S46.5: Functional capacities of extraembryonic organs in bird embryos
J. Matthias Starck
Institute of Systematic Zoology and Evolutionary Biology, University of Jena, Erbertstraße 1, D-07743 Jena, Germany, fax 49 3641 949152, e-mail starck@pan.zoo.uni-jena.de, internet http://www.zoo.uni-jena.de/~starck
Starck, J.M. 1999. Functional capacities of extraembryonic organs in bird embryos. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2836-2856. Johannesburg: BirdLife South Africa.During embryogenesis, the extraembryonic organs, the chorioallantois membrane (CAM), and the yolk sac membrane (YSM), provide the structures for gas exchange (CAM) and resorption of nutrients (YSM). Morphometric parameters such as exchange surface, diffusion barrier, capillarisation, and blood perfusion rate determine the functional capacities of both organ systems. During development of the embryo, the CAM and YSM supposedly adjust their functional capacities to match the metabolic demands that increase with the growth of the embryo. A failure of the structural or functional parameters to match the embryonic demands may ultimately limit the patterns of embryonic growth and development because of limitations of the embryonic energy metabolism. The CAM reorganises twice to increase its functional capacity: (1) The diffusion barrier between blood and air is reduced, while (2) the capillary vascularization increases. Comparative data of altricial and precocial species (Gallus gallus, Coturnix japonicus, Melopsittacus undulatus, Columba livia) suggest that the functional capacity of the CAM and associated structural modifications play a major role in the evolution of avian developmental pathways. The development of the YSM is not so well known. Morphometric studies suggest that the resorptive surface of the YSM increases significantly during embryogenesis. However, yolk resorption is independent of a diffusion barrier because yolk is primarily resorbed by endocytosis. The data reviewed in this paper indicate the necessity for some extension of Rahn et al.'s (1974) 'diffusion capacity model' of avian embryogenesis. The study of functional capacities of CAM and YSM may add key functions to the developing egg.
INTRODUCTION
Bird embryos grow and develop in a semiclosed container, the egg. Only gases (oxygen, carbon dioxide, water vapour) can be exchanged through the egg shell. All other materials (water, minerals, nutrients) necessary for avian development must be deposited in the egg before laying. The physical properties of the egg shell, i.e., egg shell area, porosity, thickness, the specific permeability of the egg shell membrane, and the partial pressure of respiratory gases, determine a series of constraints for embryonic gas exchange (Wangensteen & Rahn 1970; Wangensteen et al. 1970; Paganelli et al. 1974; Hoyt & Rahn 1980; Rahn 1984; Ar & Rahn 1985; Booth & Seymour 1987; Rokitka & Rahn 1987; Tųien et al. 1988). Under a given incubating situation none of these parameters can be changed and the maximum gas exchange is set to a fixed value. Therefore, Rahn et al. (1974) suggested that avian embryonic development is diffusion limited, i.e., that incubation terminates when the increasing metabolic demands of the growing embryo exceed the invariable diffusion parameters of the egg shell (for review see Vleck & Bucher 1998).
However, for the developing embryo the constraints on gas exchange due to the egg shell are only one partial aspect. Embryonic gas exchange is provided by the chorioallantois membrane (CAM). In addition to the diffusion limitation through egg shell, the functional capacities of the CAM determine gas exchange parameters on the level of organ and tissue. The CAM develops through a intimate fusion of the chorion and the allantois. The chorion originates from the outer sheet of the amniotic folds and is composed of extraembryonic ectoderm and mesodermal components. The allantois develops from the embryonic gut consisting of an (outer) sheet of the splanchnopleura and an inner endodermal layer. When chorion and allantois fuse to form the CAM, embryonic materials from different sources combine to form a functional organ consisting of external ectodermal epithelium, inner endodermal epithelium, and the highly vascularised mesoderm in between. Within 12 days, the CAM covers the inner surface of the eggshell membrane. Its functional capacity is determined by four parameters: (1) efficiency of the physiological transport mechanisms, (2) the dimensions of the diffusion barrier, i.e., the gas-blood barrier, (3) the overall size of the exchange surface, and (4) the density of capillarisation. When the CAM has reached its maximum size, the size dependent parameters of functional capacity of CAM cannot increase further
The yolk sac membrane (YSM) is the place of nutrient resorption. Bird embryos live exclusively of yolk, that is composed of about one third protein and two thirds lipids. The yolk lipids are taken up by the YSM and metabolised to provide lipoproteins for utilisation by the embryo. The functional capacities of the YSM may be limited by: (1) efficiency of physiological transport mechanisms, (2) the overall size of the exchange surface, and (3) the density of capillarisation.
This paper reviews data about functional capacities of the extraembryonic membranes of bird embryos and combines it with some original, yet unpublished developmental data about the chorioallantois membrane and the yolk sac membrane. The paper elaborates on ideas that reach beyond the diffusion limitation model by Rahn et al. (1974) and intends to stimulate research that considers the functional role of extraembryonic membranes of bird embryos more directly.
METHODS
Review information originates from different sources, including yet unpublished materials. Therefore, methods are considerably heterogeneous. This section provides a short overview of materials and methods used in my lab, details of methods may be found in the papers cited.
Eggs and animals
Eggs and embryos of Japanese quail Coturnix japonica and Budgerigar Melopsittacus undulatus originate from breeding stocks in the laboratory. Ostrich Struthio camelus eggs have been purchased from a commercial breeder. Quail eggs and Ostrich eggs were incubated in an automatic incubator at 37.7°C and 80% relative humidity.
