S47.1: Extreme reproductive skew in cooperatively-breeding birds: Tests of theory in White-winged Choughs
Robert Heinsohn1, Sarah Legge1 & Peter Dunn2
1Evolutionary Ecology Group, Division of Botany and Zoology, Australian National University, Canberra, ACT 0200 Australia, e-mail Robert.Heinsohn@anu.edu.au; 2Department of Biological Sciences, Lapham Hall, PO Box 413, University of Wisconsin-Milwaukee, Milwaukee, WI 53201, USAHeinsohn, R., Legge, S. & Dunn, P. 1999. Extreme reproductive skew in cooperatively-breeding birds: Tests of theory in White-winged Choughs. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2858-2878. Johannesburg: BirdLife South Africa.
The distribution of direct reproduction among members of social groups is currently receiving renewed interest. Three models that predict which individuals get to reproduce are the concession model (previously known as the optimal skew model), incomplete control models, and incest avoidance. The concession model assumes complete control by dominant individuals who can allocate reproductive opportunities to subordinates to entice them to stay, whereas the incomplete control models recognise that dominants do not always have such control. Incest avoidance is specific to the presumed costs of inbreeding, and may act in concert with either of the other two models. Unfortunately predictions derived from all three models are similar for most cooperatively breeding birds, creating difficulties in establishing support for any particular one. In this paper we review all studies of birds in which high skew (monopolisation of breeding by a single pair) has been reported using DNA-fingerprinting, and discuss the shortcomings of current theory in interpreting trends. We then report the patterns of reproduction within groups of cooperatively breeding White-winged Choughs, Corcorax melanorhamphos, under varying demographic conditions caused by a severe drought during the study. As predicted by the concession model, the extent of shared maternity and paternity (skew) as revealed by DNA-fingerprinting is dependent on the degree of relatedness between group members. However, the easing of ecological constraints (availability of other individuals for group formation) that occurred alongside of lower relatedness may also account for this trend. In either case reproduction in choughs supports the concession model over the incomplete control model. Under conditions of severe constraints prior to the drought, choughs often bred incestuously, suggesting that incest avoidance may not confound predictions from the other two models. Instead the potential for inbreeding should be included in future models of reproductive skew
INTRODUCTION
In some animal societies, reproduction is monopolised by dominant individuals whereas in others it is distributed among a number of group members. The distribution of reproduction among individuals in a group is called reproductive skew and can vary from complete monopolisation by one or a few individuals ('despotic') to equal sharing of reproduction among all reproductive individuals ('egalitarian'). The level of skew in a group can be predicted from a model that was developed in the early 1980’s (Emlen 1982; Vehrencamp 1983). This optimal skew model is based on the following assumptions: (1) dominants benefit from the presence of subordinates, (2) subordinates can be bribed to stay in the group by being allowed to reproduce, and (3) dominants have control over the extent of subordinate reproduction. Because this model involves dominants conceding reproduction to subordinates, it has recently been termed the 'concession' model (Clutton-Brock 1998).
If the assumptions are met (see below), four qualitative predictions can be made (Keller & Reeve 1994). First, the degree of skew will increase with the degree of difficulty subordinates face in dispersing to breed independently. In other words, subordinates will require less reproductive 'incentives' to stay if their dispersal options are limited. Second, such incentives will be smaller when subordinates are closely related to the dominants. This is due to the indirect component of success they receive for helping to raise related offspring. Third, incentives will decrease with the magnitude of the increase in productivity brought about by the subordinates presence. Finally, incentives will increase relative to the subordinate’s fighting ability. This is particularly true in species where individuals have weapons and/or the ability to inflict severe injuries. These predictions have received general support from a large number of cooperative vertebrates and invertebrates (e.g. Bourke & Heinze 1994; Creel & Waser 1991; Jamieson 1997; Reeve & Nonacs 1997).
As persuasive and unifying as the theory appears, a recent review has questioned whether the assumptions of skew theory can be applied across species. In his review Clutton-Brock (1998) showed that when a variety of attributes of both dominants and subordinates are considered, predictions derived from the concession model are often indistinguishable from alternative hypotheses such as inbreeding avoidance and 'incomplete control' models (Emlen 1996; Cant 1998; Reeve et al. 1998). Results from empirical studies that support the concession model can usually be interpreted in the context of these alternatives. For example, incest-avoidance may leave subordinates in kin-groups with no potential mates that are unrelated, confounding predictions when individuals are close kin (Emlen 1996).
The key assumption that dominants benefit from the presence of subordinates seems tenuous, as the presence of subordinates has no apparent effect on group productivity in many avian cooperative breeders (e.g. Gaston 1978; Monadjem et al. 1995; Zahavi 1990). Where helpers appear to increase productivity, it is often problematic to determine causality (Brown 1982). Two further complications are that helpers are sometimes only taken on when needed (e.g. Reyer & Westerterp 1985), and that helpers may only help when needed (Magrath & Yezerinac 1997). In both scenarios the helpers' effects on productivity are difficult to quantify. Another argument is that instead of increasing productivity, helpers lighten the load for breeders; however, this has rarely been demonstrated (Cockburn in press; but see du Plessis 1991). In some situations, breeding by subordinates may even lower the direct fitness of dominants (Craig 1980; Koenig et al. 1983; Komdeur 1994; Zahavi 1990; also see discussion of 'incomplete control' models below).
It seems likely that for many cooperative breeders (especially those composed of close kin), a subordinate’s motive for philopatry may not be to gain either immediate breeding or indirect breeding success through productivity gains. For example, it may simply require a safe haven while waiting to mature (e.g. improve body condition or foraging skills, Heinsohn et al. 1988), be waiting for an outside breeding vacancy rather than become a floater (Mulder et al. 1994), or be making a longer term investment such as waiting to inherit the territory or mate. Subordinates may have no expectation of breeding while the dominant is present, but may try to win favour with the appropriate-sexed breeder should a vacancy arise (e.g. Clarke 1989; Reyer 1991; Bruce et al. 1996). In extreme cases help may even be a form of rent, and have no other fitness benefits for the subordinate beyond securing a place to stay (Mulder & Langmore 1993; Dunn et al. 1995; Koenig et al. 1996). Similarly, the dominant's motive for allowing the subordinate to stay may have nothing to do with increasing future productivity; it may simply be providing its offspring a safe haven for the above reasons (parental facilitation, e.g. Brown & Brown 1984).
In the concession model, the benefit that subordinates confer to dominants is usually portrayed as an increase in productivity. Many cooperatively breeding birds clearly do not meet this criterion. However, this does not necessarily mean that the concession model cannot be applied in these cases. Indeed, the main approach of research into avian cooperative breeding has been to try and explain the paradox of sacrificed reproduction in terms of the benefits of group living. A recent review (Cockburn in press) suggests that more often these benefits are direct (e.g. territory inheritance) rather than in the form of inclusive fitness from increased group productivity. However, if the subordinate is related to the dominant and philopatry aids the subordinate's survival or future reproductive success, then its presence in the group fulfils the assumption of enhanced productivity. Without recognition and quantification of these varying benefits to philopatry and helping, testing predictions of skew theory may be difficult. In particular, the effects of extended parental care and parental facilitation should be included in future models of reproductive skew (also see Emlen 1999).
