S47.2: Problems of distinguishing among models of reproductive skew within populations of cooperatively-breeding birds
Robert D. Magrath
Division of Botany and Zoology, Australian National University, Australian National University, Canberra 0200, Australia, fax 61 2 6249 5573, e-mail Robert.Magrath@anu.edu.au
Magrath, R.D. 1999. Problems of distinguishing among models of reproductive skew within populations of cooperatively-breeding birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2879-2893. Johannesburg: BirdLife South Africa.
Four models seek to explain the degree of reproductive sharing among individuals of the same sex within breeding groups. (1) Concession models suggest that dominants allow subordinates some reproduction as a way of bribing them to remain peaceably in the group. (2) Incomplete control models explore the consequences of dominants lacking total control over subordinate reproduction. (3) Subordinates may avoid incest and thereby curtail their own reproduction. (4) Individuals of one sex may have at least partial control over reproduction amongst members of the opposite sex. I use these models to predict the qualitative effects of relatedness on reproductive sharing amongst males in cooperatively-breeding societies of birds in which groups form through natal philopatry of males. Variability of relatedness amongst adults can come about through death and dispersal, so that subordinates may be related to both dominants ('nuclear families'), or unrelated to one or both dominants. Assuming all except relatedness is constant, the predictions allow discrimination of models, even though dominants will always gain the (equal) greatest share of reproduction in nuclear families. However, as a study of white-browed scrubwrens illustrates, it is difficult in practice to distinguish among models. A major problem is covariation of subordinate age (and potentially competitiveness) with relatedness, which comes about because of the way groups form, and will affect studies of similar species. Progress can be made with the development of theory, controlling confounding variables through the choice of study species or cases, and testing assumptions underlying hypotheses.
INTRODUCTION
It is twenty years now since Sandra Vehrencamp first presented a model of optimal reproductive skew in group-living animals at the IOC in Berlin in 1978 (Vehrencamp 1980). She and others have subsequently explored a series of models that aim to explain the variation in reproductive sharing among same-sex individuals within breeding groups (Vehrencamp 1979; Emlen 1982; Vehrencamp 1983a; Vehrencamp 1983b; Keller & Reeve 1994; Reeve & Keller 1995; Emlen 1996; Reeve & Keller 1996; Emlen 1997; Cant 1998; Reeve et al.
1998). As Heinsohn et al. (1999) have outlined, societies can range from being despotic to egalitarian. In despotic groups, a single individual monopolises reproduction despite others of the same sex being present; in egalitarian groups, members share reproduction equally.Reproductive skew models were applied from the start to both cooperatively-breeding birds and insects (Vehrencamp 1980), and the primary aim was to explain variation in reproductive skew across species. Recent work on the subject continues to seek models of reproductive sharing that are applicable across a diversity of species, as well as within species and even within single populations (Keller & Reeve 1994; Reeve et al. 1998).
In this paper, I will focus on variation in reproductive skew within populations of cooperatively-breeding birds. Looking at variation within populations potentially allows powerful tests of skew theories, because this approach avoids the complexities of comparing different species, with different evolutionary histories, different life-histories and living in different environments. Problems of comparisons across species are both practical, such as getting comparable and unbiased measures of reproductive skew from different studies (Tsuji & Tsuji 1998), and theoretical, such as justifying the assumption that reproductive sharing in very different species can be explained by similar models (Clutton-Brock 1998).
Cooperatively-breeding birds themselves have diverse social organizations, so I will simplify the paper by focusing on the common and simple 'helper-at-the-nest' species with discrete breeding groups. Groups in such species usually form through natal philopatry of adult offspring, who become the subordinate members of the group. Furthermore, I will concentrate the discussion on reproductive skew amongst males, because there are potentially important differences between the sexes (Cant 1998).
This paper consists of four parts. First, I outline hypotheses that have been used to explain the degree of reproductive sharing. Second, I examine what predictions these models make about the effects of relatedness on the sharing of reproduction among males in cooperatively-breeding groups that form through natal philopatry but undergo subsequent changes of group composition. Third, I attempt to distinguish among hypotheses in a case study of reproductive sharing among males in White-browed Scrubwrens. I find that it is not possible to distinguish among hypotheses, primarily because of the joint problems of covariation of subordinate age with relatedness and the problem of relatedness asymmetry. The final section is devoted to a general discussion in which I suggest that these problems will apply to many species of cooperatively-breeding birds, and suggest ways in which we can make progress in understanding the sharing of reproduction.
Models to explain reproductive sharing
I will not use the term 'reproductive skew' or 'optimal reproductive skew' to refer to a particular type of model, since the degree of reproductive sharing is what all models try to explain, and all hypotheses could be cast in an optimality framework. Instead, I use names that reflect the mechanisms influencing skew.
It is difficult to draw up a simple conceptual framework. What are considered different models by some authors are considered to fall within the same model by others. For example, incest avoidance could influence reproductive skew as could competition amongst individuals. Whether incest avoidance should be identified as a distinct hypothesis depends on whether incest is considered to affect the fitness of offspring, and whether it is best seen as a constraint or simply another factor affecting reproductive payoffs to individuals. Here I separate hypotheses because I aim to highlight factors that could influence reproductive skew but have not necessarily been included in optimality models.
