S46.4: Functions, costs, and benefits of the immune system during development and growth
Kirk C. Klasing & Tatiana V. Leshchinsky
Department of Avian Sciences, University of California, Davis, California, 95616 USA,
fax 530-752-0175, e-mail kcklasing@udavis.com Klasing, K.C. & Leshchinsky, T.V. Functions, costs, and benefits of the immune system during development and growth. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2817-2835. Johannesburg: BirdLife South Africa.Immunocompetence requires the development and education of many interacting cell types. The macrophage-phagocyte system (innate immunity) is functional early during embryogenesis, is the predominant effector mechanism of the neonate, permits fast response to novel pathogens, but elicits the partitioning of resources away from muscle and skeletal development and toward the immune response. Further, innate immunity lacks memory and the large nutritional expense of a response is repeated upon subsequent challenges by the same pathogen. Lymphocyte-mediated adaptive immune responses are highly protective, specific, minimise auto-immunity, and have memory phenomena that reduce nutritional costs over a bird's lifetime; but adaptive immunity requires lengthy developmental processes driven by environmental antigens that are not encountered until post-hatch and it has a high maintenance cost even when unused. Life-history traits effect the developmental pattern and balance of innate and adaptive defence systems, with fast development, short lifespan, infrequent or unrelated pathogen challenges, and predictable food resources favouring a strong and persistent innate defence system. Factors such as slow development, long-life span, and repetitive pathogen challenges favour greater investment in a robust adaptive immune system. Finally, rapid evolution of pathogens exerts pressure for continued innate immunity throughout life, despite its high nutritional costs when invoked.
INTRODUCTIONThe contents of the egg and the tissues of the chick are excellent growth media for a wide variety of microorganisms. However, the internal environment of the avian egg and the resulting chick are sterile. Various protective factors, especially the immune system, insure the preservation of the sterile state. However the embryo and the chick rely on different systems of immunity. The developing embryo and the newly hatched chick depend mostly on innate immunity, which includes non-specific, non-adaptive cellular responses and maternal antibodies (Board & Fuller 1974; Seto 1981). Mature birds maintain the non-specific components of the embryo, and develop pathogen-specific, adaptive immune responses that are mediated by lymphocytes (acquired immunity). The developmental processes that provide functionally protective lymphocytes occur slowly, even in precocial chicks. The development of immunocompetence requires the differentiation and selection of lymphocytes so that all possible non-self antigens can be recognised with minimal errors that cause autoimmunity. These developmental processes require time and resources for completion. However, once complete, specific immunity retards pathogens with much less cost to the bird than the cellular components of non-specific immunity.
Immunology has surfaced as an active area of investigation by physiological ecologists. Yet, even a superficial understanding of comparative avian immunology is clearly lacking. A quick search of BIOSIS reveals that about 1% of recently published papers on immunology are related to Aves and virtually all fundamental studies of developmental immunology have used Chickens Gallus gallus or infrequently Domestic Mallards Anas platyrhynchos. In this paper we use the lessons learned from these species, together with morphological data from a much greater diversity of species, to explore the tradeoffs inherent in developing a robust specific immune system. We then examine the costs of developing an immune system, the costs of maintaining this system, and the expense of using the immune system to fight an infectious challenge in terms of pathology, nutrients consumed, and lost growth. Finally we speculate on the relationships between different developmental patterns and strategies for immunocompetence. In these discussions we use the term specific immunity to refer to responses mediated by B- and T-lymphocytes and non-specific immunity refers to responses mediated by the mononuclear and polymorphonuclear phagocytic systems, including macrophages, monocytes, and heterophils.
DEVELOPMENT OF PROTECTIVE IMMUNITYImmediately upon hatching a chick faces a bewildering number of challenges from opportunistic bacteria and parasites as well as potentially pathogenic organisms. This is a period of time when commensal populations of microflora are established on the epithelia of the skin and digestive tract. Certainly highly precocial chicks (e.g., Galliformes and Anseriformes) have almost adult levels of pathogen challenges as they explore the world to find food, yet they have very poor specific immune responses at hatching and are dependent upon innate immunity and immunoglobulin (Ig) of maternal origin (Higgins 1996; Larsson et al. 1993; Rose & Orlans 1981). Altricial chicks, such as Pigeons Columba livia and Blue and Gold Macaws Ara ararauna, require an even longer period for development of protective levels of specific immunity and also require protection from maternal immunity (Lung et al. 1996a; Lung et al. 1996b; Selvaraj & Pitchappan 1988).
Obviously, maximum protection against these challenges requires an investment in an immune system that can respond against all potential pathogens as specifically and immediately as possible. All avian species studied to date are capable of mounting specific immune responses within several weeks of hatching, but adult levels of immunocompetence are often not present for several months. Several basic parameters govern the minimum number of lymphocytes required for immunocompetence and the maximum rate at which immunocompetence is obtained. This discussion will focus on B-lymphocytes because the leading role played by the avian bursa in the academic discipline of immunology has provided a sound mechanistic and quantitative understanding of this arm of the immune system (Vainio & Imhof 1995). A similar approach to understanding comparative aspects of the T-lymphocyte arm or the specific immune system must await elucidation of quantitative aspects of the development of the antigen-recognition diversity and the rates that the various classes of T-lymphocyte emigrate from the thymus.