The concession model also makes the critical assumption that dominants are able to control the reproductive contributions of the subordinates, although it does specify that a higher share of reproduction may be surrendered if the subordinates have fighting abilities that give them additional leverage. Clearly there are other circumstances amongst cooperatively breeding birds that would also make complete control by dominants unlikely. The most likely is the potential for surreptitious copulations with the breeding female (e.g. Davies 1992). In recognition of these situations, models of 'incomplete control' have recently been devised (Cant 1998; Reeve et al. 1998). These have provided an important boost to the field because in some cases they offer alternative predictions to those from the concession model.
Cant's (1998) incomplete control model is specifically about the effect of brood size on the decisions made by dominants and subordinates over whether to contribute to reproduction. He specifies that by contributing to a clutch, a subordinate female reduces the per capita fitness of a dominant's young. This leads to the interesting situation in which predictions based on relatedness are similar to those from the concession model, but for entirely different reasons. That is, subordinates will share less reproduction with relatives because to breed would lower their inclusive fitness. Importantly, this model is specific to females, and is unlikely to apply to situations where males are competing for a proportion of a fixed pool of reproduction (e.g. fertilisation of a clutch). In contrast, the net benefit of breeding to subordinate males is an increasing linear function of their level of paternity, with the implication that they should always attempt to gain the greatest possible share. Thus skew among males will always be determined by the degree of control one individual has over another's reproduction (Cant 1998).
A more general series of incomplete control models developed by Reeve et al. (1998) specifies a range of additional predictions that take the degree of symmetry in relatedness between the parties (i.e. whether they have equal relatedness to each other's offspring) into account. One is that reproductive skew should either decrease with, or be insensitive to, the degree of relatedness when relatedness is symmetrical (e.g. siblings breeding together, Reeve & Keller 1996). However, when relatedness is asymmetrical, the incomplete control model predicts no reproduction by subordinates under any circumstances. The latter situation is the most common amongst cooperatively breeding birds, (i.e. social groups comprised of parents and offspring), but unfortunately this prediction is not easily distinguishable from that of the concession model in which subordinate reproduction decreases with increasing relatedness (Keller & Reeve 1994). Reeve et al. (1998) do state that, all else being equal, reproduction by subordinates in parent-offspring groups is more consistent with concession models. We are however left in the unsatisfying predicament in which the lack of breeding by subordinates in parent-offspring associations can equally be explained by three different models (i.e. incest avoidance, concession, and incomplete control).
Cooperatively breeding birds with highly skewed reproduction
In this paper we discuss avian species that show extreme reproductive skew. These species tend to have less variation to explore, and, therefore, testing between the alternative models (concessions, incomplete control, incest avoidance) is problematic. However, we take the approach of surveying these species to see if they generally conform to the predictions of the concession model. We then examine a population of White-winged Choughs Corcorax melanorhamphos, a species that normally has high reproductive skew, but fortuitously (for us) underwent major demographic changes that both reduced high levels of relatedness and relaxed the ecological constraints normally in force.
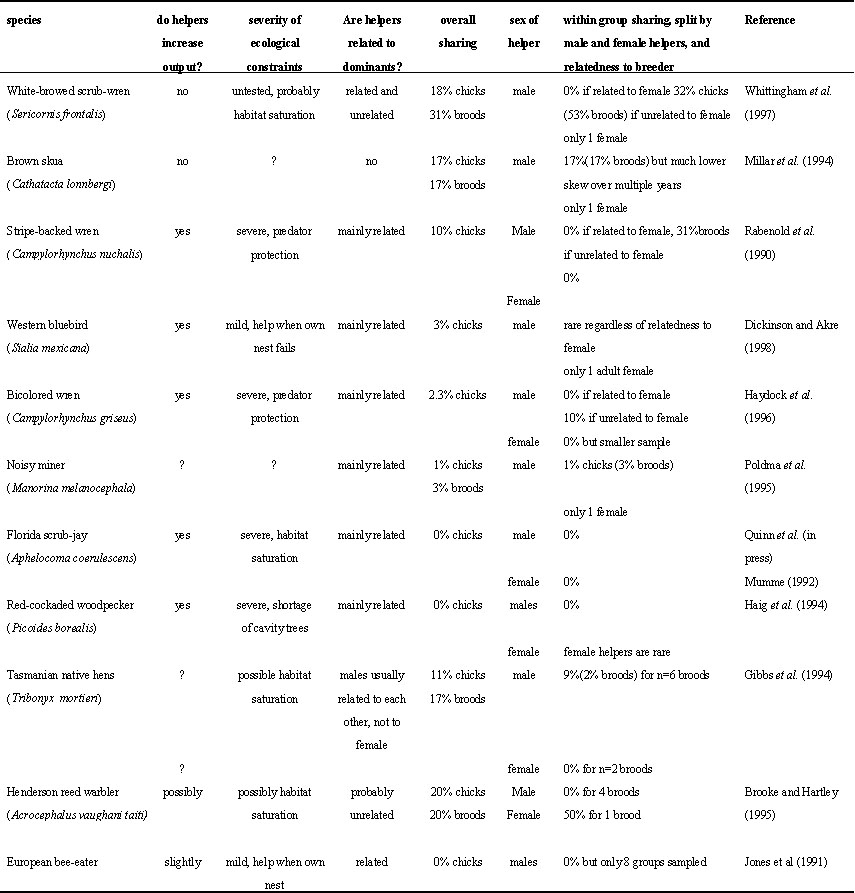
Tests of skew theory rely on molecular analysis of parentage within cooperative groups but relatively few studies (<25) using molecular techniques have been conducted on cooperatively breeding birds. We defined skewed societies (arbitrarily) as those in which 80% or more of offspring were direct descendants of one male in multi-male groups or one female in multi-female groups. We are aware of 12 studies that have confirmed such skewed societies in natural populations (Table 1). Of these, four report information from only eight or fewer broods (Gibbs et al. 94; Jones et al. 1991; Brooke & Harley 1995; Bruce et al. 1996), and consequently must be interpreted with caution. Studies using protein electrophoresis analysis were also excluded because of their limited power to assign parentage when the potential breeders are related. Obviously, our knowledge of the genetic mating system in avian cooperative breeders is still rather limited. Nonetheless, five general points can be made.
First, if we look at the eight species for which data are more substantial, it appears that these highly skewed societies are predominantly comprised of kin, as predicted by concession models. Given that most cooperative breeders live in groups of close kin (Emlen 1997), this is not too surprising. An exception is the brown skua, which appears to have high skew but unrelated helpers (Millar et al. 1994). However, there is a strong suggestion (from a small sample) that 'subordinate' males may monopolise breeding efforts in subsequent years. Thus it is dangerous to calculate skew values from single broods, and it may be necessary instead to consider total reproductive success over longer periods (e.g. the breeding season for multi-brooded species, or multi-year associations). In the four species with limited data, the Henderson reed warbler and bushtit both appear to defy predictions of the concession model, having unrelated helpers yet high skew. Although the small fingerprinting sample does not reveal sharing, the bushtits are suspected of multiple maternity and paternity from behavioural data (Sloane 1996), but as other species testify, behavioural and genetic information can be wildly conflicting (e.g. noisy miners, Dow & Whitmore 1990; Poldmaa 1995).
Second, five out of eight studies report that the presence of helpers increases production of young, thus conforming to the assumption of the concession model. However, as mentioned previously, measuring the effect of helpers in cooperative systems is fraught with confounding effects. The remaining three species highlight the difficulties of quantifying the effects of help. In white-browed scrubwrens helpers appear to have no effect (Magrath & Yezerinac 1997), in noisy miners the social system is too complex to isolate the helper effect (Dow & Whitmore 1990), whereas the advantage of co-operation in brown skuas remains elusive (Millar et al. 1994).