1. Reproductive concession model ('optimal skew model')
In both Vehrencamp’s original models and Emlen’s (1982) concept of 'fitness forfeiting', dominant individuals may attempt to maximise their inclusive fitness by bribing subordinates to stay in social groups. Dominants have total control over the reproduction of subordinates, but adjust the size of the bribe to entice the subordinate to stay and co-operate peacefully. The minimum size of the bribe they need to offer, to make it in the interests of the subordinate to stay, depends on their relatedness to the subordinate, the subordinate’s effect on the group’s reproductive productivity, and the subordinate’s other options and competitive ability (Keller & Reeve 1994). The bribe is modelled as the amount of direct reproduction the dominant permits the subordinate. Thus the size of the bribe determines the amount of skew. This is often called the 'optimal skew model', but I think Tim Clutton-Brock’s (1998) term 'concession model' nicely encapsulates the principle of the dominant making concessions to entice the subordinate to stay in the group and to co-operate.
Under this model, a dominant can be despotic when the subordinate is closely related, has a large effect on the group’s productivity, has little chance of reproducing independently, and is a poor competitor compared with the dominant. At the other extreme, the dominant may have to allow equal reproduction when the subordinate is unrelated, has a limited effect on group productivity, has a good chance of reproducing elsewhere, and is a strong competitor. Intermediate levels of relatedness, contribution to group success, constraint and competitive ability lead to intermediate levels of skew.
An important twist to this model comes from asymmetrical relatedness (Reeve & Keller 1995). In a trio consisting of a father and son (or two brothers) with an unrelated female, each male is related to the offspring of the other by the same amount (r = 0.25); relatedness is symmetrical. However, in a nuclear family consisting of a father and son with the son’s mother, the father is less closely related to offspring sired by the son (r = 0.25) than the son is to offspring sired by his father (r = 0.5). Hence, other things being equal, the father will offer fewer concessions when his son’s mother is present, because of asymmetrical relatedness.
2. Incomplete control
Recent models by Cant (1998) and Reeve et al. (1998) have explored the consequences of dominants not having the ability to control subordinate reproduction to the minimum that just compensates them for staying. Thus these models change one major assumption of concession models. Cant’s (1998) model explicitly deals with reproductive sharing by females who contribute to the same clutch. His models predict similar trends to concession models; in fact the association between close relatedness and increased skew can be exaggerated. There is, however, some debate about whether this model really does encapsulate the problem of incomplete control (S. L. Vehrencamp pers. comm.).
The models by Reeve and colleagues (1998) are important because they are designed to be applicable to both sexes, and are more complex in structure and make complex predictions. These models assume that a given productivity is divided between competing reproductives, and that there is a cost, in terms of reduced group productivity, following from resources channelled into competition rather than reproduction. Dominant individuals have access to greater resources, or are more efficient at using resources, so have some advantage in competition with subordinates. Group productivity is assumed to be a linear function of the amount of resources that are not devoted to competition.
The predictions about the effect of relatedness derived from these models are sensitive to the particular form of the model and qualitative effects can vary over values of parameters. For example, if one assumes symmetrical relatedness, small group sizes and less efficient resource use by subordinates, kinship is predicted to have little or no effect on reproductive skew. On the other hand, if competing males have stored resources, yet equal efficiency of using them, reproductive skew initially declines with increasing relatedness but then becomes constant. Overall, in any one model, skew is either not affected by relatedness or declines with relatedness. This contrasts with concession models, which predict increasing skew with increasing relatedness. In common with concession models, skew is predicted to be higher when relatedness is asymmetrical than when it is symmetrical.
As with concession models, Reeve et al.’s models predict that reproductive skew will increase as the competitive differences between dominants and subordinates increase.
One problem with Reeve et al.’s (1998) predictions is that an assumption underlying their models may not be appropriate for sharing of paternity in cooperatively-breeding birds. A male subordinate’s share of reproduction may depend on contests during the female’s fertile period, weeks before there are young to feed (the time when subordinates are most likely to exert any effect on reproductive productivity of the group). It therefore seems unlikely that there is a substantial, linear cost of competition on group productivity, a fundamental assumption.
Overall, the conclusions of the two papers attempting to model optimal reproductive skew in situations of incomplete control (Cant 1998, Reeve et al. 1998) have reached opposite conclusions regarding the effect of relatedness. Furthermore, Cant’s models deal exclusively with sharing by females, and, while the models of Reeve and colleagues are designed to be more general, they make general assumptions that may not be appropriate to paternity sharing in birds.
3. Incest avoidance
If a subordinate male is in a group with his mother, or other female relatives, he may voluntarily avoid incest and thereby increase reproductive skew. In this case, there is no need for the dominant male to bribe or constrain the subordinate, and there should be no competition among males.
4. Female control of reproductive skew among males
Reproductive skew amongst males can be affected by conflicts of interest with breeding females and may therefore at least partly reflect female control. This is particularly clear in dunnocks, in which groups are composed of adults that are unrelated to each other. In a polyandrous trio of this species, whether a beta male helps feed nestlings is influenced by whether he copulates with the female, so it is in the female’s interest to copulate with both males and she appears to take active steps to achieve that goal (Davies 1992). On the other hand, it is in the alpha male’s interest to try to stop the beta copulating with the female. Thus there is conflict between the sexes and reproductive skew is partly the result of female control. This is quite different from preceding models, all of which assume that reproductive skew results entirely from conflict or 'negotiation' within a sex.