There are three principle classes of immunoglobulins in birds: IgM, IgY (the ancestor of mammalian IgG and IgE) and IgA. These Ig classes are produced by B-lymphocytes that develop in the bursa of Fabricius. In the chicken, bursal rudiments appear between E 3 and E 5 as a dorsal evagination of the cloacal wall and epithelial folds appear on day 10 (Glick 1994; Glick 1995; Paramithiotis & Ratcliffe 1996a; Ratcliffe & Jacobsen 1994). The bursa becomes seeded with precursors between E 7 and E 15 (Fig. 1). Within the bursa, B cells undergo gene conversion to diversify the variable region of their Ig genes and then begin to proliferate (see below). This somatic gene diversification is necessary to provide tens of thousands of lymphocyte lineages, each of which recognises unique antigens. IgM-bearing cells can first be detected in low numbers at E-10, IgY-bearing cells by E 14, and IgA-bearing cells by E 16. The bursa is well formed by E 17 and 90% of cells are IgM+, IgG+ or both (Seto 1981; Sharma 1997). B-lymphocytes begin to leave the bursa and migrate to peripheral lymphoid tissues several days before hatch, but most leave during the first month following hatching. The bursa reaches maximum size at about 10 weeks after hatching with 8000-12000 follicles, each with 105 lymphocytes, but at this time it has taken on the role of a secondary lymphoid tissue where immune responses to antigens in the gastrointestinal tract occur.
The lack of functional B-lymphocytes for several weeks after hatching is mitigated by Ig provided by mothers via the egg's yolk and albumin. Maternal serum IgY is actively transferred into the egg yolk during folliculargenesis and reaches concentrations of around 20-25 mg/ml in hen's yolk (Loeken & Roth 1983). IgM and IgA are accumulated in the hen's oviduct secretions (Rose & Orlans 1981) and are present at low concentrations in the egg white (0.15 mg/ml for IgM and 0.7 mg/ml for IgA). IgY is transported across the yolk sac membrane at a slow rate beginning on E 7 and released into the chicks circulation, reaching highest concentrations just prior to hatch (Kowalczyk et al. 1985a). IgA and IgM provide protection in the intestines following consumption of albumin late in embryonic development (Kaspers et al. 1996; Noor et al. 1993). In chickens, the concentrations of total and specific Ig in the yolk are highly proportional to their concentration in the hen's serum (Larsson et al. 1993). A similar senario exists for IgY specific to bovine serum albumin in Blue and Gold Macaws (Lung et al. 1996b) and to Plasmodium falciparum in Black-footed penguins (Spheniscus dermersus; Graczyk et al. 1994). The half-life of IgY in hatchling Chickens and in Blue and Gold Macaws is about 4 days, and maternal IgY is virtually undetectable by 4 weeks of age. Maternal IgY has been demonstrated to be effective in protecting the chicken against a variety of different bacterial, viral, and parasitic challenges during the first week or two post-hatch (Ling et al. 1998; Shawky et al. 1994; Shawky et al. 1993; Smith et al. 1994; Sugita-Konishi et al. 1996), but its protective capacity is in question in Psittaciformes (Phalen et al. 1995; Ritchie et al. 1992).
An Ig response can occur as early as the last few days of embryonic development in chickens and, although they are of little help for current protection, they provide some protection during a subsequent exposure to the same challenge (Noor et al. 1995). Functional levels of antibody responsiveness occur around 2 weeks of age in Chickens, and the full B-cell repertoire and mature levels of responsiveness are reached by 6 weeks of age. Maternal IgY gradually loses effectiveness during this period and the Chicken has a 'window of vulnerability' to pathogens between the 2nd and 3rd weeks of life (Rose & Orlans 1981; Smith et al. 1994).
Trade-offs in developing a robust specific immune response
In their thought provoking paper 'The Protection: The Unit of Humoral Immunity Selected by Evolution,' Cohn & Langman used computer based modelling exercises to determine the minimum number of B-cells that represent all of the functional properties of the humoral immune system (Cohn & Langman 1990). Additional modelling analysis (Langman & Cohn 1993) extends their observations to Chickens. Although all modelling exercises are necessarily over-simplifications, the concepts derived provide insight into the evolutionary pressures that determine the numbers of B-lymphocyte lineages necessary for a functionally robust repertoire of Ig specificities (diverse Ig repertoire) and the number of cells needed within each lineage to accomplish a sufficiently rapid Ig response to eliminate pathogens. The model predicts that the development of a competent Ig response is constrained by boundaries set by, and tradeoffs between: the required diversity of the Ig repertoire; the percentage of this repertoire that responds in any given challenge; the length of time for an effective antibody response; the expense of developing and maintaining high lymphocyte densities; and the probability of an auto-immune response. The boundaries set by these factors may not be breached without serious consequences for immunocompetence and disease resistance. Understanding the optimisation of and tradeoffs between these factors is vital to gaining an appreciation of the impact of pathogens on the evolution of developmental strategies across the precocial-altricial spectrum.