Third, in making cross-species comparisons, it is easier to compare some attributes than others. The nature and severity of ecological constraints are often unknown for cooperative breeders, and difficult to compare across species. For example, some species are considered to be constrained by the lack of available habitat (e.g. Florida scrub-jays Aphelocoma coerulescens, Woolfenden & Fitzpatrick 1990) while others need helpers to overcome predation (e.g. strip-backed wrens, Rabenold 1990). Another parameter of the concession model is aggression and fighting ability, for which information is even more scarce. Again, aggression rates are species specific, with some apparently peaceable birds still maintaining strict dominance hierarchies (e.g. Zahavi 1990), and other reproductively egalitarian species engaging in costly disputes (e.g. Jamieson 1997). Thus we have not attempted to compare ecological constraints and aggression between species.
Fourth, males appear to share reproduction more than females. Eight of the twelve species listed in Table 1 have breeding groups with more than one female, although in some of these female help is uncommon (e.g. Haig et al. 1994). Within these species, males usually outnumber females, and there is no confirmed evidence of female sharing. A possible exception is the Henderson reed warbler, but the information is from a single brood (Brooke & Hartley 1995). Mating may be more easily divisible for males. For example, subordinate males only need to copulate with the breeding female, and may succeed in doing so surreptitiously, but if a second female adds to the clutch she is likely to be detected and may push the clutch size above the optimum, to the detriment of all (Koenig et al. 1983; Cant 1998). Some communal females do share reproduction in this way (e.g. Koford et al. 1990), but this has been implicated in heightened competition (Jamieson 1997) and destructive practices (e.g. egg tossing, Koenig et al. 1995) between females. That subordinate females in these despotic societies are either non-existent or barred from reproducing, and that it is often mothers that chase their daughters from the natal territory (e.g. Mulder 1995), suggests a widespread unwillingness of dominant females to share reproduction, and perhaps of subordinate females to help.
Finally, although some of the attributes of these highly skewed avian societies support predictions made by the concession model, they are also consistent with alternative models. For example, inbreeding avoidance may generally explain high skew in kin groups. In all species where sharing occurred in Table 1 the breeding female mated with an unrelated subordinate male, suggesting incest avoidance. For both Camplylorhynchus wrens, sharing sometimes occurred when subordinates were unrelated to the dominant female, but related to the dominant male (Rabenold et al. 90, Piper & Slater 1993, Piper et al. 1995). These observations suggest that dominant males cannot control fully the reproductive behaviour of their subordinates. If we remove the problem of incest avoidance by looking only at those cases where subordinates are unrelated to the breeding female, the concession model predicts that sharing will increase when the subordinate is also unrelated to the dominant male. Only the data of the white-browed scrubwren (Whittingham et al. 97) are detailed enough to test this prediction. Subordinate males never mate with their mother, they father 19% of young (in 33% of broods) if they are related to the dominant male but not the female, and father 46% of young (in 78% of broods) if they are unrelated to both dominants. Taken together these results suggest that although incest avoidance is important, concessions based on relatedness may also be operating.
In summary, our understanding of the relationship between social systems and mating systems in avian cooperative breeders is hampered by lack of information. Genetic relatedness and parentage needs to be examined in more species, and probably in greater depth. For example, to test predictions from incomplete control models we need to compare the level of sharing when brothers live together rather than father-son associations (Emlen 1996, Reeve & Keller 1996).
Constraints on reproduction in White-winged Choughs
White-winged Choughs are large (350-450g) passerines in which unassisted pairs are incapable of reproducing, and reproductive success increases linearly across all group sizes (4-16 birds, Heinsohn 1991a, 1992). All group members assist the dominant pair in all aspects of reproduction from nest-building, nest defence, incubating eggs (3 weeks), feeding nestlings (4 weeks), to extended care of fledglings (up to 8 months). Cooperative breeding is linked to the acquisition of foraging skills, which improve markedly over their four year adolescence. Choughs sift through leaf litter and dig holes in soil in search of invertebrates, and the difficulty of foraging lies in knowing where to search and when to give up in an unproductive patch (Heinsohn unpublished). Young choughs (1-2 years old) are inefficient foragers and have difficulty in sustaining their own energy budgets, but help raise young within their limited capacity (Heinsohn et al. 1988; Heinsohn 1991b). Evidence that choughs of this age would not be able to raise young independently comes in the form of the costs they incur while helping. First year choughs lose body mass in proportion to the length of time they spend incubating eggs, at rates they would not be able to maintain if they were in unassisted pairs (Heinsohn & Cockburn 1994). Natal philopatry and extended parental care are essential for choughs over their period of adolescence (e.g. Heinsohn 1987; Heinsohn 1992). Evidence that foraging is indeed the limitation on reproductive success for small groups and young birds alike was provided in a series of food supplementation experiments. When food was provided ad lib young choughs fed at the same rate as older choughs, and groups of all size produced more fledglings (Boland et al. 1997).
Choughs of both sexes typically remain in their natal groups for life, although Rowley (1978) observed that occasionally groups would fragment upon the death of a breeder (which he assumed was the male, but see below). These fragments would coalesce with others to form new breeding groups. Another way groups acquire unrelated members is by kidnapping and raising fledglings from other groups which then become helpers (Heinsohn 1991c).
Although kidnapping has been frequent in the Canberra population (>20 cases noted in over 100 group-years), we had never observed fragmentation of groups as reported by Rowley (1978) before 1994. All groups under observation have shown stable membership over multiple years, although some entire groups have disappeared from the study area (Heinsohn 1992). We first noted mixing of group membership during a period of severe drought in late 1994 that reduced the population of colour-banded choughs by about 20%. This drought occurred over the normal period of breeding (September-February), and was preceded by the coldest winter on record (150 years) in the Canberra region (Bureau of Meteorology, Canberra, pers. comm.). The drought was known to affect adversely the breeding attempts of a variety of bird species, including superb fairy-wrens, Malurus cyaneus (A. Cockburn pers. comm.), crimson rosellas Platycercus elegans, (E. Krebs pers.comm.), white-browed scrubwrens, Sericornis frontalis (R. Magrath pers. comm.), and laughing kookaburras, Dacelo novaeguineae (S. Legge pers. comm.).
METHODS
Groups of White-winged Choughs have been colour-marked and studied in Canberra, Australia, from 1985 to 1989 (Heinsohn 1992), and again since 1993. For our study of the chough mating system we collected blood samples (about 100µl each) for DNA fingerprinting from 170 birds living in 26 groups in two areas 7 km apart (Campbell Park and Black Mountain, A.C.T. Australia) from 1993 until 1996, and from one group in an area 30km away (Tidbinbilla, Table I). Within these areas groups of choughs use undefended foraging areas of 20 ha during nesting to 1000 ha after they fledge their young (Heinsohn 1991a). Only the area immediately surrounding the nest is defended actively. The home ranges used by different groups often overlap considerably, leading to frequent contact between groups (Heinsohn 1987, 1988). All groups were censused at least once every 10 days from the beginning (September) to the end (March) of each breeding season.