Light microscopy
All tissue material was preserved in 5% paraformaldehyde solution in 0.1m phosphate buffer (Sųrensen buffer) at pH 7.4. After dehydration in graded ethanol, specimens were embedded in resin (Historesin®), sectioned (section thickness 3 µm), and stained with a solution of 0.1% methylene blue and 0.1% thionin in 3:1 water and glycerol. For cryomicroscopy, incubated eggs were frozen without further treatment at about –10°C. Before sectioning, frozen eggs were transferred to the cryostat to cool down to –18°C. Complete series through entire eggs were made; section thickness was 8 µm. Sections were dried and stained with a combination of Sudan black and aldehyde fuchsin.
Electron microscopy
Tissue samples were preserved in 2.5% glutardialdehyde in 0.1m phosphate buffered saline. After washing the specimens were treated with osmiumtetroxide 1% for 12h. Embedding in epon and ultrathin sectioning followed standard protocols for electron microscopy.
Magnetic resonance imaging and spectroscopy
For magnetic resonance imaging and spectroscopy a 1.5 Telsa Vision®, Siemens was used. Sessions of MRI and MRS in average lasted 3 hours. A T1-weighted 3D-sequence (echo time TE = 20 ms, repetition time of TS = 6s) was used during the first three weeks of incubation. Continguous series of images of 0.7 mm slice thickness were made. Between 6th and 9th week, T1-and T2-sequences (for T2: TE = 120 ms; TR = 4465 ms) were applied to record continguous series through the entire egg with a slice thickness 2 mm (for details see: Grodd, in press; Starck and Grodd, unpublished manuscript).
Morphometry
A PC-based morphometry software (Jandel Sci.®) was used to measure morphometric parameters such as diffusion distance and resorptive surface.
The chorioallantois membrane
The formation of the amnion, the chorion, and the allantois follows more or less the same pattern in all birds. The cavities of the amnion and the chorion are formed by folds of extraembryonic ectoderm and somatic mesoderm that arise around the embryo. The amnion cavity encloses the embryo, and the chorion cavity extends to the inner shell membrane. Minor differences in the onset of amnion formation may result in heterochrony of the closure of the amniotic folds. Only few such differences have been reported and no obvious functional interpretation may be given. The allantois buds from the embryonic hind gut into the cavity of the chorion. As a diverticle of the embryonic gut, it consists of entodermal and splanchnopleuric material. After about five days, the allantois bud reaches the chorion epithelium. Both tissues fuse intimately and attach to the inner surface of the shell membrane. The fusion of both tissues forms the CAM. Because blood vessels develop only in tissues that contain splanchnic mesoderm, the addition of the allantois tissues to the chorion is essential for the development of blood vessels and the later function of the CAM for gas exchange. While the CAM functions as gas exchange organ, the cavity of the allantois functions as a sink and depot for nontoxic metabolic waste products. Metabolic waste products may be actively transported into the volume of the chorioallantois where there may be enriched above blood plasma level (Epple 1992).
Fitze-Gschwind (1973) studied the structural and functional development of the CAM in domestic chickens and showed that the CAM undergoes a dramatic structural reorganisation around the tenth day of incubation. This structural reorganisation results in a considerable reduction of the blood-air diffusion barrier (i.e., distance from capillary blood volume through endothelial cells, pericytes, connective tissue of chorion, chorion epithelium, extracellular matrix of shell membrane, the egg shell). During this reorganisation, the blood capillaries move from a topographic position below the chorion epithelium to a position between or even above the epithelial cells of the chorion, closer to the eggshell membrane (Fig. 1a). These data have been indirectly confirmed by Auspruck et al. (1974), who studied the cellular differentiation of the vascular epithelium in chicken eggs. In addition she showed, that cell proliferation of the CAM, particularily of the endothelial cover of the blood vessels, declined at day 11 of incubation, just when the CAM has reached its maximum extension. An identical structural reorganisation has been observed in the Muscovy Duck Cairina moschata, the Budgerigar, Melopsittacus undulatus (Arnoldi 1998) and Japanese Quail (Coturnix japonica; Arnoldi and Duncker, personal communication; Fig. 1b). Morphometric data available from Fitze-Gschwind (1973) show that the thickness of the diffusion barrier decreases from an average of 4.3 µm at day 8 to 0.8 µm at day 12 and 0.5 µm at day 14 (Fig. 2). Following Fick's law of diffusion, gas exchange depends not only on the exchange area but also on the thickness of the diffusion barrier. Therefore, the reorganisation of the CAM may be understood as a mechanism to impove gas exchange when the area of the CAM has reached its maximum size and no further size increase is possible. Figure 2 shows, that the reduction of the gas-blood diffusion barrier occurs right at the time (embryonic day 11) when the CAM has reached its maximum extension and cell proliferation activity ceases. These processes are paralleled by a constant increase of capillary volume (Fig. 2) as determined by Fitze-Gschwind (1973); thus, the functional capacity of the CAM for gas exchange may increase even further, when parameters of size and diffusion barrier can not further be changed.