Numbers of B-lymphocytes:
According to Langman & Cohn (1993), the minimum number of B-lymphocytes in the body is a function of the number of antigenic epitopes that must be recognised and the number of responding cells required to mount an effective Ig response. The number of lineages of B-lymphocytes expressing functionally different Ig specificities (diversity of Ig repertoire) required to provide sufficient recognition of possible antigens inherent in all potential pathogens is about 5x104. Although the exact value of this number is hotly debated (Kaufman & Salomonsen 1993), the minimum number is independent of species or size because all birds, hatchlings to adults and hummingbirds to ostriches, face the same diversity of antigens (pathogens, food, pollen etc.). In Chickens, Common Quail Coturnix coturnix, Mallards, Muscovy Ducks Cairina moschata, Pigeons, Turkeys Meleagris gallopavo, Neotropical Cormorants Phalacrocorax olivaceous, and Black Hawks Buteogallus urubitinga, gene conversion and induced point mutations of Ig genes in dividing bursal stem cells accomplish this diversity of Ig repertoire (Funk & Tompson 1996; Higgins & Warr 1993; McCormack & Thompson 1993). Once a productive recombination is accomplished in a cell, it may repeatedly divide to provide a lineage of daughter cells that migrate to tissues and provide Ig needed for protective immunity. For a protective level of immunocompetence there must be sufficient numbers of lymphocytes in each lineage to provide 10 to 100 ng of Ig per ml of extracellular fluid within 6 days time of a challenge by a pathogen. According to Cohn & Langman (1990), this requires a total population of antigen responsive B-lymphocyte of 106 cells (excluding plasma cells) per ml of extracellular fluid. A population of 106 B-lymphocytes containing 5x104 antigen recognition lineages is called '1 protecton'.
Theoretically, an adult of a species that weighs 4 g has about 1 ml of extracellular fluid (Medway & Kare 1959) and possesses 1 protecton of B-lymphocytes dispersed among its immune tissues. An adult of another species that weighs 40 g would have 10 protectons (107 antigen responsive B-lymphocytes). This theoretical density is similar to the number observed in a variety of species regardless of size (Cohn & Langman 1990). Functionally, this is because B-lymphocytes have to be present in sufficient numbers to quickly establish a specific concentration of Ig in plasma, lymph, and interstitial fluids; thus their population density is set by volume of extracellular fluid, which approximates a constant % of body weight. In other words, the number of the B-lymphocytes is predicted to scale to body weight with an allometric constant of 1. The number of lineages of functional B-lymphocytes that must be developed (Ig repertoire) is predicted to be independent of body weight. Consequently, the allometric constant for the size of the repertoire is 0 because the population of lymphocytes in all birds, regardless of size, has to recognise the same number of epitopes.
Time required for development:
The recombination, gene conversion, and mutation processes (Ig gene rearrangement) that generate the diverse Ig repertoire is inefficient and more than 90% of the rearrangements are unproductive resulting in deletion of the cell (Lassila 1989). This inefficiency derives from the highly random nature of the Ig rearrangement process and is necessary to generate diversity of the variable region of Ig. The high rate of deletion of lymphocytes with unsuccessful Ig gene rearrangements results in a lengthy time for establishment of the 5x104 clones needed for a protecton. In chickens, this process starts around day E 15 (Fig. 1) and requires 4 weeks to reach 95% completion (Reynaud & Weill 1996). Thus, this is a continuous process with 1-week occurring in the embryo (E 15-E 21) and 3 weeks or more occurring post-hatch. A hummingbird's bursa must accomplish a similar number of rounds of stem-cells replication and rearrangements of Ig genes as the bursa of an Ostrich because a similar size repertoire of antibody specificities is required by all species. As cell division rates are independent of body weight, the time period for developing the B-lymphocyte repertoire is predicted to be independent of body size – unless tradeoffs are made by relaxing the stringency of deletion of auto-reactive clones. However, strict boundaries for acceptable levels of auto-reactive clones predict that this trade-off would occur to a very limited degree (Cohen & Langman 1990). Another strategy would be to decrease the size of the Ig repertoire and accept a greater probability that an infectious challenge can not be controlled by specific immunity. Such species would be predicted to inhabit low pathogen niches, such as arctic tundra or exposed islands (Piersma 1997).
Once a lymphocyte stem cell has successfully completed Ig gene rearrangement to diversify its antibody specificity, it repeatedly divides within the bursal follicle to form a lineage that seeds the peripheral tissues. The burst of clonal proliferation that follows an infrequently successful Ig rearrangement event is thought to be highly productive because deletion events are minimal (Paramithiotis et al. 1995; Paramithiotis & Ratcliffe 1994). Consequently, with a cell replication time of 10 hours clonal proliferation should not be a primary limiting factor for the time required to expand each lineage to populate the peripheral tissues. B-lymphocytes arising from the first lineages with successfully rearranged Ig genes begin leaving a chicken's bursa at day E 18 (Cooper et al. 1969). At the time of hatch, the chick's repertoire is very narrow (i.e., few lineages) and the number of cells in each lineage is insufficient to mount a response in sufficient time to impart significant protection during a challenge by a pathogen. Chickens bursectomised at hatch eventually achieve normal plasma levels of Ig, but the number of antigen specificities is very small.