A female’s perspective on reproductive skew among males might also involve genetic costs and benefits. For example, a subordinate male may gain some net benefit from offspring of an incestuous mating with his mother even if those offspring are of lower fitness than offspring of the dominant; however, it seems less likely to be in the interests of the female to raise her son’s inbred offspring in comparison with the dominant’s outbred offspring. Thus females may impose constraints on incest.
As far as I know, there has been no formal modelling of the effects on reproductive skew of the opposite sex, despite the fact that the problem was identified in early discussions of reproductive skew by both Vehrencamp (1979, 1980) and Emlen (1982).
VARIABILITY IN RELATEDNESS AND PREDICTED SKEW
I now return to simple 'helper-at-the-nest' cooperatively-breeding birds, and see how we can use variability in relatedness to test models of reproductive skew. A common type of group of such a species is the 'nuclear family', consisting of the dominant pair and their adult offspring. However, deaths and social re-arrangements can lead to groups with variable relatedness. Consider a trio consisting of a female, a dominant (alpha) male and a subordinate (beta) male who is the son of both. If the female dies or disperses and is replaced by an immigrant, then a 'stepmother' group is formed. Similarly, replacement of the alpha male leads to a 'stepfather' group. Another single change in the trio can result in an 'unrelated' group in which each adult is unrelated to either of the others. Thus replacement of individuals can result in groups in which beta males are full-siblings of offspring of the dominant pair, to groups in which they are half-siblings and even to groups in which they are unrelated.
Variability in relatedness between subordinate males and dominants among groups in a population is relevant to testing all of the hypotheses that have been put forward to account for variation in reproductive skew. Furthermore, examining the effect of variation in relatedness seems the most promising way to test models of skew, because relatedness can be quantified more easily than can competitiveness and ecological constraints.
How common are these changes in group composition, and therefore how much variation in group composition is there in species in which groups usually form through natal philopatry? At least for some such species, replacement families are common, and Emlen (1997) even suggests that the idea of a stable family is largely a myth. For example, amongst species in which male subordinates have been shown to gain paternity within the group, about 67% of stripe-backed wren groups had one or more helpers unrelated to the female (Piper & Slater 1993) and 47% of superb fairy-wren helpers were in groups with unrelated females (Dunn et al. 1995).
In common with white-winged choughs (Heinsohn et al. 1999), most comparisons both within and among species have shown higher skew in groups of closer relatives (Vehrencamp 1983a; Keller & Reeve 1994; Emlen 1996; Emlen 1997; Clutton-Brock 1998; Reeve et al. 1998). This pattern is consistent with the concession model, but it is also consistent with other models (Emlen 1997; Clutton-Brock 1998; Reeve et al. 1998); see Discussion.
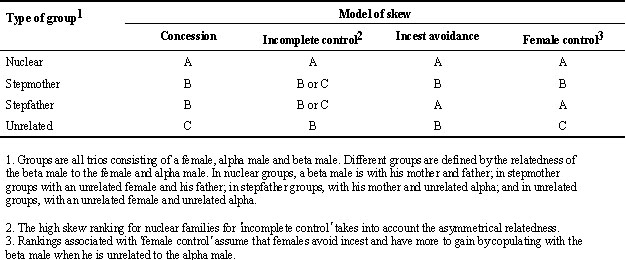
Table 1 summarises the qualitative predictions of different models for reproductive skew among males. I do not use quantitative predictions because they are relevant only to specific situations and depend on factors in addition to relatedness. The Table assumes all except relatedness is held constant, and ranks across different types of group the degree of skew expected within each model. A rank of 'A' means that, within that model, that type of social group would have the most extreme skew or equal most extreme skew. The ranking protocol is that rank 'B' is used after rank 'A', even if more than one type of group has the rank 'A'; the same applies for rank 'C'. The rankings within a model do not have absolute meaning, so that they should not be compared across models. For example, a rank of 'B' for one model does not imply greater skew than a rank of 'C' for another model.
I explain the rationale for the ranked predictions of each model in turn. (1) In the concession model, skew is predicted to be higher when offspring of the alpha male and female are closer relatives (it is also higher in nuclear families because relatedness is asymmetrical). The offspring are full sibs in nuclear groups, half-sibs for stepmother and stepfather groups, and unrelated for unrelated groups. (2) In the incomplete control model, relatedness asymmetry leads to a prediction of high skew in nuclear families. Reeve et al. (1998) predict that skew will remain constant or increase with a decrease in relatedness, so the remaining groups will have similar ranking, or 'unrelated' groups will have higher skew. (3) Incest avoidance predicts no reproduction (high skew) when the subordinate is with his mother, but sharing when the mother has been replaced. (4) The rankings under female control are speculative, and are based on the assumptions that females avoid incest and have more to gain from subordinate reproduction when subordinates are least related to offspring of the female and alpha male. The rationale for the second assumption is discussed below. Thus groups in which mating would be incestuous have high skew, and 'unrelated' groups have the least skew.