It seems appropriate now, to amend the diffusion limitation model of Rahn et al. (1974), who considered embryonic gas exchange as invariably determined by the egg shell, with dynamic, embryo oriented data: Within the egg, the embryo can sufficiently supply its gas and energy needs during the first 4 – 8 days of its life by exchange through the vascularised area of the yolk sac (Reeves 1984). Later on, the CAM establishes the embryonic respiration organ that grows with increasing respiratory demands of the embryo until about day 11; then the CAM has reached its maximum area and covers the entire internal surface of the egg. Now, while the embryonic demands are still increasing, the reduction of the blood-gas diffusion barrier may provide a second process of improving the performance of the CAM until day 14. When changes of the size parameters (exchange surface) and diffusion distance have been exhausted at day 14, the functional capacity of the CAM may still further improve by a continued increase of capillary volume until day 19. Then, the beginning aeration of the lungs slowly shifts the embryonic gas exchange from CAM to the lung breathing. Also, the blood oxygen affinity increases continuously from day 8 to hatching (Reeves 1984). As pointed out by Rahn et al. (1974), the eggshell conductance and partial pressure differences of oxygen determine an external physical envelope within which the embryo and its extraembryonic tissue develop. The static diffusion-limited model of Rahn et al., which has been based on the physical parameters of diffusion, partial pressure of respiratory gases, metabolism, and incubation time, has to be extended by aspects of the CAM development and a more embryo-oriented view at the functional capacities and the limitations of tissues and organs themselves. Although there are not enough data available yet for a detailed extension of Rahn's diffusion limited model, it may well be, that it is not the physical container that constraints embryonic development but the functional capacity of the CAM.
The study of the CAM may offer interesting research avenues to understand some aspects of the size dependencies of avian embryonic metabolism and incubation time. Besides the physical container of the egg shell, developmental limitations through surface-volume ratio, embryonic blood oxygen affinity, blood-gas diffusion barrier of the CAM, and the density of capillarisation provide an interesting framework for the study of evolutionary diversification and constraints in avian embryonic development. Many of these ideas remain speculative, yet, but a deeper understanding of evolutionary diversification of avian embryonic growth, length of incubation period, and developmental stage at hatching hinges on these problems, and we must integrate structural analysis, morphometry, and physiology to solve them.
The yolk sac membrane
The yolk sac membrane develops from the hypoblast of the early germinal disk. The hypoblast contributes entoderm and mesoderm to the embryo as well as to the extraembryonic yolk sac. A clear distinction of entodermal and mesodermal material is not possible yet for the YSM but the dual origin is clearly recognisable in its potential to form resorptive epithelium (entodermal origin), mesenchyme, and blood vessles (mesodermal origin). Beginning immediately after the formation of the hypoblast, extraembryonic material overgrows the yolk sphere to form the YSM.
In principle, one would expect that the surface of the YSM, physiological transport characteristics, the diffusion barrier from yolk to blood, and the capillary density determine the functional capacity of the YSM similar to the gas exchange of the CAM. The size and form of the yolk are important structural parameters because the maximum resorptive surface is determined by the surface of the yolk. Widespread presumptions in textbooks about the yolk sac and its membrane, which date back to descriptions and drawings of Duval (1889) and Witschi (1959), depict the yolk as an unchanged ball and the YSM as an intestine-type surface with many villi or folds (Walker & Liem 1994; Kardong 1995; Klasing 1998). Following such presumptions, one would expect that the resorptive surface of the YSM decreases during embryogenesis as a function of yolk consumption and decreasing yolk volume. As a consequence of the increasing nutrient demands of the embryo a conflict will arise when nutrient resorption by the YSM cannot satisfy the nutrient demands of the embryo. As a working hypothesis, the structural differentiation and morphometrics of the yolk sac epithelium, size and form of the yolk deposit, and physiological transport mechanisms are the major parameters to study the functional capacities of the YSM.
The epithelium of the yolk sac membrane
The epithelia of the YSM and the epithelium of the embryonic gut show distinct cytological differentiations. While the epithelium of the early embryonic gut is composed of flattened cells, the extraembryonic yolk sac epithelium consists of highly differentiated cylindrical cells, which are actively taking up yolk as determined by the numerous cytoplasmatic vesicles containing yolk (Fig. 3). The transition from embryonic entoderm to extraembryonic yolk sac epithelium is sharp and clear. Epithelial differentiation occur early and may be observed even before blood islands and blood vessels have been developed (Fig. 3). Within two days, first blood islands and vessels appear, and the second main function of the yolk sac arises, i.e., erythropoiesis. The blood vessels develop in the splanchnic mesoderm. The early capillaries bulge slightly into the lumen of the yolk sac. The histological sections reveal a large number of haemopoietic stem cells differentiating in the edothelial wall of the capillaries, particularily the venous capillaries (Fig. 4).
Later in embryonic stage 31, the cytology of the epithelium of the YSM and the blood vessels has changed. Within the resorptive epithelium, the number of large vesicles has increased considerably. Only few vesicles contain stained yolk globules while the contents of most other vesicles do not stain, thus indicating changed enzymatic processes. The capillaries of the YSM arrange in internal folds of the YSM, with a central arterial capillary surrounded by a dense layer of curled venules. The central arterial vessles contain a few blood cells of different stage of differentiation while the venous vessles are highly loaded with erythropoietic stem cells (Fig. 5). The endothelial wall of yolk sac capillaries is the major source of blood cell formation in the early embryo.
In stage 31, each YSM artery is surrounded by a dense network of venous capillaries. The wall of these venous capillaries contains numerous proliferating haemopoietic stem cells, thus appears thickened as compared to the arterial wall (Fig. 5). The vessles run in folds of the YSM that reach into the yolk. However, such folds produce only a slight surface enlargement as compared with the villi of the gut (no morphometric data available yet) and they do not reach deeply into the yolk. From a histological and developmental point of view, the resorptive epithelium of the YSM and the gut are different and cannot be compared.