Chicks of many species continue to grow for long periods after their B-lymphocytes lineages differentiate and mature levels of specific immunity are established (about 3-4 weeks post-hatch in the Chicken). In these chicks, the bursa must continue to provide additional protectons so that the concentration of lymphocytes in extracellular fluids remains at 106. Consequently, from about 3 weeks post-hatch onward, the primary function of the Chicken's bursa is to provide additional protectons to supply the growing chick at a rate that is proportional to its growth rate. At 10 weeks of age the bursa of chickens begins to decrease in size and function until it almost completely involutes as growth plateaus around 20 weeks of age. Following regression of the bursa, the B-cell lineages are self-renewing in the peripheral tissues. The bursa of other species with similar incubation lengths and growth periods, such as domestic ducks, Chuckars Alectoris chuckar and Ring-necked Pheasants Phasianus colchicus, undergo very similar time kinetics of bursal size and the onset of maturity (Hashimoto & Sugimura 1976; Mercer-Oltjen & Woodard 1987). The size of the bursa is highly correlated with rate of growth in these species. Ostriches and emus need to continue to supply additional protectons during the duration of their very long growth period and their bursa remain large and active for the entire 16 month growth interval (Von Rautenfeld & Budras 1981).
Cockatiels are interesting because they hatch after a similar length of incubation (19 days) as chickens, but their altricial mode of development permits very fast growth that plateaus at 3 weeks post-hatch (Itchon 1994). This is about the time that full diversity of the B-lymphocytes repertoire is predicted to occur and, as expected, the bursa begins to regress at this time. This species does not reach sexual maturity until 9 months later and it is doubtful that the involution is related to sex hormones.
Tradeoffs for highly altricial species:
Altricial species that hatch following a short incubation time represent a challenge to the developmental model worked out in Galliformes. Maternal IgY levels of altricial chicks are not expected to be any greater than those in precocial chicks, and lower amounts of egg yolk available to altricial chicks would suggest even less maternal immunity from IgY. Development of specific immunity by 2 weeks after hatch would seem to be important to combat pathogens following loss of maternal immunity, but this process takes 3 to 4 weeks post E-15 in Chickens. Many altricial species hatch as early as E10 or E11, several days before stem cells have differentiated in the Chicken's bursa. Hatchling White King Pigeons and Mourning Doves Zenaida macroura lack significant lymphocyte populations in the bursa (Shields et al. 1979) and the bursa of hatchling Starlings Sturnus vulgaris are at an early developmental stage relative to hatchling Chickens (Glick & Olah 1982). Domestic pigeons also have poorly developed bursa and they do not mount effective Ig responses at 4 weeks of age (Selvaraj & Pitchappan 1988). Consequently, there is no indication that highly altricial species accelerate lymphocyte differentiation by significantly advancing bursal development. Accelerated development of the B-lymphocyte repertoire could also be accomplished by less stringent deletion of self-reacting clones, or by generating a smaller number of antigen-specific lineages prior to their clonal proliferation and seeding of peripheral tissues. The model of Cohn & Langman (1990) predicts that these tradeoffs are unlikely and limited. Taken together, the evidence suggests that development of the antigen recognition repertoire of the specific immune system is related to elapsed time for development regardless of whether or not hatching has occurred. If this proves to be true, highly altricial species would not have competent Ig protection until more than a week later than Chickens. Clearly investigations specifically designed to examine the tradeoffs between altricial development and immune system development are needed.
The cost of developing, maintaining and using an immune system
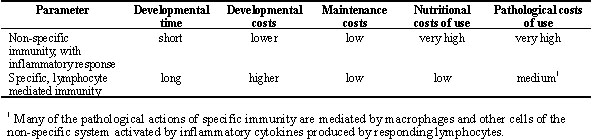
An analysis of the costs of immunity can be subdivided into those costs for development of the immunologic repertoire, the cost of maintaining this system under normal 'housekeeping' conditions, and the cost of using the system to thwart invasive pathogens. The costs of development of the immune system are primarily the expenditure of energy to fuel the inefficient process of developing antigen recognition diversity in B and T lymphocytes and to provide substrates (e.g., amino acids and lipids) for the initial burst of leukopoiesis needed to supply the late embryo and hatchling with leukocytes. The costs of maintaining the immune system are related to allocating nutrients for the continued production of leukocytes, Ig, and other plasma proteins to replace those lost during normal turnover of cells and extracellular proteins. The costs of using the immune system to thwart the invasion of potential pathogens come in two primary forms. First, there are losses in tissue function that result from damage incurred when leukocytes engage their effector mechanisms and damage tissue integrity and host cell viability (collateral damage). Second, there are nutritional costs of mobilising the responding cell types and fuelling their effector functions (Table 1).