All models predict the (equal) greatest skew in nuclear families, but there are differences in the predicted skew among the other types of group which appear to make it possible to distinguish among models. For example, incest avoidance by beta males would lead to no reproduction (high skew) whenever the mother is present, but shared reproduction when she has been replaced by an unrelated immigrant; by contrast, skew will also be affected by relatedness to the alpha male in other models.
I now turn to a study of reproductive sharing among males in a population of white-browed scrubwrens that I have been studying since 1992.
TESTING HYPOTHESES FOR REPRODUCTIVE SKEW IN SCRUBWRENS
Study population
White-browed scrubwrens Sericornis frontalis are small (c. 11-15 g), largely-insectivorous passerines in the family Pardalotidae (Sibley et al. 1988), and are common throughout southern and eastern Australia in habitats with dense undergrowth (Blakers et al. 1984). Females, which have brown lores, lay three eggs in a clutch and fledge up to three broods during the breeding season (July-March). Males, which have black lores, do not help with nest-building or incubation but often feed young, which remain in the nest for about 15 days (Magrath & Yezerinac 1997). All adults and their offspring in the population were marked with unique combinations of colour-bands and had a blood sample taken to enable DNA-fingerprinting (Whittingham et al. 1997). The study population in the Australian National Botanic Gardens in Canberra ranged from 35 to 53 breeding groups per year.
Scrubwrens most commonly bred in pairs or trios, with trios consisting of a single breeding female, a alpha male and a beta male (Magrath & Yezerinac 1997). Dominance was stable during breeding attempts and from year to year, with older males dominant to younger ones. Males often remained as subordinates on their natal territory, sometimes for many years, while females always dispersed in their first year.
Over the years 1992 to 1997 the adult population ranged from 58 to 67% male, and a shortage of females appeared to be a proximate constraint on subordinate male dispersal. Yearling males never gained a breeding vacancy as a dominant male in a multi-male group, and rarely gained a position as a pair male (2/106 resident yearlings).
Estimating relatedness
I used pedigrees to estimate relatedness of beta males to the breeding female and dominant male. Band-sharing from DNA-fingerprinting was used to determine relatedness for a subset of broods in 1992 and 1993 for which pedigree data were unavailable. DNA-fingerprinting methods are detailed in Whittingham et al. 1997. Where both were available, pedigree and fingerprinting data were consistent in identifying whether the resident female was a subordinate’s mother (n = 29 subordinate:female dyads). Furthermore, DNA-fingerprinting of 137 nestlings in 31 social groups revealed no egg-dumping in this population (Whittingham et al. 1997). Thus pedigree analyses are accurate in determining relatedness to the breeding female.
Pedigree analyses are less accurate in assigning paternity (Magrath & Whittingham 1997). The pedigree analyses assumed that offspring raised by a group were first-order relatives (sons or brothers) of all resident males. This assumption will lead to overestimation of relatedness between subordinate and dominant males. Where both were available, twelve of 15 socially-related dyads were also genetically related. The three inconsistencies (20%) arose because these beta males were sired by a male in the natal group which fingerprinting revealed to be unrelated to current alpha male. Extra-group paternity could also introduce errors in estimating relatedness among males, as 12% of a sample of 137 nestlings from 1992 and 1993 were the result of extra-group paternity (Whittingham et al. 1997).
DNA-fingerprinting suggests that males lower in the hierarchy than beta males never gained paternity (Whittingham et al. 1997), so in examining group composition and reproductive skew, I focus on alpha and beta males. Work in progress (S. M. Yezerinac & R. D. Magrath) will extend the genetic analyses to most groups over the period 1992 to 1996.
Variability in group composition and reproductive skew
In only 44% of breeding groups were beta males in 'nuclear families' with both their genetic mother and probable genetic father (some may be brothers). Thirty-nine percent were in a 'stepmother' group with an unrelated female and a probable genetic father, and 17% were unrelated to both the female and dominant male. Overall, 56% of beta males were in a group with an unrelated female. These data come from 109 group-years over the 6 years 1992-1997.
The variability in group composition came about through a combination of death and dispersal (Magrath & Whittingham 1997). Females that died or dispersed were replaced by immigrants that were unrelated to the males. Males occasionally dispersed to become subordinates in groups of individuals to which they were unrelated. Changes occasionally involved more than one individual; for example, males sometimes dispersed to join a female after evicting the resident male.
Beta males never gained paternity when with their mother, but sometimes did so when with an unrelated female (Fig. 1). Sharing appeared to be more equitable when the beta male was with an unrelated alpha male than when he was with his father, although the sample size was inadequate to allow direct statistical comparison.
The pattern of reproductive sharing and relatedness in scrubwrens is consistent with the concession model. When the beta male is with his mother and genetic father, offspring of the alpha male will be his siblings, and so the alpha male will need to provide the fewest concessions to entice the beta to stay peaceably. In stepmother groups, the alpha male’s offspring will be half-sibs, and so the alpha may need to make some concessions. The greatest concessions would be expected in groups in which beta is related to neither the female or alpha male, since he will be unrelated the alpha male’s offspring.