In all embryonic stages, the epithelium of the YSM is characterised by a number of small yolk vesicles at the apical end of the cells, very large central vesicles, and a number of smaller vesicles at the basis. The epithelial cells have no brush border. The yolk vesicles in the apical portion of the epithelial cells have the same size and appearance as those yolks globules in the yolk mass. Cryostat sections and Sudan black stains indicate that the lipid composition is unchanged as compared to the yolk. It is therefore highly probable that the yolk is absorbed by endocytosis of the epithelial cells. Although transmission electronmicrographs are not yet available, it appears highly probable that microvilli are absent. Epithelial cells of the YSM are different from enterocytes of the intestine, in structure and function.
During the late phases of embryonic development, erythopoiesis ceases from the venous capillaries. The cells of the resorptive epithelium increase in size, develop extremely large vesicles and disintegrate from the tissue; cell nuclei are barely recognisable (Fig. 6). Apical yolk globules cannot be recognised in the resorptive cells indicating that the endocytotic activity has slowed down. Vascularisation as declined and most capillaries have collapsed. The histology reveals a disintegrated tissue with probably low functional capacity.
To sum up, the epithelium of the yolk sac is highly differentiated and specialised for uptake of yolk. Cytological evidence and preliminary labelling experiments with fluorescent labelled microspheres suggest that yolk uptake is driven by an endocytotic processes. As concluded from histological studies enzymatic processing of the yolk occurs in large vesicles within those cells that incorporated the yolks globules. To the end of the embryonic period, the epithelium of the YSM disintegrates. It is assumed, that the uptake capacity of the YSM declines with cytological disintegration of the epithelium. There is no open connection between the yolk sac and the gut.
Changes of size and shape of the yolk
When the egg is formed the yolk is deposited as a spherical ball. However, during embryogenesis the yolk ball undergoes considerable changes in size and shape. Starck & Grodd (1997) and Starck et al. 1997 studied developmental changes of the yolk ball in the African Ostrich using magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), and colour-coding image analysis for morphometry. Interestingly, the yolk undergoes considerable and unexpected changes of size and form within short time after start of incubation (Fig. 7). During the first three weeks of incubation, the volume of the yolk increases to almost twice the volume it had immediately after laying (Starck & Grodd 1997). Later on, the volume slowly declines. However, when the chick hatches, the volume of the yolk is still larger than it was when the egg was laid. Such changes the of yolk volume are accompanied by changes of the form. When the egg is laid and before incubation the yolk is a ball (Fig. 8). After two weeks of incubation the yolk has flattened and represents a thick disk that extends through the entire egg. Magnetic resonance imaging reveals two different compartments of the yolk, that can clearly be distinguished by their different MR-signal intensity (Fig. 9). The upper compartment is in immediate contact with the embryo and the growing yolk sac and gives a bright MR-signal. The MR-signal from the lower compartment resembles that obtained form the yolk before incubation. Both compartments are clearly recognisable over five weeks incubation, later, they get increasingly mixed. After five weeks, they cannot be separated anymore.
A similar development of the yolk ball has been described by Ragosina (1961) for the domestic chicken, who reported and documented considerable changes of the form of the yolk sac and yolk sac membrane. Falen et al. (1991) used a high resolution MR-technology to study the structure of the yolk in the developing chicken egg. Their study has focused on the concentric rings of the yolk and not on the more general form changes of the yolk deposit. However, their published images show very clearly that after 8 days the yolk has flattened and forms a plug across the entire egg. A compartmentalisation of the yolk may be recognised from their images, but it has not been discussed in the paper.
Recent studies of Japanese Quail yolk sac development using serial frozen sections through entire eggs (Fig. 10a, b) show the very same pattern as discussed above for Ostrich and domestic chicken (Starck, unpublished material). Briefly after beginning of incubation the yolk flattens and changes into a plug that extends horizontally across the longitudinal axis of the egg. A compartmentalisation of the yolk may be recognised after few days of incubation. These data are in contrast to the paradigmatic drawings of Duval (1889), Witschi (1959) and their successors. In contrast to the conventional view, the yolk changes its form and shape considerably during embryogenesis. Furthermore, the yolks is not only compartmentalised into two fractions, it also increases its overall volume and surface (see below), thus providing a larger resorption surface for the YSM.
Composition of yolk
Starck & Grodd (1997) performed MRS to study the material composition, i.e., water and lipid contents, of the two compartments they found using MRI. Before incubation, the water to lipid ratio is approximately 1:1 (cf. Mehner 1983 for chicken). During the following two weeks of incubation the two yolk compartments undergo significant changes of water contents. The lower yolk compartment increases its water content to a ratio of about 4:1. The upper yolk compartment reaches a water to lipid ratio of 25:1. Papers by Ronen et al. (1995) and Görke & Kimmich (1995) using a MR-diffusion imaging technique (diffusion-weighted spin-echo sequences) have shown that large quantities of water are mobilised from the albumen and transported to the yolks during early embryogenesis, thus blowing up the yolk. Although the phenomenon has not yet studied in detail, and evidences come from different sources, the data suggest the idea that the original yolk ball may be a highly condensed storage that cannot be used by the embryo. When incubation begins, water from the albumen is moved to the yolk and modifies it to become available for resorption by the embryo.