Obviously the cost due to pathology and necrosis induced by a pathogen can be fatal if the immune system does not engage in defence against an effective challenge (e.g., active tolerance or ignorance due to insufficient repertoire) or if it engages ineffectually. On the other hand a maximal response of all arms of the immune system can also be fatal as exemplified by anaphylaxis or septic shock. It is likely that various species have adopted differing strategies for responding to pathogens, including intensity of their response and type of response. The balance of these immunoregulatory decisions should depend upon the tradeoffs between the cost of the response and the cost of not responding. Estimates of the pathology induced by inadequate or over-exuberant immune responses are detailed in every textbook on avian pathology, but estimates of the nutritional costs of a successful and appropriate immune response are poorly characterised. Studies in physiological ecology and theoretical immunology often refer to the 'costs' in terms of resources such as energy to mount an immune response, or the tradeoffs between immunocompetence and other nutrient requiring functions. An accounting of these costs must be accurately made in birds for these theories and arguments to have a sound quantitative basis.
Examples of developmental costs:
Strictly from a size perceptive, the bursa and thymus are very small relative to organs such as the heart, kidneys, etc., but they are by far the largest lymphoid organs of the embryo and hatchling. Although their relative size is small, the rate of cell division is among the highest of any tissue, with a cell division rate of once every 10 hours for lymphocytes in the bursa of the late embryonic chick (Lydyard et al. 1976). This results in the production of about 107 cells per day at E18, or about 0.01% of body weight per day. In the late embryo, the bursa grows slowly because most of the B-lymphocytes produced undergo apoptosis (deletion) and the total cell number increases slowly. Thus, during the early stage of bursal development most of the nutrients in the daughter cells are 'recycled'. That is they undergo apoptosis and are then phagocytosed by macrophages and hydrolyzed to amino acids and fatty acids, which can be re-used (Funk & Tompson 1996). A similar scenario occurs in the thymus (Gobel 1996) where more than 95% of the thymocytes die by apoptosis. For this reason, the primary cost of expanding the lymphocyte repertoires is energy and not amino acids. As time progresses, the decrease in lymphocyte deletion and increase in export of lymphocytes represents a nutrient investment for amino acids, fatty acids and energy for synthesising and exporting these cells, but still this expense is not quantitatively great. In Chickens, Chuckars, and Ring-necked Pheasants the bursa reaches a maximum relative size of 0.45 % of body weight at about 3-4 weeks of age and then declines in relative size (Betti & Sesso 1989; Mercer-Oltjen & Woodard 1987). Quantitatively, the bursa of a 4 week old chicken produces 5x108 cells per day, representing 0.04% of its body weight. The relative size of the thymus follows a similar, although slightly delayed, time course during development as the bursa. If it produces a similar number of cells each day, lymphopoeisis represents less than 0.1% of the body weight of the chicken each day.
The costs of development of macrophages, natural killer cells, and heterophils responsible for innate immunity are difficult to calculate due to their very wide tissue distribution. The stem cells that give rise to these cells are produced early in development (<E 6) and granulocytopoiesis occurs in spleen and bone marrow. Prior to this time, phagocytosis is predominantly accomplished by a wide variety of embryonic cells. The embryonic yolk sac, the aorta, the spleen and liver rudiments are the earliest sites of macrophage activity. Following E 7 the phagocytic function become concentrated in the liver, spleen, lung, kidney, bone marrow, and other areas of the mononuclear phagocyte system (Kent 1961). Following hatching, the numbers of macrophages and other phagocytes in lymphoid tissues (e.g., spleen, gut associated lymphoid tissues, lungs, harderian gland, thymus, bursa) are typically well below the numbers of lymphocytes (Del Cacho et al. 1993a; Del Cacho et al. 1993b; Jeurissen et al. 1992; Jeurissen et al. 1994). Two factors indicate that there is not a transiently large cost for development of the cells involved in non-specific immunity. First, there does not appear to be a sudden burst of proliferation for supplying tissues in a catch up manner, as there is for lymphocytes. Additionally, diversity generating cell divisions and high rates of cell deletion are not required because receptors for foreign material (mostly carbohydrates) do not undergo gene rearrangement. For these reasons it is likely that the nutritional costs to the late embryonic and post-hatch chick for the development of these cells is less than that of lymphocytes.
Secondary lymphoid tissues form almost exclusively after hatching and require 4-8 week to obtain adult proportions in Chickens. These are the areas where antigen driven immune responses occur and include spleen, Harderian gland, cecal tonsils, Meckel's diverticulum, Peyer's patches, cloaca lymphoid aggregates, the rudimentary cervical lymph nodes, the palatine tonsils, and pharyngeal submucosal nodular aggregates. The cecal tonsils in Chickens are rudimentary at hatching but expand in size rapidly when foreign antigens from the diet and colonising microorganisms arrive (Gomez Del Moral et al. 1998; Honjo et al. 1993). Peyers patches are not histologically definable at hatching but begin to develop quickly after day 7 (Pope 1991). Differential proportions of leukocytes in these tissues have been accurately quantified and show that lymphocytes are present in greater numbers than all other cells combined, but little is known about the size and rate of growth of secondary lymphoid tissues. Clearly an accurate assessment of the post-hatch costs of continued development of the immune system requires an accurate assessment of the size and growth rate of secondary lymphoid organs. It will also require an estimate of the proportion of lymphocytes in these tissues that arrive from the thymus and bursa versus those that arise from proliferation in the tissue itself.