The major problem with the concession model in scrubwrens is that it is not clear that the alpha male benefits from the presence of the beta male, as there is no detectable effect of either subordinate helping at the nest or group size on the reproductive productivity of the group (Magrath & Yezerinac 1997). However, DNA-fingerprinting has identified a possible cryptic benefit to the alpha male. The incidence of extra-group paternity was lower in breeding groups (6% of young) compared to pairs (24% of young), and one interpretation is that the presence of an additional male causes this difference (Whittingham et al. 1997). It seems very unlikely, nonetheless, that this benefit would be sufficient to account to the high sharing with unrelated beta males. Overall, although the pattern of increased paternity sharing with lower relatedness consistent with the concession model, more data are required on possible benefits to alpha males of having subordinates in the group.
Problems in distinguishing among models of reproductive skew
Although the pattern of reproductive sharing and relatedness is consistent with the concession model, the data are also consistent with every other model! This section explains why.
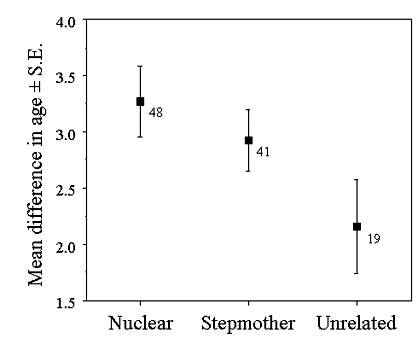
Assuming no death or dispersal of group members, a male who remains on his natal territory will join his mother and father. As years pass, it is likely that one or both of the dominants will be replaced. Thus, because of the way groups with different relatedness form, beta males should be older, on average, when with an unrelated female and probably alpha male. This is true of scrubwrens (Fig. 2). Furthermore, the difference in age between alpha and beta males is greatest in nuclear families compared with groups in which the beta is unrelated to the female (Fig. 3).
Thus, as Emlen (1996) points out, the competitive ability of beta males may covary with their relatedness to the dominant pair. Not only do age differences support this argument, but the fact that some of the beta males in 'unrelated' groups had been able to join groups of non-relatives suggests that they might be superior competitors, independent of age. There is therefore a problem in interpreting the data on reproductive skew in scrubwrens because higher reproductive sharing could result from the inability of the alpha male to suppress beta reproduction. Thus a monotonic decrease in skew with a decrease in relatedness could come about through a correlated change in competitiveness of the beta male. It is therefore not possible to distinguish between concession and incomplete control models (Table 2). While the ranking of skew remains the same for concession models as in Table 1, the ranking for incomplete control may change such that beta males gain a greater share in 'unrelated' groups because they are on average better competitors than those in 'stepmother' groups. The high skew in nuclear families in both the concession and incomplete control models could come about because of low competitive ability of males in such groups, as well as close relatedness and relatedness asymmetry (the relatedness of the beta male to the alpha male’s offspring [0.5] is closer than the alpha to the beta’s offspring [0.25]).
Emlen (1996) points out that in many species it may be difficult to distinguish between incest avoidance and concession models. This problem applies to scrubwrens, although this relates more to weakness in the data rather than theoretical problems. If beta males or females avoid incest, then beta males should gain no paternity in nuclear families, but should gain paternity in the other groups, as was found. Theoretically, it is possible to distinguish incest avoidance from concession models: incest avoidance predicts no difference in sharing between the two group types in which the female is unrelated to the beta male, whereas concession models predict that sharing may be intermediate in stepmother groups (Table 1). Unfortunately, the sample sizes are currently insufficient to decide this issue.
Finally, the pattern of reproductive sharing is also consistent with female control over male reproduction. First, females may avoid incest even if their sons do not. Second, if females seek multiple paternity to increase genetic variability among their young, they will have greatest to gain from multiple paternity when the sires are genetically different. Thus female choice could explain the most equitable sharing of paternity in groups in which the males are unrelated. Finally, subordinate male scrubwrens provide more care to nestlings if they have gained paternity (Whittingham & Dunn 1998), and so again females may seek copulations with beta males particularly when beta males have the least indirect fitness to gain via alpha paternity. This final suggestion depends on there being some, as yet undetected, benefit to the female of having subordinates contribute to the care of nestlings. The end result is that female control may lead to high skew in nuclear families, because of incest avoidance by the female, and least skew in 'unrelated' groups, so that the ranking of skew becomes the same as for the concession and incomplete control models.
The end result of these complexities is that the current data do not permit rejection of any of the models of reproductive skew (Table 2). Three models make identical predictions about qualitative variation in skew, while data are currently insufficient to reject the incest avoidance model.
DISCUSSION
Problems of distinguishing among models of skew
This survey of models of reproductive skew, combined with the case study of scrubwrens, has identified several difficulties in trying to distinguish among models using within-population variation in reproductive skew and relatedness. I believe that these problems will apply to many other cooperatively-breeding birds. I discuss the problems identified so far.
(1) Covariation of competitiveness and relatedness
A major difficulty in scrubwrens was that the competitive ability of beta males was plausibly higher in groups with lower relatedness, because in these groups beta males were older and more similar in age to alpha males. This covariation of relatedness and subordinate age arose primarily from the way groups form through natal philopatry. As this mode of group formation is common in cooperatively-breeding birds, this problem is likely to be general (Emlen 1996), and compounds the difficulty of distinguishing among models. Even without this covariation of age and relatedness, relatedness asymmetry will lead to high skew in nuclear families under both concessions and incomplete control models (Reeve et al. 1998). It can also be difficult to distinguish between reproductive competition and incest avoidance (Emlen 1996; Koenig et al. 1998). The effect of the covariation of age and relatedness is that qualitative predictions of concessions and incomplete control models, that are already similar for some group types (Table 1), potentially become indistinguishable over all group types (Table 2).