Fate of the yolk residues
Briefly before hatching, all bird embryos incorporate the residues of the YSM into their belly (Fig. 11). The relative amount of yolk residues depends on egg size and mode of development, i.e., in general, precocial hatchlings have a larger yolk residues than altricial hatchlings (Schmekel 1960; Carey 1983; Ar et al. 1987). The conventional wisdom assumes that yolk residues are used as energy store for the hatchlings first few days after hatching (e.g., Witschi 1959; Van Tyne & Berger 1976; O'Connor 1984; Welty & Babtista 1988; Bezzel & Prinzinger 1990). However, a number of observations suggests that there might be other, potentially more important functions associated with the yolks residues. A simple test of the nutrient storage hypothesis may be performed by restricting food consumption of bird hatchlings and compare the yolk residues of the restricted birds with those of non-restricted birds. The storage hypothesis would predict that the group experiencing food restrictions should use up yolk residues faster that the untreated controls. Early studies published by Schilling & Bleeker (1928), Parker (1929), Schmekel (1960), Noy et al. (1996) and unpublished data by Starck are in contrast to the predictions. These studies could not reveal significant difference in yolk mass of food restricted and control birds. Unexpectedly, the mass of the residual yolk and residual YSM decreased at the same rate in restricted and unrestricted birds. Noy et al. (1996) found that yolk was resorbed at faster rate when chicks were fed. Thus, there are indications that the rate of yolk resorption is independent of the nutritional state of the chick. This is not surprising if one takes into account that all bird hatchlings have considerable subcutaneous fat deposits (no quantitative data available), which may serve as energy stores for the first few days after hatching.
One may ask now, what other functional aspects might be associated with the residues of the yolk and the YSM. Both, the yolk and the embryonic yolk sac have more and different functions than just keeping food available for the growing embryo. As pointed out above, the embryonic yolk sac is the major place of embryonic haematopoiesis (Schmekel 1962, Dieterlen-Ličvre 1987, Cumano et al. 1995). Liver, spleen, and bone marrow, develop erythropoietic activity just at the end of the embryonic period when erythropoiesis in the yolk sac membrane ceases. However, Schmekel (1962) showed that yolk sac hematopoiesis in the Swift Apus apus, Budgerigar Melopsittacus undulatus, Pigeon Columba livia f. dom. and all songbirds studied so far, continues at a high level until the end of the first week of postnatal live. Thus, at least in those species the residues of the yolk sac membrane function as hematopoietic organs in addition to liver, spleen, and bone marrow as in other birds. It would be tempting to speculate that persistence of the yolk sac membrane as a functional erythropoietic organ is related to the fast growth of these highly altricial species and that the additional source of red blood cell is necessary to sustain the high demands for blood cells by the growing chick that, otherwise could not been matched by liver, spleen and bone marrow.
Another hypothesis focuses on the developing immunocompetence of embryos and chicks. The growing embryo has no immune competence of his own, except for cellular immune defence which occurs during later phases of embryonic development (see Klasing, this volume). In young birds, specific or acquired immunoglobulin mediated immunity is not attained before the second week after hatching. However, the yolk contains considerable concentrations of maternal immunoglobulins. Maternal immunoglobulins (IgG) are transferred to the yolk during egg formation in the ovary. In addition to yolk immunoglobulins the albumen contains IgA and IgM which are deposited in the oviduct when the egg passes the magnum (Lösch 1996). Maternally derived antibodies are the major source of immunoprotection during the early phases post hatching (review in Apanius 1998). One may hypothesise, that yolk residues are rather for mother's immunoglobulins than for the chicks food.
DISCUSSION
Today, extraembryonic organs are neglected organs, although they position key roles for many functions during avian embryonic development. A small number of publications presents data that help to develop a picture of the functional capacities of extraembryonic membranes in birds. For the CAM, it becomes obvious that Rahn et al.'s (1974) 'diffusion limitation model' describes only the invariable envelope within which avian incubation time is determined by a relationship of metabolic demand and the invariable physical properties of the egg. However, the morphometric data suggest that within such invariable envelope, the exchange capacities are adjusted to match the embryonic oxygen demands. Our comparative knowledge about the mechanisms that improve performance is still limited to a few species.
Until today, the YSM is an almost unknown organ. Widespread presumptions about its structure and function are based on late 19th century misinterpretations and oversimplifications. In this paper, data have been presented that reveal the YSM as an organ whose cells are specialised for endocytosis of yolk. The YSM is a no-gut digestive organ, but cytology and microscopic anatomy of tissues have solved the problems in a different way from intestinal tissue. The resorptive cells of the YSM pick up yolk globules by endocytosis rather than by membrane-bound transport systems. A brush border (microvilly) is missing in YSM epithelial cells. The surface magnification of the YSM by blood vessels being submersed into the yolk is probably low. Yolk residues may have different function than just being nutrient reserves. Because the nutrient storage hypothesis cannot predict the constant rate of yolk resorption, that has been observed independent of the nutritional state of chicks, erythropoiesis and maternally transmitted immunocompetence have been suggested as two functions of the yolks sac that advocate for a maintenance of the yolk beyond hatching and for a smooth transition form embryonic to more mature function.
ACKNOWLEDGEMENTS
I gratefully acknowledge unpublished materials of the CAM development in Budgerigars and Muscovy made available by Duck Hans-Rainer Duncker and Barbara Arnoldi. I thank Sybille Koch and Margret Roser for technical support. Research was supported by grants from the German Research Foundation (STA 345/2-2 and STA 345/5-1,5-2) and the Research Committee of the German Ornithological Society.
REFERENCES
Apanius, V. 1998. Ontogeny of immune function. In: Starck, J.M. & Ricklefs, R.E. (eds) Avian growth and development. Evolution within the altricial-precocial spectrum. New York; Oxford University Press: 203–222.
Ar, A. & Rahn, H. 1985. Pores in the avian eggshells: Gas conductance, gas exchange, and embryonic growth rate. Respiration Physiology 61: 1–20.
Ar, A., Arieli, B., Belinsky A. & Yom-Tov, Y. 1987. Energy in avian eggs and hatchlings: utilization and transfer. Journal of Experimental Zoology Supplement 1: 151–164.