Although choughs are monomorphic we were able to sex them by amplifying a homologue of the CHD gene which is sex-linked in many non-ratites (Griffiths et al. 1997). We aged, to the nearest 6 months, birds up to four years old based on eye colour (Rowley 1975). Birds older than four years old were all assigned the same age (4+) as eye colour does not change after this. Choughs do not usually reach sexual maturity until they are four (Rowley 1978), but we included all three year-olds in our search for possible parents of younger birds within groups. Multilocus DNA fingerprints were made using the per and 33.15 probes following methods in Mulder et al. (1994).
Because we are interested in the conditions under which reproduction is shared, we restrict our analysis of reproductive skew to group reproductive efforts for which we obtained blood from more than one young in a brood. The data for reproductive skew presented in this paper are thus from 17 groups with all (n= 6) or most (n= 11) of the birds sampled (Table 2 and Table 3). In eight cases, both parents were caught and we were able to determine parentage using the presence of novel fragments and band-sharing similarity (S of Lynch 1990). In the remaining groups we were able to establish with band-sharing coefficients (within 99 per cent confidence intervals) whether nestlings within broods were closely related and therefore produced by two or more parents. Four groups that either failed to breed or produced only one young are included in Table 2 and Table 3 to show group composition. We also use band-sharing between individuals separated by 30 km (Canberra vs. Tidbinbilla) to calculate band-sharing between unrelated individuals. Choughs are weak fliers and do not travel more than a few kilometres from their natal group (Heinsohn unpublished).
We attributed nestlings or fledglings to a pair of putative parents if the DNA profile of the parents contained all of the bands found in a nestling or fledgling (no novel fragments) and both parents shared a greater proportion of their bands with the nestling than an unrelated bird would have shared (see below). We estimated the mean similarity index (S ) within all 15 groups to examine genetic relatedness within groups, and to test predictions about reproductive skew under varying conditions. The standard error of S was corrected for non-independence in cases where we used overlapping pairs of individuals (Lynch 1990; see Results).
RESULTS
Band-scoring
The mean (± SD) number of scorable bands above 2 kb was 17.2±3.5 with per (range = 9 to 26) and 12.7±3.8 with 33.15 (range = 5 to 20). A segregation analysis of bands in a family of 12 young revealed that, in general, bands were transmitted independently and were inherited stabley over 5 generations.
Mating system before the drought
In three groups sampled in 1993 and 1994 we were able to identify the parents directly (Table 2; Orange at Black Mountain, Green and Blue at Campbell Park). There were no novel fragments when we compared the profiles of these putative parents and offspring, and thus we considered them parents. Although we sampled most of the individuals in five other groups (Table 2), we were not able to catch one or both of the oldest birds, which are most likely to be parents.
The following example involving the orange group at Black Mountain demonstrates our calculations and logic in deducing the relatedness between group members. In this group (Table 2), the two parents shared 0.68± 0.10 (mean±SD; range = 0.51-0.79, N=12) and 0.66± 0.10 (range = 0.48-0.81, N=12) of their bands (per and 33.15 probes averaged) with the 12 other birds in the group, three of which were sufficiently old (at least 4 years old) to be reproductively active, and nine were at least 1 to 4 years younger than the parents. All other combinations of adults produced at least 2 novel fragments in comparisons between these possible parents and the nestlings. The two parents shared 0.54 of their bands (mean of both probes) with each other. The lower 99% confidence limit (one-tailed) of band similarity between parents and offspring was 0.42 for one parent and 0.45 for the other parent (average S from both probes). The upper 99% confidence limit (one-tailed) for individuals in different areas, which we presumed were unrelated, was 0.33. These results suggest that the breeding pair were close relatives (r>0.25), although we cannot state for certain if they were first order relatives (r=0.5) with band-sharing alone, because we lack a known pedigree with various levels of relatedness, and distributions of band similarity among various types of relatives probably overlap considerably (see Lynch 1991; Haig et al. 1993).
In the Blue group at Black Mountain band-sharing was also high (Table 2), but we failed to collect blood from one of the two birds of breeding age before it died. Nevertheless, because we could detect which bands must have come from the dead parent, and very few consistently shared bands were necessary from the other parent, the data were consistent with a single pair producing all young over four years (1990 to 1993). In the Small Blue group at Campbell Park we identified the parents of four younger (3 months-2 years old) birds, that also shared 0.42 of their bands with each other, suggesting that they were also related. Our only example of unrelated parents occurred in the Green group at Campbell Park, in which the parents of two younger birds shared only 0.23 of their bands. We found that only pairs bred in the four groups for which we could determine the number of parents over 13 group-years of breeding prior to the drought. Brood sizes and number of parents for reproductive efforts producing more than one young in 1993, 1994 and early 1995 are shown in Table 2.
Based on band sharing from known relatives, 6/9 groups contained only close relatives (i.e. S> 0.42; Table 2). One of the three remaining groups contained only one non-relative, while another had two non-relatives, one of which was kidnapped. All of these unrelated individuals were relatively young (1-2 yrs old). Only the White group in Campbell Park was composed of two unrelated subgroups of six and four relatives (Table 2). Further evidence that dispersal is generally low and reproduction is kept within groups for long periods is that band similarity within groups was high relative to that between groups, with neighbouring groups as different from each other as from a group in another population 30km away (unpubl. data).
Mating system after the drought
The breeding season of 1994 occurred during the severe drought described above. The main effect was that no groups at Campbell Park attempt to breed during the usual period from September-January, although two groups bred successfully very late between January and March 1995. On Black Mountain, the orange group (13 members) produced one fledgling in October 1994.
The second obvious effect of the drought was the disappearance of some individuals from most groups under observation in 1993 and 1994. In all cases (n=5) where breeders were known it was the female who disappeared, and in 4 of the 5 cases the group fragmented into two or more portions. Fragments ranged from one to five individuals in size, and in most cases were of one sex. For example, of the original 14 members of the Orange group at Black Mountain, three (including the breeding female) disappeared in late 1994, and the remainder dispersed in fragments consisting of five males, three males, and three females. Similarly, the five colour-banded members of the red group disbanded into two fragments of three males and two females respectively. However, the white group with 13 members of varying degrees of relatedness showed an entirely different pattern, with one fragment of six birds forming a new breeding group (WOB), while the remaining seven individuals dispersed singly. In all cases except the WOB fragment of the white group, fragments from group break-ups united with other individuals or fragments to establish new breeding groups.
The data dealing with group fragmentation and reformation are generally complex, and will be presented in full elsewhere. Here we present one example (Fig. 1) of how the constraints and incentives for staying in the natal group normally experienced by choughs became relaxed after the repeated group break-ups during and after the drought. The small orange group of Campbell Park initially contained four members, but the breeding female and one other disappeared after a failed breeding attempt in 1994. The remaining two members, (OOO and BBO), who were (probably) unrelated males (S=0.357) had both joined another group of five (White/Oranges) that attempted to breed in early 1995. Both acted as helpers in a failed breeding attempt. OOO then left and joined the reproductive attempt of the white group (10 members), in which he helped raise two young, but failed to sire any of the young himself.
BBO remained with the White/Oranges and helped in the subsequently successful production of two young in late 1995. However he did not gain any paternity at that time either. He then left to join three other birds, two females that were related to each other and one unrelated male, and gained paternity of one of two young produced. Meanwhile, OOO’s group fragmented and he remained with five of them (WOB). Their first breeding effort failed, but OOO shared paternity in a successful breeding attempt by the end of 1995. The movements of OOO and BBO that led to their successfully becoming breeders are shown in a flow diagram in Figure 1.
