fall on or closer to the line at Otokia than at Shakespear, indicating lower skew. It also shows that when two major females laid exceptionally large clutches at Otokia, the respective unrelated minor females also laid large clutches relative to the modal clutch range of 5 - 6 eggs of single females. By contrast, when four major females at Shakespear laid clutches larger than the modal range of 4 - 5, the minor females never laid more than four eggs, suggesting that minor females were more likely to limit the size of their clutch when nesting with a relative.
Reproductive skew was relatively high in related co-breeding Common Moorhen females and similar to that of the Shakespear population of Pukeko where co-breeders were also related (Table 1), but that is where the similarities end. Joint clutches of moorhens were, on average, almost twice (1.9) the size of single female clutches and, unlike Pukeko, only a minority of these (33%) was of a size smaller than the expected modal clutch range of joint clutches (MCRj) (Table 2). There was no significant difference in the frequency distribution of clutch sizes between major or minor females and that of single females (Fig. 1; Kolmogorov-Smirnov one sample test, Dmax = 0.22 and 0.20 respectively, both P values > 0.10). Minor female moorhens were extremely variable in their clutch sizes (range 1 to 8) compared to major females (range 5 to 9), giving rise to a bimodal distribution in sizes of joint clutches (Fig. 3), a pattern that goes undetected using the conventional approach of simply calculating average skew.
Only 33% of minor female moorhens laid a clutch smaller than MCRs compared to 83% for minor female Pukeko at Shakespear (Table 2). Similarly, 25% of major female Pukeko laid clutches smaller than the MCRs versus 0% of moorhens. While a few minor female moorhens did lay small clutches, others laid clutches as large as the largest of major females, and most clutches within pairs of co-breeders were closely matched (Fig. 2). Thus the majority of minor female Common Moorhens appeared to be less sensitive to the costs of enlarged clutches and less likely to adjust their clutch sizes compared to co-breeding (related) female Pukeko at Shakespear. Daughters that breed with their mothers in Common Moorhens tend to be offspring of late broods from the previous season (McRae 1996). Minor female Pukeko at Shakespear tend to be either daughters, sisters, or half-sisters that remain on their natal territory initially as non-breeding helpers but do not become breeders until three years of age or older (Craig & Jamieson 1988, 1990). Thus age-related differences in clutch size doesn't explain why older (minor) female Pukeko are more likely than younger (minor) moorhens to lay smaller clutches than normal when nesting jointly. It may be that Pukeko are more likely to adjust their clutches because of declining fitness benefits associated with larger joint clutches (Jamieson & Quinn, unpubl. MS).
The joint clutch size of Tasmanian Native Hens is only 1.4 times as large as a single female clutch with 81% of these smaller than the expected modal clutch range of joint clutches (MCRj) (Table 2). This suggests that either the minor female or both the minor and major female are adjusting their clutch size downward. Unfortunately, eggs of individual females can not be distinguished (A. Goldizen, pers. comm.). However, the social organisation of native hens and the distribution of clutch sizes for single versus joint-nesting females are most similar to those of the Shakespear population of Pukeko (Fig. 3) and thus I would hypothesise that minor females are reducing their clutch size resulting in a relatively high reproductive skew. This hypothesis remains to be tested, perhaps by means of molecular techniques to determine maternity of eggs.
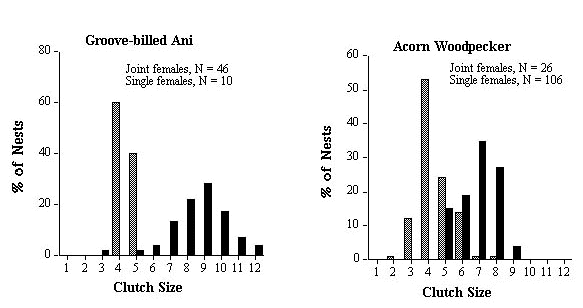
The similarities and differences between the two species that remove eggs (Groove-billed Anis and Acorn Woodpeckers) are even more intriguing. There was no significant difference in reproductive skew between the two species (Mann-Whitney test, W = 1272.5, P = 0.10), even though co-breeding females are related in one but not the other (Table 1). Mean and modal clutch range of single females is identical for both species (Table 2), and both remove similar numbers of eggs. First-laying female anis had 2.1 ± 0.16 eggs destroyed in 93% (n = 46) of nests and second-laying females lost 1.0 ± 0.0 in only 6% of nests. First-laying female woodpeckers lost 2.1 ± 0.37 eggs in 92% (n = 12) of nests while second-laying females lost 1.5 ± 0.5 eggs in 17% of nests.