Arnoldi, B. 1998. Histologisch-quantitative Untersuchungen der Chorioallantoismembran bei Moschusente und Wellensittich. PhD Thesis, Justus-Liebig-University, Giessen, Germany.
Auspruck, D.H., Knighton, D.R. & Folkman, J. 1974. Differentiation of vascular endothelium in the chick chorioallantois: A structural and autoradiographic study. Developmental Biology 38:237–248.
Bezzel, E., & Prinzinger R. 1990. Ornithologie. 2nd edition. Stuttgart. UTB für Wissenschaft Große Reihe, Eugen Ulmer Verlag.
Booth, D.T. & Seymour R.S. 1987. Effect of eggshell thinning on water vapor conductance of Malleefowl eggs. The Condor 89: 453–459.
Carey, C. 1983. Structure and function of avian eggs. Current Ornithology 1:69–103.
Cumano, A., Garcia-Porrero, J., Dieterlen-Ličvre F. & Godin I. 1995. Hématopočse intra-embryonaire chez la souri. Compte Rondue Société de Biologie 189: 617–627.
Dieterlen-Ličvre F. 1987. Hemopoietic cell progenitors in the avian embryo: Origin and migration. Annals New York Academy of Sciences 511: 77–87.
Duval, M. 1889. Atlas d'Embryologie. Paris.
Epple, A., Gill, T.S. & Nibbo, B. 1992. The avian allantois: A depot for stress-released catecholamines. General and Comparative Endocrinology 85: 426–476.
Fahlen, S.W., Szeverenyi N.M., Packard, D.S. & Ruocco, M.J. 1991. Magnetic resonance imaging study of the yolk in the developing avian egg. Journal of Morphology 209: 331–342.
Fitze-Gschwind, V. 1973. Zur Entwicklung der Chorioallantoismembran des Hühnchens. Advances in Anatomy, Embryology, and Cell Biology 47: 1–52.
Görke, U., Weis, J. & Kimmich, R. 1995. NMR-Microscopy of tranmsport in fertilized bird eggs. In: 3rd International Conference on Magnetic Resonance Microscopy. Heidelberg: 118.
Grodd, W. 1998. Introduction to magnetic resonance imaging and spectroscopy. Zoology — Analysis of Complex Systems 101: 174-199.
Hoyt D.F. & Rahn, H. 1980. Respiration of avian embryos — a comparative analysis. Respiration Physiology 39: 255–264.
Kardong, K. 1995. Vertebrates: Comparative anatomy, function, evolution. Dubuque, Iowa; WM. C. Brown Publishers.
Klasing, K. 1998. Comparative avian nutrition. New York, CAB International.
Lösch, U. 1996. Wie kommen die Antikörper ins Hühnerei. Altex 13: 15-17
Mehner, A. 1983. Eibildung. In: Mehner A., & Hartfiel, W. (eds) Handbuch der Geflügelphysiologie; Vol. 2. Jena, VEB Fischer Verlag: 953–1044.
Noy, Y., Uni, Z. & Sklan, D. 1996. Routes of yolk utilisation in the newly hatched chick. British Poultry Science 37: 987–995.
O'Connor, R. 1984. The growth and development of birds. Chichester, John Wiley & Sons.
Paganelli, C.V., Olszowka, A. & Ar, A. 1974. The avian egg: Surface area, volume, and density. The Condor 76: 319–325.
Parker, S.L. 1929. Effects of early handicaps on chickens as measured by yolk absorption and body weight to twenty weeks of age. Hilgardia 4.
Ragosina, M.N. 1961. Embryonic development of the domestic chicken in relation to the yolk and extraembryonic membranes. Moscow; Academy of Sciences (in Russian).
Rahn, H. 1984. Factors controlling the rate of incubation water loss in bird eggs. In: Seymour, R.S. (ed.) Respiration and metabolism of embryonic vertebrates; Dordrecht; Dr W Junk Publishers: 271–288.
Rahn, H. Paganelli, C.V. & Ar, A. 1974. The avian egg: air-cell gas tension, metabolism, and incubation temperature. Respiration Physiology 22: 297–309.
Reeves, R.B. 1984. Blood oxygen affinity in relation to yolk-sac and chorioallantois gas exchange in the developing chick embryo. In: Seymour, R.S. (ed.) Respiration and metabolism of embryonic vertebrates; Dordrecht; Dr W Junk Publishers: 231–244.
Rokitka M.A. & Rahn, H. 1987. Regional differences in shell conductance and pore density of avian eggs. Respiration Physiology 68: 371–376.
Ronen, I., Ar, A. & Navon, G. 1995. Identification of water compartments in developing bird embryos by NMR microscopy. In: 3rd International Conference on Magnetic Resonance Microscopy. Heidelberg: 42.
Schilling, S.J. & Bleeker, W.L. 1928. The absorption rate of the reserve yolk in baby chicks. Journal of the American Veterinary Medical Association 72.
Schmekel, L. 1960. Daten über das Gewicht des Vogeldottersackes vom Schlüpftag bis zum Schwinden. Revue Suisse de Zoologie 68: 103–110.
Schmekel, L. 1962. Embryonale und frühe postnatale Erythropoiese in Leber, Milz, Dottersack und Knochenmark der Vögel. Revue Suisse de Zoologie 69: 559–615.
Starck J.M. & Grodd W. 1997. Magnetic resonance imaging (MRI) anatomy of developing ostrich embryos (Struthio camelus Struthioniformes). Verhandlungen der Deutschen Zoologischen Gesellschaft 90:215.
Starck J.M., Grodd W., & Rombock S. 1997. Magnetic resonance imaging of the development of ostrich (Struthio camelus Struthioniformes) embryos. Journal of Morphology 232:327.