Although quantitative aspects of the development of the immune system are incomplete, the current picture suggests that the costs of development of specific immunity are considerably greater than the costs for non-specific immunity. Costs to the embryo are low because most development, including thymic and bursal activities, occur post-hatch even in precocial species such as chickens.
Maintenance costs:
The overall maintenance costs of owning an immune system can be put in perspective by examining the weight of immune tissues relative to body weight. Estimates of the size of the immune system including bone marrow components requires the use of data collected in from a variety of species (Klasing 1998b) but reveals that a little less than 0.5% of the body is made up of leukocytes and their progenitors. Accessory cells such as reticular, dendritic, and stromal cells should also be charged to the immune system, but accurate quantitative estimates of their contribution are not yet available. Even in the unlikely case that these accessory cells contribute similar mass to the immune system as leukocytes, the cellular components of the immune system probably does not exceed 1% of the body weight.
Nutritional costs of the immune system must also consider turnover rates of cells and macromolecules. Direct measurements of the rate of protein turnover indicates that bursa and spleen have turnover rates that are 50% higher than liver and the thymus is similar to liver (Klasing & Austic 1984). Cell lifespan measurements provide a similar conclusion. About 60% of the peripheral B-lymphocytes in 3 week old chicks have a lifespan of only 3 days, while 35% have a lifespan of 14 days giving an average of about 7 days (Paramithiotis & Ratcliffe 1996b). These observations suggest that tissue weights underestimate the contribution of maintaining an immune system to total daily energetics, but still the total contribution of lymphocytes to the maintenance nutrient requirements must be low. Macrophages, the most prevalent cell of the non-specific immune system, have a life span of many weeks if they are not engaged in an immune response.
Normal rates of immunoglobulin synthesis in young Chickens are less than 0.02% of body weight per day (Leslie & Clem 1970). Immunoglobulin synthesis is higher in females laying eggs because of daily secretion of IgY into egg yolk; yet even in the hen laying an egg a day, daily total immunoglobulin synthesis is probably well less than 0.05% of body weight per day (Kowalczyk et al. 1985b). Other humoral components, such as complement, contribute an order of magnitude less to daily protein synthesis than do immunoglobulins based on serum concentrations and half–life estimates and most of these costs occur post-hatch. For example, total complement activity becomes detectable in the embryo at day E13 and it reaches 5 to 10 % of adult level (0.03 % body weight) at hatching (Gabrielsen et al. 1973). Thus, the costs of maintaining specific and non-specific components of the immune system in working order and awaiting a challenge from a pathogen appear low but begins early in embryogenesis.
Costs of using the immune system: An immune response to a pathogen consists of the clonal proliferation of lymphocytes, formation of germinal centers in lymphoid tissues for affinity maturation of Ig, the recruitment of new monocytes and heterophils from bone marrow, and the synthesis of effector molecules (e.g., Ig, nitric oxide, lysozyme, complement) and communication molecules (e.g., cytokines). Furthermore, most immune responses to pathogens are accompanied by a systemic inflammatory response, which includes behavioural, metabolic, and cellular responses that markedly impact the partitioning of nutrients among tissues (Klasing 1997; Klasing & Korver 1997; Klasing & Roura 1991).
Quantitative estimates of the magnitude of an immune response requires the use of mammalian data. In Humans, leukopoiesis in the bone marrow increases by about 2-fold during acute systemic infections to 0.06% of the body weight (Elgert 1996). In Mice injected with a polyclonal mitogen into the footpad, the draining lymph node doubles in size due to the formation of germinal centers (B cell proliferation) and this represents a weight increase of 1 mg in a 20 g mouse, or about 0.005% of body weight (Szakal 1989). Although the rate of synthesis of Ig specific for epitopes carried by the challenging pathogen increases dramatically during a disease challenge, the rate of synthesis of total antibodies increases only moderately. Hyperimmunization, for example, results in about a 25% increase in total serum Ig in Chickens (Leslie & Clem 1970), a level still well below albumin concentrations.
Although accurate information on the nutritional demands of a vigorous immune response are clearly imperfect, it is apparent that the costs of supplying the immune system with the substrates and energy needed for a vigorous response is very low relative to needs for growth, moulting, or egg production. For example, the weight of leukocytes and Igs predicted to be produced each day during an infectious challenge appears to be less than 2% of the total increase in body weight of a 2 week old Chicken (Klasing 1998b). Thus, a summation of cells and effector molecules consumed during an immune response suggests that use of the immune system should not have important nutritional costs. Yet this can not be true – simple observation of sick birds tells us that they lose body condition. Systemic bacterial infections often cause birds to become cachetic, waste away, and die (Goodman 1996). The missing component must be due to quantitatively important changes in non-lymphoid tissues.