Despite these similarities in clutch size parameters, anis end up with more eggs in the final joint clutch which was 1.9 times the size of single-female clutches, compared to only 1.6 for Acorn Woodpeckers (Table 2). The difference between the two species in the frequency distribution of final clutch sizes can be seen clearly in Figure 3. The difference is primarily a result of 83% of minor females and 25% major female Acorn Woodpeckers reducing their clutch size relative to the clutch size of single-nesting females compared to only 28% and 7% respectively for Groove-billed Anis (Table 2). The difference between major and minor females in the two species can be seen in (Fig. 2), and again suggests that related joint-nesting females are adjusting their clutch sizes because they are more sensitive to the costs of enlarged clutches than unrelated females.
However, genetic relatedness between co-breeders may not be the only factor affecting clutch sizes. Anis have extremely thick egg shells males are able to maintain higher than normal body temperatures when incubating at night (S. Vehrencamp, pers. Comm.). As a consequence, hatching rates for joint nests involving two or three females are as high as they are for single- nesting females. Hence ani eggs may be better adapted to the crowded conditions of joint nests than Acorn Woodpecker or Pukeko eggs (S. Vehrencamp, pers. comm.). It would be interesting to know whether similar factors and/or smaller egg to body size ratios allow Common and Dusky Moorhens to successfully incubate larger clutches than Pukeko and Tasmanian Native Hens.
In conclusion, there is partial support for Cant's (1998) hypothesis that co-breeding females that are closely related are more sensitive to the fitness costs of sharing a nest than unrelated co-breeders. However, related female Common Moorhens and unrelated Groove-billed Ani show no trend of reducing the size of individual clutches suggesting other species-specific factors may be important in affecting the costs of incubating enlarged joint clutches. More attention to the patterns of clutch adjustment in joint nesting species, rather than the degree of skew itself, and to factors that may make a species more or less adapted to joint nesting is needed to fully understand species differences in reproductive skew.
ACKNOWLEDGMENTS
I would like to thank the Co-convenors of the symposium Steve Emlen and Morne Du Plessis and for inviting me to give a talk. I would also like to thank my cooperative (unrelated) colleagues (Anne Goldizen, Walt Koenig, Sue McRae and Sandy Vehrencamp) for kindly sharing with me unpublished data, without which I would have had nothing to say. John Haselmayer and Sandy Vehrencamp greatly improved an earlier draft of the manuscript. My research and travel to the IOC conference in Durban are funded by the University of Otago. Finally, I am indebted to my partner Frances Anderson for her patience while I wrestled with this manuscript over several weekends, time that could have been spent in front of a warm fire with a bottle of wine.
REFERENCES
Brown, J.L. 1987. Helping and communal breeding in birds. Princeton; Princeton University Press.
Cant, M.A. 1998. A model for the evolution of reproductive skew without reproductive suppression. Animal Behaviour 55: 163-169.
Clutton-Brock, T.H. 1998. Reproductive skew, concessions and limited control. Trends in Evolution & Ecology 13: 288-292.
Craig, J.L. & Jamieson, I.G. 1988. Incestuous mating in a communally breeding bird: a family affair. American Naturalist 131: 58-70.
Craig, J.L. & Jamieson, I.G. 1990. Pukeko: different approaches and some different answers. In: Stacey, P.B. & Koenig, W.D. (eds) Cooperative breeding birds: long-term studies of ecology and behaviour. Cambridge; Cambridge University Press: 385-412.
Emlen, S.T. 1982a. The evolution of helping. II. The role of behavioral conflict. American Naturalist 119: 40-53.
Emlen, S.T. 1982b. The evolution of helping. I. An ecological constraints model. American Naturalist 119: 29-39.
Emlen, S.T. 1995. An evolutionary theory of the family. Proceedings of National Academy of Science USA 92: 8092-8099.
Emlen, S.T. 1996. Living with relatives: lessons from avian family systems. Ibis 138: 87-100.
Emlen, S.T. 1999. Reproductive skew in coopereatively breeding birds: an overview of the issues. In: Adams, N. & Slotow, R. (eds) Proc. 22 Int. Ornithol. Congr. Durban, University of Natal.
Garnett, S.T. 1980. The social organisation of the dusky moorhen Gallinula tenebrosa Gould (Aves : Rallidae). Australian Wildlife Research 7: 103-112.