Starck, J.M. 1998. Structural variants and invariants in avian embryonic and postnatal development. In: Starck, J.M. & Ricklefs, R.E. (eds) Avian growth and development. Evolution within the altricial-precocial spectrum. New York; Oxford University Press: 59–88.
Tųien Ų., Paganelli, C.V., Rahn, H. & Johnson, R.R. 1988. Diffusive resistance of avian eggshell pores. Respiration Physiology 74: 345–354.
Van Tyne, J. & Berger A.J. 1976. Fundamentals in ornithology. New York, John Wiley & Sons.
Vleck, C.M. & Bucher T.L. 1998. Energy metabolism, gas exchange, and ventilation. In: Starck, J.M. & Ricklefs, R.E. (eds) Avian growth and development. Evolution within the altricial-precocial spectrum. New York; Oxford University Press: 89–116.
Walker, W.F. Jr., & Liem, K.F. 1994. Functional anatomy of the vertebrates. An evolutionary perspective. 2nd. Ed. Fort Worth; Saunders College Publishing.
Wangensteen, D.O. & Rahn, H. 1970. Respiratory gas exchange by the avian embryo. Respiration Physiology 11:31–45.
Wangensteen, D.O., Wilson, D. & Rahn. H. 1970. Diffusion of gases across the shell of the hen's egg. Respiration Physiology 11: 16–30.
Welty, J.C. & Babtista, L. 1988. The life of birds. 4th edition. New York; Saunders College Publishing.
Witschi, E. 1959. Development of Vertebrates. Philadelphia; Saunders.
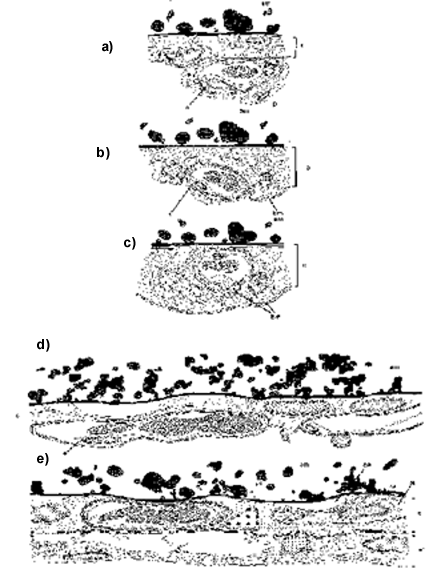
Fig. 1. Developmental changes of the chorioallantois membrane between day 8 and day 14 of embryonic development. (a) The CAM of an 8-day-old embryo of domestic chicken. The capillary is below the chorion epithelium (subepithelial position), as indicated by its position relative to the basal membrane of the chorion epithelium. (b) The CAM of a 10-day-old embryo of domestic chicken. The capillaries have moved between the cells of the chorion epithelium (intraepithelial position). (c) The CAM of a 12-day-old embryo of domestic chicken. The capillary has reached its final position close to the shell membrane; note the position of the capillaries relative to the basal membrane of the chorion-epithelium (from Starck 1998, a – c redrawn from Fitze-Gschwind 1973). (d) The CAM in a 10-day-old budgerigar embryo; (e) 17-day-old Budgerigar embryo (from Starck 1998; redrawn with kind permission of B. Arnoldi and H.-R. Duncker.) Abbreviations: c indicates chorion epithelium; e, endothelial cell of capillary; p, pericyte; sm, shell membrane.

Fig. 2. Morphometry of the chorioallantois membrane in domestic chicken; size of the exchange area (open circles), thickness of diffusion barrier (filled circles), and capillary volume (filled triangles; additional right hand axis). Data from Fitze-Gschwind (1973); redrawn from Starck (1998).

Fig. 3. Yolk sac membrane of 2-day old embryo of Japanese Quail embryo (HH Stage 14). Microphotograph; Rüdeberg-stain; section thickness 3 µm. Abbreviations: res = resorptive epithelium of the YSM, sp = extraembryonic splanchnopleural sheet.
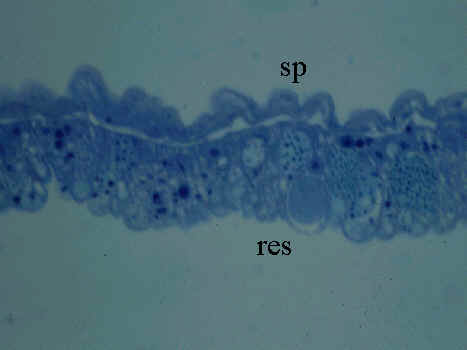
Fig. 4. Yolk sac membrane of 3-day old embryo of Japanese quail (HH stage 20). Microphotograph, Rüdeberg-stain; section thickness 3 µm. Abbreviations: a = artery, res = resorptive epithelium of the YSM, sp = extraembryonic splanchnopleural sheet, v = vein.
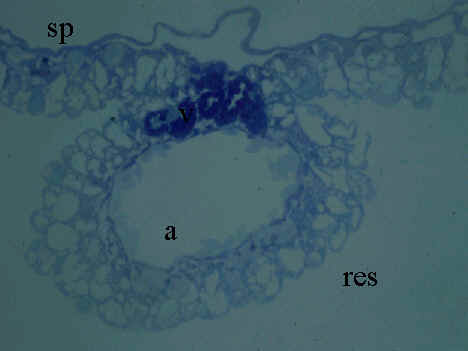
Fig. 5. Yolk sac membrane of 6-day old embryo of Japanese quail (HH stage 31). Microphotograph, Rüdeberg-stain; section thickness 3 µm. Abbreviations: a = artery, res = resorptive epithelium of the YSM, sp = extraembryonic splanchnopleural sheet, v = vein.