In contrast to the above, one case of a breeding female disappearing did not lead to group fragmentation. In the Black Mountain blue group, the breeding female and another bird disappeared, but the remaining seven group members stayed together. There was no female of breeding age to replace her, and no extra-group females were seen attempting to fill the vacancy. The blue group failed to breed in 1994, but attempted to breed in 1995. In this case a young female who was presumably the daughter of the male breeder layed a clutch
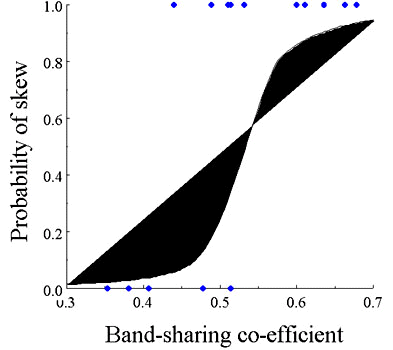
that failed to hatch. The eggs appeared to be infertile, so we could not collect DNA to confirm with fingerprinting that the old male who fathered all the previous young was again the father, this time breeding incestuously with his daughter.A major result of the remixing of groups was that mean relatedness (as measured by band-sharing) within groups was significantly lower in the 1995/96 sample of groups than it was in 1993/94 (t 27,2-tailed=2.96, P=0.006; Fig. 2). In marked contrast to data from before the drought, 7/15 groups that produced a brood with more than one young had shared paternity, and 2/15 had shared maternity (Table 3). Using all data (pre and post drought), we found that reproduction (maternity and paternity) was more likely to be shared when relatedness within groups as measured by band-sharing was lower (logistic regression, X21 = 4.3, P=0.039; Fig. 3).
DISCUSSION
White-winged Choughs represent one extreme among the range of social and mating systems found in birds. They are obligate cooperative breeders that do not breed successfully as unassisted pairs (Heinsohn 1991a, 1992). Our use of DNA-fingerprinting to examine the mating system of choughs leads to a number of insights that were not possible from observational studies. Until the last couple of years of this study, choughs had never been seen to disperse from their natal groups. Although groups clearly do break up from time to time (Rowley 1978), all observed recruitment in this study had been from reproduction and subsequent philopatry of young combined with occasional cases of kidnapping, suggesting that group fragmentation is a rare event (Heinsohn 1992).
The system changed after a period of unusual mortality in 1994, with many group break-ups occurring simultaneously within the population. The large-scale and repeated remixing of fragments from previous groups supported our contentions that the necessity of help for successful reproduction (Heinsohn 1991a; Heinsohn 1992) is normally a major constraint on dispersal and independent breeding. During the period of flux after the drought, many fragments repeatedly combined and broke up before settling into new combinations, suggesting both an unprecedented availability of other choughs with which to form groups, and a period of trial and bargaining between potential breeders.
Because our study covers a period before and after a major population restructuring, we have the unique opportunity to deduce the mating system in a period of relative population stability versus a period of extreme flux. Our data can be used to examine: (1) group structure and mating tactics when individual dispersal is severely constrained and within-group relatedness is high; (2) the strength of incest taboos under those conditions; (3) group structure and mating tactics when ecological constraints are relaxed, and (4) which models of reproductive skew, if any, apply.
Mating tactics under severe ecological constraints
Before and during the drought six of nine groups under observation were composed entirely of first order relatives (Table 2). Although these groups contained as many as five birds of breeding age (4+ years, Rowley 1978), single pairs appeared to monopolise reproduction (i.e., for 13 group years). At that stage of the project it was clear that the choughs had been living in entirely despotic societies for a number of years (Vehrencamp 1983). This result fits predictions from optimal skew theory in which high relatedness between group members, severe ecological constraints, and a strong helper effect on productivity should all make incentives to helpers unnecessary and reproduction easier to monopolise by the dominant pair (Emlen 1982; Vehrencamp 1983; Keller & Reeve 1994).
Significance of inbreeding
One unexpected and potentially enlightening result from the pre-drought results is that two of the four confirmed breeding pairs that bred over multiple years consisted of close (probably first order) relatives. This stands in contrast to the accepted wisdom that inbreeding is maladaptive and generally avoided via incest taboos (Pusey & Wolf 1996; Emlen 1997). There is strong evidence of incest taboos in many cooperatively breeding birds (e.g. Koenig & Mumme 1987), although rails appear to copulate incestuously at high rates (e.g. Craig & Jamieson 1988; McRae 1996) as do green wood-hoopoes, Phoeniculus purpureus (du Plessis 1992). However, the fitness costs of inbreeding may change over time (Barrett & Charlesworth 1991), and inbreeding may not be very costly if the genetic load of deleterious alleles has been purged by historical population crashes or by selection following repeated inbreeding (Templeton & Read 83). Thus the fitness costs of inbreeding may be reduced when it is a natural part of the social system, and this may be the case for some cooperatively breeding birds. Although many show strong incest taboos, other cooperatively breeding species appear highly inbred (e.g. Tasmanian native hens, Gibbs et al. 1994; red-cockaded woodpeckers, Haig et al. 1993).
The discovery of consistent inbreeding in choughs has important implications for skew theory, as it was previously suggested that inbreeding avoidance reinforces high skew in parent-offspring associations by shutting down intra-group mating options (Emlen 1996). Such a trend might confound the prediction from the concession model that high relatedness will encourage non-breeding by subordinates (Clutton-Brock 1998). Our data suggest that inbreeding is not always avoided in choughs and presumably arises when the dispersal options of individuals are limited. This is similar to trends reported for green wood-hoopoes (du Plessis 1992). It is interesting that such inbreeding occurred at a time when the groups were stable and there were few fragmented groups or individuals in the population searching for others to breed with. For example, three breeding-aged choughs stayed as helpers in the Black Mountain orange group until the death of the breeding female. This may represent an incest avoidance measure as all group members were highly related. However, at some time greater than four years prior to that, an individual that was closely related to the breeder filled a vacancy and occupied it for multiple years. It seems more likely that it was the enhanced potential to find new breeding opportunities, caused by the drought, that encouraged the break-up of the group at that time.
Interestingly, the four groups that broke up in this fashion all had extra birds of breeding age that could profit from joining new groups. The one that did not break up, the Black Mountain blue group, had no extra individuals of the appropriate age. The remaining seven members remained together and did not attempt to breed in 1994. By 1995, the oldest female was three years old and she attempted to breed incestuously with her father. Although it is clear that the younger group members would gain nothing by dispersing before they could either breed themselves, or at least help a breeding relative, it is harder to understand why the breeding male did not accept a new female into the vacant position. The data for choughs combine to suggest that, despite possible costs of inbreeding (e.g. lower fertility, Koenig 1982), incestuous mating is an option that must be considered in predictions from skew theory. Thus fears that incest avoidance might confound predictions, especially from the concession model (Emlen 1996) might prove unfounded, or at least not as serious as suspected (also see Emlen 1999).
Mating tactics under relaxed ecological constraints
After the drought, only one of 13 groups was composed entirely of relatives, with the remaining twelve consisting of up to three factions of relatives (Table 3). This change in population structure provided a fortuitous opportunity to test the prediction from the concession model that reproductive skew will decrease when relatedness decreases (i.e. dominants will offer incentives to subordinates to stay). The mean band-sharing coefficient for members of groups dropped significantly over this period (Fig. 2), and logistic regression showed that skew within groups was lower when band-sharing was lower (Fig. 3). In five of seven cases where breeding was shared, there was one female breeder and multiple males sharing paternity. In the remaining two cases, there were two male and two female breeders.