Jamieson, I.G. 1997. Testing reproductive skew models in a communally breeding bird, the pukeko Porphyrio porphyrio. Proceedings of Royal Society of London B 264: 335-340.
Jamieson, I.G. & Craig, J.L. 1987. Dominance and mating in a communal polygynandrous bird: cooperation or indifference towards mating competitors? Ethology 75: 317-327.
Keller, L. & Vargo, E.L. 1993. Reproductive structure and reproductive roles in colonies of eusocial insects. In: Keller, L. (ed.) Queen number and sociality in insects: 16-44. Oxford; Oxford University Press.
Keller, L. & Reeve, H.K. 1994. Partitioning of reproduction in animal societies. Trends in Evolution & Ecology 9: 98-102.
Koenig, W.D. & Mumme, R.L. 1987. Population ecology of the cooperatively breeding acorn woodpecker. Princeton; Princeton University Press: 435pp.
Koenig, W.D., Pitelka, F.A., Carmen, W.J., Mumme, R.L. & Standback, M.T. 1992. The evolution of delayed dispersal in cooperative breeders. Quarterly Review of Biology 67: 111-150.
Koenig, W.D., Mumme, R.L., Standback, M.T. & Pitelka, F.A. 1995. Patterns and consequences of egg destruction among joint-nesting acorn woodpeckers. Animal Behaviour 50: 607-621.
Koenig W.D. & Stacey, P.B. 1990. Acorn Woodpeckers: group-living and food storage under contrasting ecological conditions. In: Stacey, P.B. & Koenig, W.D. (eds) Cooperative breeding birds: long-term studies of ecology and behaviour: 413-454. Cambridge; Cambridge University Press.
Magrath, R.D. 1999. Problems of distinguishing among models of reproductive skew within populations of cooperatively-breeding birds In: Adams, N. & Stotow, R. (eds) Proc. 22 Int. Ornithol. Congr. Durban, University of Natal.
McRae, S.B. 1995. Temporal variation in responses to intraspecific brood parasitism in the moorhen. Animal Behaviour 49: 1073-1088.
McRae, S.B. 1996. Family values: costs and benefits of communal nesting in the moorhen. Animal Behaviour 52: 225-245.
Mumme, R.L., Koenig, W.D. & Pitelka, F.A. 1988. Costs and benefits of joint nesting in the acorn woodpecker. American Naturalist 131: 654-677.
Reeve, H.K. & Ratnieks, F.L.W. 1993. Queen-queen conflict in polygynous societies: mutual tolerance and reproductive skew. In: Keller, L. (ed.) Queen number and sociality in insects: 45-85. Oxford; Oxford University Press.
Reeve, H.K., Emlen, S.T. & Keller, L. 1998. Reproductive sharing in animal societies: reproductive incentives or incomplete control by dominant breeders? Behavioral Ecology (in press).
Siegel, S. & Castellan, N.J. 1988. Nonparametric statistics for the behavioral sciences. New York; McGraw-Hill Book Co.: 399pp.
Vehrencamp, S.L. 1977. Relative fecundity and parental effort in communally nesting anis (Crotophaga sulcirostris). Science 197: 403-405.
Vehrencamp, S.L. 1980. To skew or not to skew? Proceedings of 17th Congressus Internationalis Ornithologici 17: 869-874.
Vehrencamp, S.L. 1983a. Optimal degree of skew in cooperative societies. American Zoologist 23: 327-335.
Vehrencamp, S.L. 1983b. A model for the evolution of despotic versus egalitarian societies. Animal Behaviour 31: 667-682.
Vehrencamp, S.L., Bowen, B.S. & Koford, R.R. 1986. Breeding roles and pairing patterns within communal groups of groove-billed anis . Animal Behaviour 34: 347-366.
Table 1. Reproductive skew for co-breeding females in five populations of communal breeding birds.

Table 2. Incubated clutch size parameters for single (Cs) and joint (Cj) nesting females including clutches of major (Cmj) and minor (Cmn) females in five species of communally breeding birds.