A covariation of competitiveness and relatedness could come about for other reasons. Individuals which force themselves into groups of unrelated individuals may be competitively superior to those that stay on the natal territory. Second, individuals that have been unsuccessful in gaining paternity may eventually leave groups for that reason (Clutton-Brock 1998). This means that, as time goes by, subordinates are on average more competitive and therefore gain a greater share of paternity. In the meantime, the group may have changed composition, so that they are (incidentally) less related to the dominants. It is even conceivable that the opposite effect could occur, at least at some subordinate ages, if better competitors are more likely to leave the group and acquire breeding vacancies.
The problem of a covariation of fighting ability and relatedness was discussed in some detail by Reeve and Keller (1995), who considered the problem of particular importance in eusocial species of insects. In matrifilial (mother-daughter) societies, workers are usually smaller than the queen and so have lower competitive ability. By contrast, in semisocial (same generation, such as sibling) societies, individuals are of similar size. Thus competitive ability covaries with relatedness asymmetry, and so it is difficult to establish the cause of the more extreme reproductive skew in matrifilial societies. Reeve and Keller thought that the issue was not a problem in vertebrates, where subordinates are usually of similar size to dominants. Emlen (1996) counters this suggestion by noting the common association of age, experience and dominance in vertebrates. I agree that we cannot ignore the problem in vertebrates.
In a review comparing the concession model with models of incomplete control, which is focused primarily on sharing among female mammals, Clutton-Brock (1998) concludes that 'there is no unequivocal evidence that dominant female vertebrates make concessions to subordinates in return for assistance'. His conclusion is based primarily on the problem of confounding variables (including competitiveness and relatedness), and suggests that the problems identified in my review apply to both sexes and many vertebrates.
(2) Models are not exclusive
Although they are usually discussed separately, there seems no reason to believe that models of skew should be considered strict alternatives. It seems likely, as has been found with acorn woodpeckers (Koenig et al. 1998), that both incest avoidance and competition among same-sex individuals can influence reproductive skew. Indeed, the effect of incest on group productivity could potentially be incorporated into concession and incomplete control models (Vehrencamp 1983a; Emlen 1999). Similarly, it seems likely that the degree of skew in one sex will be affected by the other sex, even if there is also competition or the offering of concessions within that sex. Given that models are not alternatives, if may be difficult, or even pointless, to try to distinguish among them.
How to make progress?
There are clearly problems with determining the causes of variation in reproductive skew within populations, but I believe that there are many ways in which we can make progress in the development and testing of different models.
(1) Theory
Although the first models of skew were presented 20 years ago, formal models of skew are only beginning to address the problem of incomplete control (Reeve & Keller 1995; Cant 1998; Reeve et al. 1998). Furthermore, the only model of incomplete control relevant to males (Reeve et al. 1998), seems to make assumptions that reduce its relevance to paternity sharing in birds. The following developments would be welcome: (1) models that include the interests of the opposite sex; (2) models that explore the potential differences between the sexes in competition within that sex (Cant 1998); (3) models that focus on specific types of animals, given that it is unlikely that single models will apply to all species (Clutton-Brock 1998); (4) models that explore the simultaneous effects of more than one cause of skew (e.g. competition and incest); and (5) models that examine concessions other than reproductive sharing (e.g. parental facilitation (Brown & Brown 1984) could be seen as a concession, and younger individuals may benefit most from being allowed to stay on the territory).
(2) Controlling confounding variables
Although using variation in relatedness to test of models of skew is necessary, it is also necessary to take into account potentially confounding variables. It might be possible to quantify and control statistically for competitiveness, or select cases in which competitiveness is likely to be similar. For example, it may be possible in scrubwrens to control for beta male age, or the difference in age between the alpha and beta males, when examining the effect of relatedness on reproductive skew. It is also desirable to select species, or types of group, in which it is possible to avoid the confounding effects of asymmetric relatedness. For example, it may be easier to test models of reproductive skew in societies in which brothers form coalitions, rather than in societies where sons stay with fathers. In the case of species like scrubwrens, it may be more fruitful to focus on group types (stepmother and unrelated groups) in which relatedness is symmetrical.
(3) Testing assumptions
I suspect that the most useful approach in the field will be to focus on testing assumptions and mechanisms, and not (exclusively) predictions of different models. It can be difficult or impossible to distinguish among models on the basis of their predictions, and yet the assumptions of models differ substantially, as do the proximate causes of reproductive skew. For example, it may be possible to test the assumption that incest incurs a cost and that subordinates avoid mating with close relatives. In some species of cooperatively-breeding birds, there is evidence for incest avoidance; for example, in acorn woodpeckers, groups may curtail reproduction for years rather than engage in incest (Koenig et al. 1998). In other species, like white-winged choughs, incest is common (Heinsohn et al. 1999). If there is no incest avoidance, it is possible to dismiss one reason for high skew in closely-related groups and avoid one factor confounding the interpretation of other models (Heinsohn et al. 1999).