Unfortunately we cannot isolate lower relatedness as the change that drove reduced skew, as ecological constraints on individual dispersal were also relaxed. As illustrated in Figure 1, individuals not only dispersed either singly or with other group members, they also sometimes moved repeatedly between different groups. In a number of cases individuals kept joining new groups of choughs until they achieved some direct reproductive success. Such mobility was unprecedented in this study and suggests that new options for dispersing and evaluating different possible combinations had become available. This option for dispersal coincided with a marked reduction of the benefits of helping to raise close relatives. Direct reproductive success appeared to be the goal for many choughs, although interestingly individuals that failed in this respect rarely left the breeding effort they were assisting until its conclusion (fledged young or failure). Regardless of whether it was lower relatedness or relaxed ecological constraints (or both) that caused reduced skew, both patterns are predicted by the concession model but not by incomplete control models.
Models of reproductive skew
In summary, White-winged Choughs may prove to be an ideal species for tests of skew theory, having three important attributes that are not always available in other cooperatively breeding species. First, they have one of the strongest helper effects reported amongst birds (Heinsohn 1991a; 1992), that when combined with high relatedness within groups (the usual scenario) leaves no doubt about large indirect benefits from helping. Second, the ecological constraints that encourage group living are severe to the extent that pairs cannot breed without helpers, leaving most individuals no option but to remain philopatric. Such extremes lead to the clear prediction from the concession model that reproduction should be highly skewed. Data from the pre-drought period of this study confirm this prediction and show that reproduction can be monopolised by a breeding pair for many years, even when other breeding-aged individuals are also present. Finally, choughs appear willing to breed incestuously, thereby removing a major hurdle in distinguishing between alternative hypotheses. In particular, this allows rejection of incest avoidance as a possible cause of reproductive skew in kin groups. Thus our observation that the degree of skew increases as within-group relatedness and ecological constraints increase generally supports predictions from the concession model (Emlen 1982; Vehrencamp 1983; Reeve & Keller 1994) over those from incomplete control models (Reeve et al. 1998).
One final caveat must also be made. That is, we cannot tell whether reproduction by multiple males is driven by 'staying incentives' offered by the dominant, or by solicitation to multiple males by the female. Group size is so important for reproductive success in this species that females may benefit by offering paternity, and hence their own form of staying incentive, to a number of males (also see Magrath 1999). Such polyandry is a driving force for many cooperative species (Hartley & Davies 1994; Davies et al. 1996; Whittingham et al. 1997). The question of who allocates mating opportunities, i.e., the male through dominance, or the female through cooperative polyandry or surreptitious extra-pair copulations, may prove to be another important problem for skew theory.
ACKNOWLEDGEMENTS
We thank Stephen Emlen and Morne du Plessis for inviting our contribution to the symposium. We are also grateful to Chris Boland and Nina Cullen for assistance in the field, and to Rob Magrath for comments and discussion. Our research is funded by the Australian Research Council.
REFERENCES
Barrett, S.C.H. & Charlesworth, D. 1991. Effects of a change in the level of inbreeding on the genetic load. Nature, 353, 522-524.
Brown, J.L., Brown, E.R., Brown, S.D. & Dow, D.D. 1982. Helpers: effects of experimental removal on reproductive success. Science 215: 421-422.
Bruce, J.P., Quinn, J.S., Sloane, S.A. & White, B.N. 1996. DNA fingerprinting reveals monogamy in the bushtit, a cooperatively breeding species. Auk 113: 511-16.
Brown, J.L. & Brown, E.R. 1984. Parental facilitation: parent-offspring relations in communally breeding birds. Behavioral Ecology and Sociobiology 14: 203-209.
Boland, C.R.J., Heinsohn, R. & Cockburn, A. 1997. Experimental manipulation of brood reduction and parental care in cooperatively breeding white-winged choughs. Journal of Animal Ecology 66: 683-691.
Bourke, A.F.G. & Heinze, J. 1994. The ecology of communal breeding: the case of multiple-queen leptothoracine ants. Philosophical Tansactions of the Royal Society (London), Series B 354: 359-372.
Cant, M.A. 1998. A model for the evolution of reproductive skew without reproductive suppression. Animal Behaviour 55: 163-169.
Clarke, M.F. 1989. The pattern of helping in the Bell Miner (Manorina melanophrys). Ethology 80: 292-306.
Clutton-Brock, T.H. 1998. Reproductive skew, concessions, and limited control. Trends in Ecology and Evolution 13: 288-292
Cockburn, A. 1998. Evolution of helping behavior in cooperatively breeding birds. Annual Review of Ecology and Systematics (in press).
Craig, J.L. 1980. Breeding success of a common gallinule. Behavioral Ecology and Sociobiology 6:289-295.
Craig, J.L. & Jamieson, I.G. 1988. Incestuous mating in a communal bird: a family affair. American Naturalist 131: 58-70.
Creel, S.R. & Waser, P.M. 1991. Failures of reproductive suppression in dwarf mongooses (Helogale parvula): accident or adaptation? Behavioral Ecology 2: 7-15.
Davies, N.B. 1992. Dunnock Behaviour and Social Evolution. Oxford University Press, Oxford.
Davies, N.B., Hartley, I.R., Hatchwell, B.J. & Langmore, N.E. 1996. Female control of copulations to maximize male help: a comparison of polygynandrous alpine accentors, Prunella collaris, and dunnocks, P. modularis. Animal Behaviour 51: 27-47.
Dickinson, J.L. & Akre, J.J. 1998. Extrapair paternity, inclusive fitness, and within-group benefits in western bluebirds. Molecular Ecology 7: 95-105.
Dow, D.D. & Whitmore, M.J. 1990 Noisy miners: variations on the theme of communality. In: Stacey, P.B. & Koenig, W.D. (eds) Cooperative breeding in birds: 559-592. Cambridge; Cambridge University Press.
Dunn, P.O., Cockburn, A. & Mulder, R.A. 1995. Fairy-wren helpers often care for young to which they are unrelated. Proceedings of the Royal Society London, series B 259: 339-43.
du Plessis, M.A. 1991. The role of helpers in feeding chicks in cooperatively breeding green (red-billed) woodhoopoes. Behavioral Ecology and Sociobiology 28: 291-295.
du Plessis, M.A. 1992. Obligate cavity-roosting as a constraint on dispersal of green (red-billed) woodhoopoes: consequences for philopatry and the likelihood of inbreeding. Oecologia 90: 205-211.
Emlen, S.T. 1982. The evolution of helping. II. The role of behavioral conflict. American Naturalist 119: 40-53.
Emlen, S.T. 1996. Reproductive sharing in different types of kin associations. American Naturalist 148: 756-63.
Emlen, S.T. 1997. Predicting family dynamics in social vertebrates. In: Krebs, J.R. & Davies, N.B. (eds) Behavioural ecology: an evolutionary approach: 228-253. 4th ed. Oxford; Blackwell Science.
Emlen, S.T. 1999.Reproductive skew in cooperatively breeding birds: an overview of the issues. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2922-2931. Johannesburg: BirdLife South Africa.
Gaston, A.J. 1978. The evolution of group territorial behaviour and cooperative breeding. American Naturalist 112: 1091-1100.