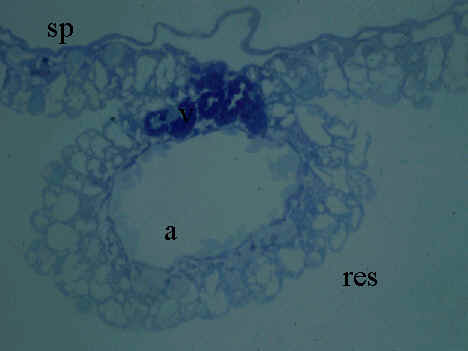
Fig. 6. Yolk sac membrane of Japanese quail hatchling. Microphotograph, Rüdeberg-stain; section thickness 3 µm. Abbreviations: a = artery, res = resorptive epithelium of the YSM, sp = extraembryonic splanchnopleural sheet, v = vein.
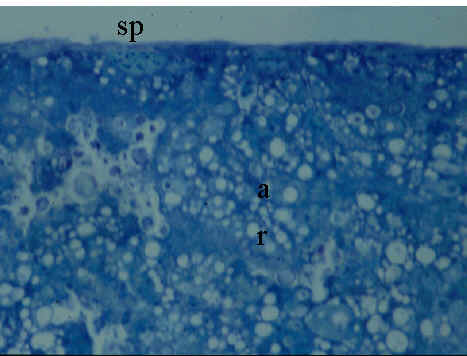
Fig. 7. Morphometry of the yolk sac volume during embryogenesis of the Africal Ostrich (Struthio camelus). Complete series of magnetic resonance images were used as basis for morphometry of the yolk sac and its compartments (Compare Fig. 8 and Fig. 9 for MR-Imaging; from Starck & Grodd, 1997).
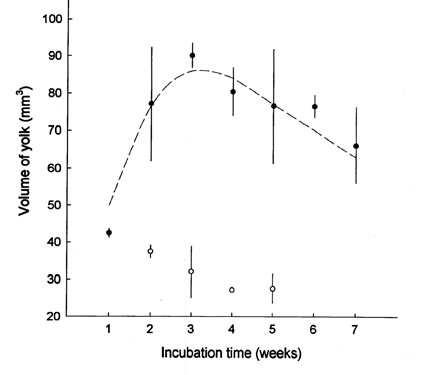
Fig. 8. Magnetic resonanc image of an ostrich egg before incubation. Abbreviations: y = yolk, n = nucleus of Pander.
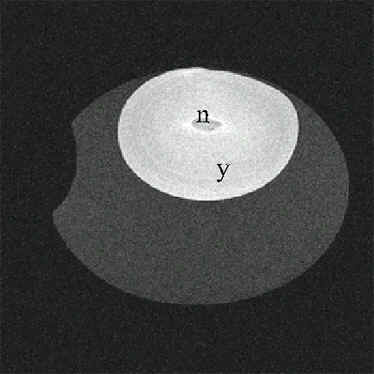
Fig. 9. Magnetic resonanc image of an ostrich egg after 1 week incubation. Note the changes of size and form of the yolk. Two different compartments are recognisable in the yolk. Abbreviations: y1 = dense yolk compartment (high lipid, low water content), y2 = diluted yolk compartement (low lipid, high water content).
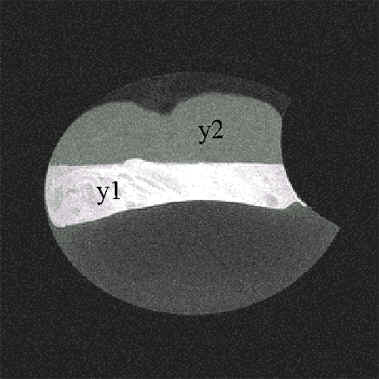
Fig. 10. Cryostat sections of Japanese quail eggs (a) before incubation; (b) after 10 days incubation. Note the flattening of the yolk and the compartmentalisation into a densely stained lower part and a less dense stained part. Section thickness 8µm, Sudan black stain. Abbreviations: a= albumen, e = embryo, y = yolk.
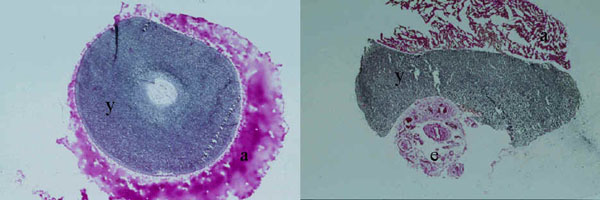
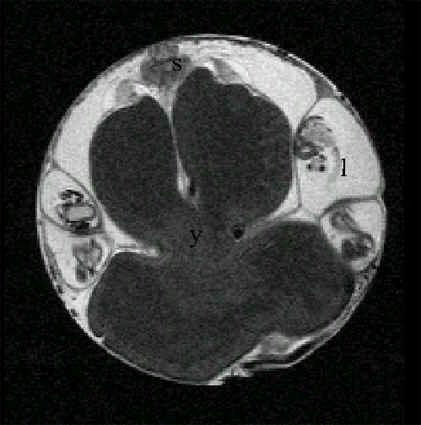
Fig. 11. Magnetic resonance image of an African Ostrich embryo 2 days before hatching incorporating the yolk sac into its belly. In this image, the yolk has been incorporated half into the belly (Starck and Grodd, unpublished manuscript). Abbreviations: y = yolk, l = leg, s = spinal cord.