Most infections are accompanied by various degrees of an inflammatory response. The systemic manifestations induced by an inflammatory response and especially its acute-phase components are highly conserved through the evolution of vertebrates and are observed in lizards, birds, and mammals (Kluger 1979). An acute phase response is orchestrated by inflammatory cytokines (interleukins 1 and 6, tumor necrosis factor, interferon gamma) produced by activated macrophages and is characterised by well-defined behavioural, metabolic, and cellular changes. In Chickens, behavioural changes include anorexia, increased sleep, and reduced socialisation (Dantzer et al. 1993; Johnson et al. 1993). Metabolic changes include hepatic synthesis of acute phase proteins, fever, skeletal muscle catabolism, and accelerated whole-body protein turnover. Cellular changes include increased heterophil concentrations in blood and increased rates of leukopoiesis. In young chicks, these alterations are expressed as decreased growth and have significant implications on nutritional requirements. The qualitative and quantitative aspects of these changes in Chickens have been reviewed elsewhere (Elsasser et al. 1995; Johnson 1997; Klasing 1994; Klasing & Korver 1997) and will not be detailed here.
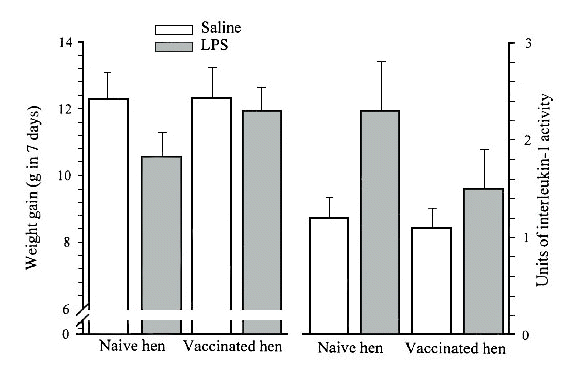
It is clear that the costs that result from anorexia and the systemic changes in metabolism associated with an acute phase response are an order of magnitude greater than the costs that can be accounted for by immune components. For example, alternate day injections of the classic inflammatory agent, Escherichia coli lipopolysaccharide (LPS), into hatchling Japanese Quail chicks elicits a sustained inflammatory response and reduces growth rate by 14% (Fig. 2). If hens supplying the eggs for the experiment are previously vaccinated against the LPS, maternal immunity provided to the chick markedly reduces the inflammatory response as indicated by decreased levels of interleukin-1 activity. In Quail chicks with high levels of maternal immunity, the depression in growth due to the LPS challenge was only 4%. A decreased inflammatory response due to maternal IgY is presumably through preventing the LPS from interacting with macrophage LPS receptors, which trigger the release of inflammatory cytokines. The systemic components of the inflammatory response of Quail challenged with LPS can be blocked by infusing interleukin-1 receptors, which neutralise this pro-inflammatory cytokine (Klasing 1998a). Blocking hormone-like actions of pro-inflammatory cytokines ameliorates the anorexia and fever, permitting the chick to grow faster.
About 70% of the decreased growth rate that occurs due to a systemic inflammatory response is due to anorexia and the remaining 30% is from metabolic inefficiencies or possibly behavioural changes (Klasing et al. 1987). Fever and increased protein turnover may account for much of the increased inefficiency (Baracos et al. 1987). Fever is protective in that it up-regulates leukocyte functions and rates of cell division, whereas increased protein turnover heightens surveillance for intra-cellular infection by viruses and bacteria.
Most complex antigens stimulate macrophages to release inflammatory cytokines in the process of stimulating specific immune responses. Antigens that induce T-lymphocyte responses (Th1) are thought to induce greater levels of inflammatory cytokines than those that induce Ig responses (Th2). In this regard, cell-mediated immune response are thought to have a greater nutritional cost and a greater impact on growth than an Ig response (Klasing & Korver 1997). However, the division of CD4 lymphocytes into Th1 and Th2 populations remains to be verified in Aves.
The high cost of an inflammatory response likely explains why a variety of avian species (Connors & Nickol 1991; Korver et al. 1997; Sheldon & Verhulst 1996) respond to challenges with live bacterial and viral pathogens, and sometimes even relatively benign parasites, with changes in body condition and energy metabolism that are much greater than can be reconciled by summation of the substrates needed for a specific immune response. Even benign antigens like sheep red blood cells induce increased heat production, protein turnover, and fever, which result in a slightly decreased rate of growth (Klasing & Austic 1984; Siegel et al. 1982). In Chickens, the frequency that the immune system must respond to challenges by opportunistic microorganisms is inversely proportional to the rate of growth and the efficiency of dietary energy use for tissue deposition (Roura et al. 1992).