There appears to be no direct evidence that subordinate dispersal can be manipulated by reproductive concessions made by dominants (Clutton-Brock 1998), and yet this is a fundamental assumption of concession models. Similarly, it is important to know at a proximate level whether dominants could potentially exclude subordinates from reproduction and whether they have precise control over the magnitude of skew, should they permit subordinate reproduction. What behavioural mechanisms would allow precise control?
Other assumptions that deserve close scrutiny include the assumption that dominants benefit from the presence or help (or both) of subordinates. In many species of cooperatively-breeding birds, there is no clear evidence that helping by subordinates benefits dominants (Cockburn 1998), and yet this is a fundamental assumption of all but the incest model (but see Cant 1998 for an exception). Even if dominants do benefit, quantifying the benefit is difficult, particularly in species that live on all-purpose territories (Koenig & Mumme 1990; Magrath & Yezerinac 1997).
All models of skew tacitly assume some degree of kin 'recognition' - some behavioural rules which allow differential treatment of individuals of different (average) kinship. This raises the issue of what mechanisms are involved, and what precision of 'recognition' is possible. Incest avoidance by a male in species with a single female in the breeding group only entails recognising whether the resident female has been replaced or not. By contrast, other models may require very precise kin 'recognition'; for example, distinguishing between older brothers and fathers is relevant to concessions and incomplete control models and yet there may be no mechanism by which this could be achieved. Depending on the behavioural constraints on kin 'recognition', the predictions of incest and other models may become even more similar.
ACKNOWLEDGMENTS
I would like to thank Morne du Plessis and Steve Emlen for organising the symposium, inviting me to contribute and for commenting on the manuscript. I thank Rob Heinsohn, Sarah Legge and Sandra Vehrencamp for stimulating discussions about reproductive skew and for useful comments on the manuscript. Tim Clutton-Brock, Andrew Cockburn, Steve Emlen and Rob Heinsohn kindly forwarded unpublished manuscripts. Many people have worked with me on the scrubwrens over the years, including Linda Whittingham, Peter Dunn, Stephen Yezerinac who worked both in the lab and field, and Beth Bobroff, Janet Gardner, Tony Giannasca, Ashley Leedman, James Nicholls, Anjeli Nathan and Lynda Sharpe who were invaluable in the field. To all, and to others who have helped in various ways, I am grateful. My research on scrubwren social behaviour, which cultivated my interest in reproductive sharing, has been supported by grants from the Australian Research Council.
REFERENCES
Blakers, M., Davies, S.J.J.F. & Reilly, P.N. 1984. The Atlas of Australian Birds. Melbourne: Melbourne University Press.
Brown, J.L. & Brown, E.R. 1984. Parental facilitation: parent-offspring relations in communally breeding birds. Behav. Ecol. Sociobiol. 14: 203-209.
Cant, M.A. 1998. A model for the evolution of reproductive skew without reproductive suppression. Anim. Behav. 55: 163-169.
Clutton-Brock, T.H. 1998. Reproductive skew, concessions and limited control. TREE 13: 288-292.
Cockburn, A. 1998. Evolution of helping behavior in cooperatively breeding birds. Ann. Rev. Ecol. Syst. 29: in press.
Davies, N.B. 1992. Dunnock Behaviour and Social Evolution. May, R.M. & Harvey, P.H. (eds) Oxford Series in Ecology and Evolution. Oxford; Oxford University Press.
Dunn, P.O., Cockburn, A. & Mulder, R.A. 1995. Fairy-wren helpers often care for young to which they are unrelated. Proc. R. Soc. Lond. B 259: 339-343.
Emlen, S.T. 1982. The evolution of helping. II. The role of behavioral conflict. Am. Nat. 119: 40-53.
Emlen, S.T. 1996. Reproductive sharing in different types of kin associations. Am. Nat. 148: 756-763.
Emlen, S.T. 1997. Predicting family dynamics in social vertebrates. In: J. R. Krebs & N. B. Davies. (ed.) Behavioural ecology: an evolutionary approach: 228-253. Oxford; Blackwell Science.
Emlen, S.T. 1999. Reproductive skew in cooperatively breeding birds: an overview of the issues. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2922-2931. Johannesburg: BirdLife South Africa.
Heinsohn, R.H., Dunn, P.O. & Legge, S. 1999. Extreme reproductive skew in cooperatively-breeding birds: tests of theory in white-winged choughs. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2858-2878 . Johannesburg: BirdLife South Africa.
Keller, L. & Reeve, H.K. 1994. Partitioning of reproduction in animal societies. Trends Ecol. Evol. 9: 98-102.
Koenig, W.D., Haydock, J. & Stanback, M.T. 1998. Reproductive roles in the cooperatively breeding acorn woodpecker: incest avoidance versus reproductive competition. Am. Nat. 151: 243-255.
Koenig, W.D. & Mumme, R.L. 1990. Levels of analysis and the functional significance of helping behavior. In: Bekoff, M. & Jamieson, D. (eds), Explanation, Evolution, and Adaptation: 268-303. Oxford; Westview Press.
Magrath, R.D. & Whittingham, L.A. 1997. Subordinate males are more likely to help if unrelated to the breeding female in cooperatively-breeding white-browed scrubwrens. Behav. Ecol. Sociobiol. 41: 185-192.