Gibbs, H.L., Goldizen, A.W., Bullough, C. & Goldizen, A.R. 1994. Parentage analysis of multi-male social groups of Tasmanian native hens (Tribonyx mortierii): genetic evidence for monogamy and polyandry. Behavioral Ecology and Sociobiology 35: 363-71.
Griffiths, R., Daan, S. & Dijkstra, C. 1996. Sex identification in birds using two CHD genes. Proceedings of the Royal Society London, series B 263: 1171-76.
Haig, S.M., Belthoff, J.R. & Allen, D.H. 1993. Examination of population structure in red-cockaded woodpeckers using DNA profiles. Evolution 47: 185-94.
Hartley, I.R. & Davies, N.B. 1994. Limits to cooperative polyandry in birds. Proceedings of the Royal Society London, series B 257: 67-73.
Heinsohn, R.G. 1987. Age-dependent vigilance in winter aggregations of cooperatively breeding white-winged choughs (Corcorax melanorhamphos). Behavioral Ecology and Sociobiology 20: 303-306.
Heinsohn, R.G. 1988. Inter-group ovicide and nest destruction in cooperatively breeding white-winged choughs. Animal Behaviour 36: 1856-1858.
Heinsohn, R.G., Cockburn, A. & Cunningham, R.B. 1988. Foraging, delayed maturity and cooperative breeding in white-winged choughs (Corcorax melanorhamphos). Ethology 77: 177-86.
Heinsohn, R.G. 1991a. Evolution of obligate cooperative breeding in white-winged choughs: a statistical approach. Acta XX Congressus International Ornithologie 3: 1309-1316.
Heinsohn, R.G. 1991b. Slow learning of foraging skills and extended parental care in cooperatively breeding white-winged choughs. American Naturalist 137: 864-81.
Heinsohn, R.G. 1991c. Kidnapping and reciprocity in cooperatively breeding white-winged choughs. Animal Behaviour 41: 1097-1100.
Heinsohn, R.G. 1992. Cooperative enhancement of reproductive success in white-winged choughs. Evolutionary Ecology 6: 97-114.
Heinsohn, R. & Cockburn, A. 1994. Helping is costly to young birds in cooperatively breeding white-winged choughs. Proceedings of the Royal Society London, series B 256: 299-303.
Jamieson, I.G. 1997. Testing reproductive skew models in a communally breeding bird, the pukeko, Porphyrio porphyrio. Proceedings of the Royal Society London, series B 264: 335-40.
Jones, C.S., Lessells, C.M. & Krebs, J.R. 1991. Helpers-at-the-nest in European bee-eaters (Merops apiaster): a genetic analysis. In: Burke, T., Dolf, G., Jeffreys, A.J. & Wolff, R. (eds) DNA fingerprinting: approaches and applications: 169-192. Basel: Birkhäuser Verlag.
Keller, L. & Reeve, H.K. 1994. Partitioning of reproduction in animal societies. Trends in Ecology and Evolution 9: 98-102.
Koenig, W.D. 1982. Ecological and social factors affecting hatchability of eggs. Auk 99: 526-36.
Koenig, W.D., Mumme, R.L. & Pitelka, F.A. 1983. Female roles in cooperative breeding acorn woodpeckers. In: Wasser, S.K. (ed.) Social Behaviour of Female Vertebrates: 235-261. New York: Academic Press.
Koenig, W.D. & Mumme, R.L. 1987 Population Biology of the Cooperatively Breeding Acorn Woodpecker. Princeton; Princeton University Press.
Koenig, W.D., Mumme, R.L., Stanback, M.T. & Pitelka, F.A. 1995. Patterns and consequences of egg destruction among joint-nesting acorn woodpeckers. Animal Behaviour 50: 607-21.
Koenig, W.D., Van Vuren, D. & Hooge, P.N. 1996. Detectability, philopatry, and the distribution of dispersal distances in vertebrates. Trends in Ecology and Evolution 11: 514-17.
Koford, R.R., Bowen, B.S. & Vehrencamp, S.L. 1990. In: Stacey, P.B. & Koenig, W.D. (eds) Cooperative Breeding in Birds: 333-356. Cambridge: Cambridge University Press.
Komdeur, J. 1994. Experimental evidence for helping and hindering by previous offspring in the cooperative-breeding Seychelles warbler Acrocephalus sechellensis. Behavioral Ecology and Sociobiology 34: 175-86.
Lambert, D.M., Millar, C.D., Jack, K., Anderson, S. & Craig, J.L. 1994. Single- and multilocus DNA fingerprinting of communally breeding pukeko: do copulations or dominance ensure reproductive success? Proceedings of the National Academy of Science USA 91: 9641-45.
Lynch, M. 1990. The similarity index and DNA fingerprinting. Molecular Biology and Evolution 7: 478-484.
Lynch M. 1991. Analysis of population genetic structure by DNA fingerprinting. In: Burke, T., Dolf, G., Jeffreys, A.J. & Wolff, R. (eds) DNA fingerprinting: approaches and applications: 113-126. Basel: Birkhaüser Verlag.
Magrath, R.D. 1999. Problems of distinguishing among models of reproductive skew within populations of cooperatively-breeding birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2879-2893. Johannesburg: BirdLife South Africa.
Magrath, R.D. & Yezerinac, S.M. 1997. Facultative helping does not influence reproductive success or survival in cooperatively breeding white-browed scrubwrens. Journal of Animal Ecology 66: 658-70.
McRae, S.B. 1996. Family values: costs and benefits of communal nesting in the moorhen. Animal Behaviour 52: 225-45.
Millar, C.D., Anthony, I., Lambert, D.M., Stapleton, P.M., Bergmann, C.C., Bellamy, A.R. & Young, E.C. 1994. Patterns of reproductive success determined by DNA fingerprinting in a communally breeding oceanic bird. Biological Journal of the Linnaean Society 52: 31-48.
Monadjem, A., Owen-Smith, N. & Kemp, A.C. 1995. Aspects of the breeding biology of the arrowmarked babbler Turdoides jardineii in South Africa. Ibis 137: 515-18.
Mulder, R.A. & Langmore, N.E. 1993. Dominant males punish helpers for temporary defection in superb fairy-wrens. Animal Behaviour 45: 830-33.
Mulder, R.A., Dunn, P.O., Cockburn, A., Lazenby-Cohen, K.A. & Howell, M.J. 1994. Helpers liberate female fairy-wrens from constraints on extra-pair mate choice. Proceedings of the Royal Society London, series B 255: 223-229.
Mulder, R.A. 1995. Natal and breeding dispersal in a co-operative, extra-group-mating bird. Journal of Avian Biology 26: 234-40.
Mumme, R.L. 1992. Do helpers increase reproductive success? An experimental analysis in the Florida scrub jay. Behavioral Ecology and Sociobiology 31: 319-28.
Piper, W.H. & Slater, G. 1993. Polyandry and incest avoidance in the cooperative stripe-backed wren of Venezuela. Behaviour 124: 227-247.
Piper, W.H., Parker, P.G. & Rabenold, K.N. 1995. Facultative dispersal by juvenile males in the cooperative stripe-backed wren. Behavioral Ecology 6: 337-42.
Pöldmaa, T., Montgomerie, R. & Boag, P. 1995. Mating system of the cooperatively breeding noisy miner Manorina melanocephala, as revealed by DNA profiling. Behavioral Ecology and Sociobiology 37: 137-43.