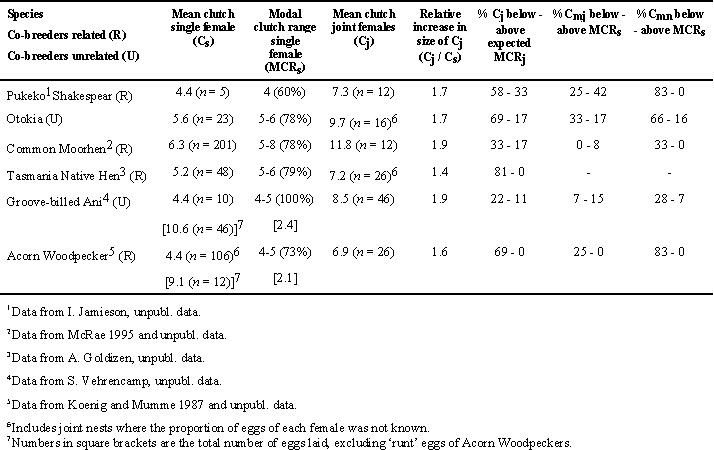
Fig. 1. Frequency distribution of clutch sizes of single nesting females (shaded bars) and major (black bars) and minor (open bars) joint-nesting for five populations/species of communally breeding birds.
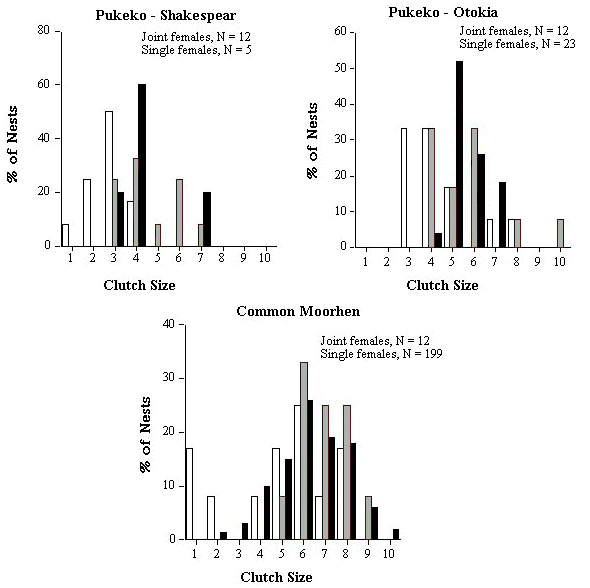
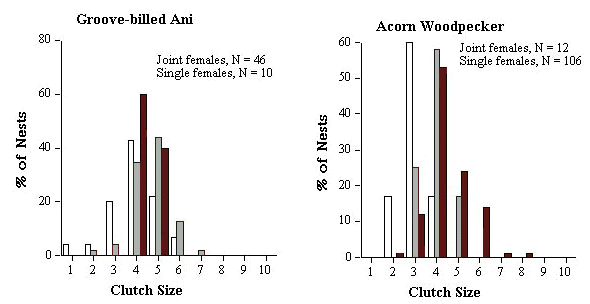
Fig. 2. Relationship in clutch size between major and minor joint-nesting females for five populations/species of communally breeding birds.
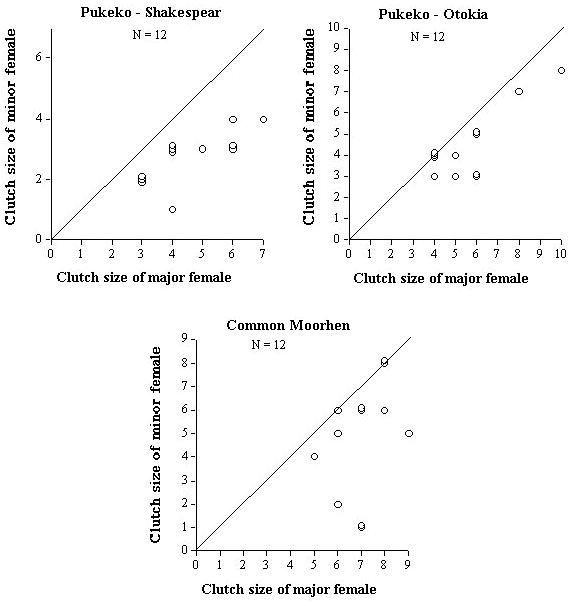
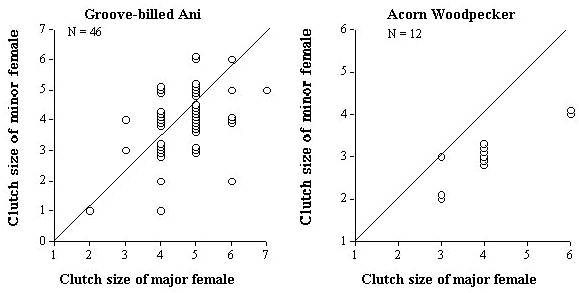
Fig. 3. Frequency distribution of clutch sizes of single nesting females (shaded bars) and joint- nesting females (black bars) for six populations/species of communally breeding birds.