Magrath, R.D. & Yezerinac, S.M. 1997. Facultative helping does not influence reproductive success or survival in cooperatively-breeding white-browed scrubwrens. J. Anim. Ecol. 66: 658-670.
Piper, W.H. & Slater, G. 1993. Polyandry and incest avoidance in the cooperative stripe-backed wren of Venezuela. Behaviour 124: 227-247.
Reeve, H.K., Emlen, S.T. & Keller, L. 1998. Reproductive sharing in animal societies: reproductive incentives or incomplete control by dominant breeders? Behav. Ecol. 9: 267-278.
Reeve, H.K. & Keller, L. 1995. Partitioning of reproduction in mother-daughter versus sibling associations - a test of optimal skew theory. Am. Nat. 145: 119-132.
Reeve, H.K. & Keller, L. 1996. Relatedness asymmetry and reproductive sharing in animal societies. Amer. Natur. 148: 764-69.
Sibley, C.G., Ahlquist, J.E. & Monroe, B.L. 1988. A classification of the living birds of the world based on DNA-DNA hybridization. Auk 105: 409-423.
Tsuji, K. & Tsuji, N. 1998. Indices of reproductive skew depend on average reproductive success. Evol. Ecol. 12: 141-152.
Vehrencamp, S.L. 1979. The roles of individual, kin, and group selection in the evolution of sociality. In: Marler, P. & Vandenberg, J.G. (eds), Handbook of behavioral neurobiology: social behavior and communication: 351-394. New York; Plenum.
Vehrencamp, S.L. 1980. To skew or not to skew? In R. Nöhring. (ed.) XVII Congressus Internationalis Ornithologici: 869-874. Berlin 1978: IOC.
Vehrencamp, S.L. 1983a. A model for the evolution of despotic versus egalitarian species. Anim. Behav. 31: 667-682.
Vehrencamp, S.L. 1983b. Optimal degree of skew in cooperative societies. Amer. Zool. 23: 327-35.
Whittingham, L.A. & Dunn, P.O. 1998. Male parental effort and paternity in a variable mating system. Anim. Behav. 55: 629-640.
Whittingham, L.A., Dunn, P.O. & Magrath, R.D. 1997. Relatedness, polyandry and extra-group paternity in the cooperatively-breeding white-browed scrubwren (Sericornis frontalis). Behav. Ecol. Sociobiol. 40: 261-70.
Table 1. Qualitative predictions from different models of degree of skew amongst males assuming that all except kinship are constant. The degree of skew is ranked within models from greatest (A) to least (B or C depending on the model). Ranks have no absolute values, and so are not comparable between models. Where the model predicts no difference, the same rank is given. The text gives a full rationale of ranking.

Table 2. Qualitative predictions from different models of degree of skew amongst male scrubwrens, taking into account the likely covariation of beta competitive ability with kinship. The degree of skew is ranked within models from greatest (A) to least (B or C depending on the model). Ranks have no absolute values, and so are not comparable between models. Stepfather trios did not occur, and so were not ranked.
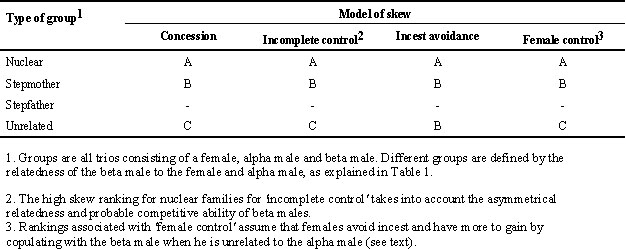
Fig. 1. Sharing of paternity between alpha and beta males in White-browed Scrubwrens. Sample sizes are numbers of broods, but percentages were calculated on total nestlings fingerprinted (34 nestlings from nuclear groups, 26 from stepmother groups and 24 from unrelated groups). Five nestlings resulting from extra-group paternity were excluded before calculating percent sharing. Group types are defined in Table 1. Data are taken from Table 2 in Whittingham et al. 1997.
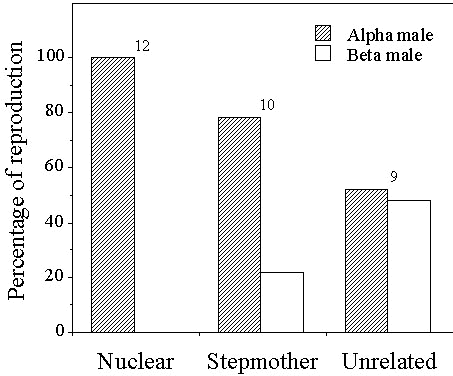
Fig. 2. Minimum age of beta male scrubwrens in different types of breeding groups. Groups are defined in Table 1. Sample sizes are group years.
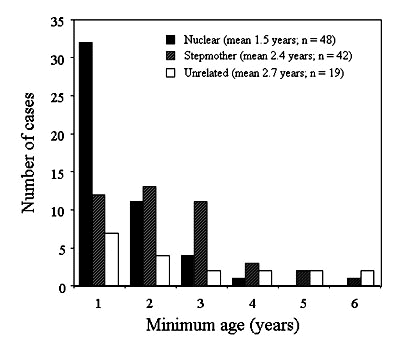
Fig. 3. Number of years difference in age between alpha and beta male scrubwrens in different types of breeding groups. Groups are defined in Table 1. Sample sizes are group years.