Pusey, A. & Wolf, M. 1996. Inbreeding avoidance in animals. Trends in Ecology and Evolution 11: 201-206.
Quinn, J.S., Woolfenden, G.E., Fitzpatrick, J.W. & White, B.N. 1998. Multi-locus DNA fingerprinting supports genetic monogamy in Florida scrub-jays (in press).
Rabenold, K.N. 1990. Campylorhynchus wrens: the ecology of delayed dispersal and cooperation in the Venezuelan savanna. In: Stacey P.B. & W.D. Koenig (eds) Cooperative Breeding in Birds. Cambridge: Cambridge University Press; 157-196.
Rabenold, P.P., Rabenold, K.N., Piper, W.H., Haydock, J. & Zack, S.N. 1990. Shared paternity revealed by genetic analysis in cooperatively breeding tropical wrens. Nature 348: 538-40.
Reeve, H.K. & Keller, L. 1996. Relatedness asymmetry and reproductive sharing in animal societies. American Naturalist 148: 764-69.
Reeve, H.K. & Keller, L. 1997. Reproductive bribing and policing evolutionary mechanisms for the suppression of within-group selfishness. American Naturalist 150: S42-S58.
Reeve, H.K., Emlen, S.T. & Keller, L. 1998. Reproductive sharing in animal societies: reproductive incentives or incomplete control by dominant breeders? Behavioral Ecology 9: 267-278
Reyer, H.-U. 1990. Pied kingfishers: ecological causes and reproductive consequences of cooperative breeding. In: Stacey, P.B. & Koenig, W.D. (eds) Cooperative Breeding in Birds: 527-558. Cambridge: Cambridge University Press.
Reyer, H.-U. & Westerterp, K. 1985. Parental energy expenditure: a proximate cause of helper recruitment in the pied kingfisher (Ceryle rudis). Behavioral Ecology and Sociobiology 17: 363-69.
Rowley, I. 1975. Bird Life. Sydney; Collins.
Rowley, I. 1978. Communal activities among white-winged choughs Corcorax melanorhamphos. Ibis 120: 178-97.
Sloane, S.A. 1996. Incidence and origins of supernumeraries at bushtit (Psaltriparus minimus) nests. Auk 113: 757-70.
Templeton, A.R. & Read, B. 1983. The elimination of inbreeding depression in a captive herd of Speke's gazelle. In: Schoenwald-Cox, M., Chambers, S.M., MacBryde, B. & Thomas, L. (eds) Genetics and conservation: 241-261. Menlo Park; Benjamin Cummings.
Vehrencamp, S.L. 1983. Optimal degree of skew in cooperative societies. American Zoologist 23: 327-35.
Whittingham, L.A., Dunn, P.O. & Magrath, R.D. 1997. Relatedness, polyandry and extra-group paternity in the cooperatively-breeding white-browed scrubwren (Sericornis frontalis). Behavioral Ecology and Sociobiology 40: 261-70.
Woolfenden, G.E. & Fitzpatrick, J.W. 1984. The Florida Scrub Jay: Demography of a Cooperative-Breeding Bird. Princeton: Princeton University Press.
Zahavi, A. 1990. Arabian babblers: the quest for status in a cooperative breeder. In: Stacey P.B. & W.D. Koenig (eds) Cooperative Breeding in Birds: 103-130. Cambridge; Cambridge University Press.
Table 1. Characteristics of cooperatively breeding species with high degree (<20% sharing) of reproductive skew.

Table 2. Composition of all groups under observation in 1993, 1994, and early 1995, the number of fledglings produced (i.e. size of brood used in skew analysis), and the effects of drought. Those groups marked with (BM) are from Black Mountain, and the remainder are from Campbell Park. 'N relatives' refers to the number and size of factions of related individuals.
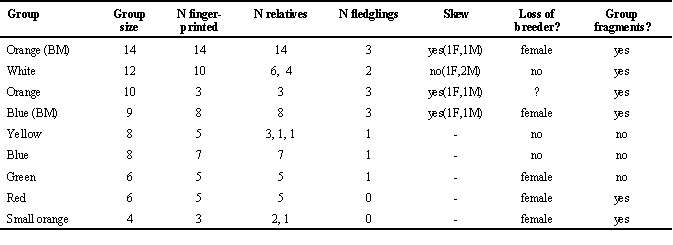
Table 3. Composition of all groups under observation in late 1995 and early 1996, the number of fledglings produced (size of brood used in skew analysis), and the extent of reproductive skew. Those groups followed by (BM) are from Black Mountain, and the remainder are from Campbell Park.
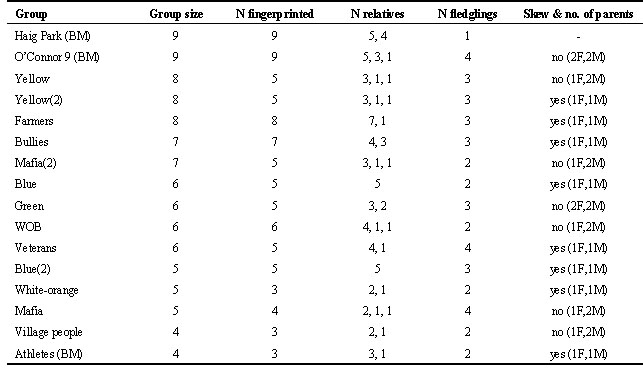
Fig. 1. A flow diagram depicting the movements of two adult males, OOO and BBO.
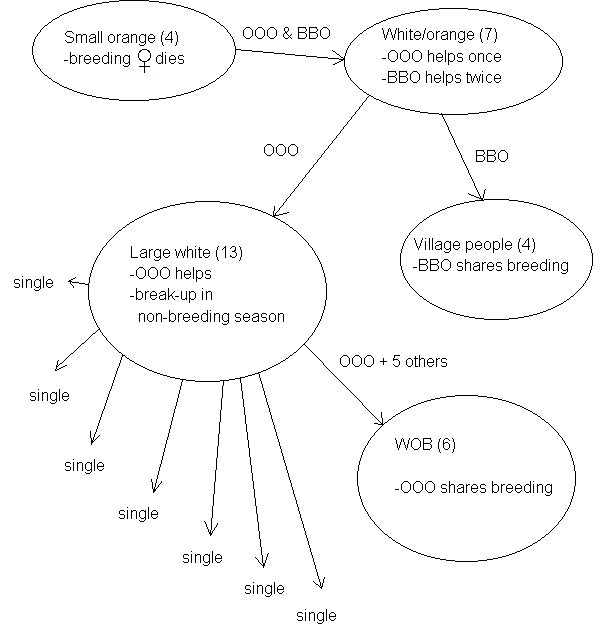
Fig. 2. Mean band-sharing coefficients in 1993/1994 (before and during drought) and 1995/1996 (after drought).
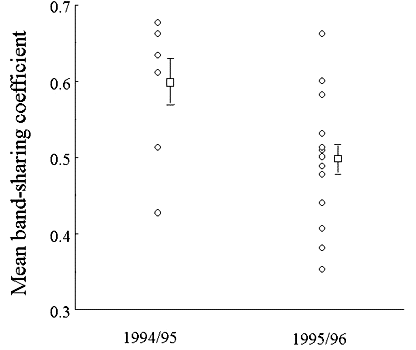
Fig. 3. Logistic regression showing whether reproduction was 100% skewed (1) or shared (0) against mean band-sharing coefficients for cooperative groups.