It is often stated that the depression in growth and reproductive performance that is associated with an immune response is due to the diversion of nutrients away from productive processes for use by the immune system to mount an immune response. A quantitative analysis of the processes involved indicates that this theory should be modified. The depression in growth and reproductive performance that is associated with an immune response is due mostly to anorexia and changes in nutrient use by the bird's major organs with only minor use by the cells of the immune system to support their modest anabolic functions.
Implications for developmental strategies
The relationships between the ontogeny of the innate and specific components of the immune system and the costs of development, maintenance, and usage of these components should have important implications for the precocial-altricial spectrum of development. Below we suggest 12 parameters that should be important influences on what are probably very diverse strategies that avian species have adopted for development of immunocompetence. (1) The egg is normally a sterile environment for the embryo and foreign antigen is not available to initiate specific immunity until after hatch. (2) Development of the cellular components of innate immunity, such as macrophages, heterophils, and NK-cells, at protective levels of maturation occurs early in embryogenesis. (3) Once the bursa becomes functional in the embryo (approximately E12) gene rearrangement events necessary to form an Ig repertoire that is sufficiently diverse for an effective Ig response requires several weeks. This timeline can not be markedly accelerated without severe autoimmune consequences for the bird. (4) Maternal IgY is critical for protecting the hatchling prior to the development of effective specific immunity. (5) Development of the repertoire of T- and B-lymphocytes is energetically more expensive than development of the cellular components of innate immunity. (6) Following development, maintenance of the immune system is relatively inexpensive. (7) Use of specific immunity is not very costly in terms of synthesis of leukocytes or Ig. (8) Use of cellular components of innate immunity by the growing chick is costly because a systemic inflammatory response causes anorexia and diverts nutrients from growth related processes towards the acute-phase response. (9) Use of innate immunity by the growing chick is more likely to cause pathology relative to use of specific immunity because effector events invoked by macrophages and heterophils to kill pathogens are more likely to be destructive than the effector mechanisms of specific immunity. (10) Maternal Ig markedly decreases the costs of immunity following hatch. (11) The macrophages, heterophils and other cells of the non-specific system respond quickly even the first time that a pathogen is encountered; however subsequent engagements of the same pathogen result in the same high costs as the first encounter. (12) The specific immune system requires many days to produce protective levels of Ig or T-cells the first time a pathogen is encountered. Subsequent encounters with the same pathogen evoke quick responses with minimal costs.
Each of these parameters requires considerably more quantitative and comparative refinement and a few may prove to be unique to chickens, ducks, and other precocial species. Still, these 12 parameters should be useful for forming and testing hypothesis on comparative aspects of the development of the immune system. Below are an example of such hypothesis.
Universal aspects of development:
The immunologic parameters listed above predict a few patterns that should be common to all developmental strategies. For example, all birds must rely on innate immunity and on yolk IgY for the specific component of their immune system prior to hatching and for at least a week afterward. Even if long incubation periods permit the entire B and T lymphocyte repertoires to be developed and available at the time of hatch, antigens are not available to drive Ig production until after hatch. Nearly a week is required for effective levels of IgY to be produced following exposure to novel antigens. This principle explains why species with long incubation periods, such as ratites, still have high levels of maternal IgY even though they have plenty of time to develop a robust repertoire of B-lymphocytes in ovo.
Yolk IgY extends the maternal specific immune system to the hatchling. When a hatchling is challenged by pathogens similar to those that it's mother had been exposed to previously, maternal IgY is effective at protecting the chick for a period of time post-hatch. This provides a window of time for efficient and rapid growth because the chick does not need to invoke its own non-specific immune defences.
An encounter with many novel pathogens is controlled mostly by non-specific phagocytes such as macrophages and heterophils and is predicted to have a high cost in terms of pathology and nutrition. Specific immunity developed following a challenge by a novel pathogen has minimal value during the early stages of novel challenges, but provides quick protection with relatively low levels of pathology against subsequent exposures. For the first few weeks post-hatch 'novel' refers to pathogens that the chick's mother had not recently been exposed. Following loss of maternal IgY, 'novel' means pathogens that the chick itself has not encountered before.
Immunocompetence versus incubation time:
The immunologic parameters listed above (# 2, 3) predict differences in the developmental pattern of immunocompetence depending upon the length of incubation. Species with short incubation periods are expected to require more time post-hatch to develop effective specific immunity than species with long incubation times. Therefore, the shorter the incubation period, the longer the period of time that the hatchling must rely on innate immunity, including maternal Ig and non-specific inflammatory responses. It would seem likely that chicks with very short incubation periods would be provided with higher levels of IgY with a longer half-life, but to date there is no evidence to support this supposition. In fact, the lower quantity of yolk provided to altricial species suggest less maternal IgY. In highly altricial species, a long window of vulnerability between the dissipation of maternal immunity and the development of effective specific immunity would argue for a greater reliance on non-specific immune mechanisms during this time period. As non-specific immunity is expensive when used, there must be an advantage to completing the growth period before the effectiveness of maternal antibody wanes. Stated in another way, an advantage of highly altricial development is that the growth period is mostly finished prior to complete loss of maternal IgY and before the expenses from use of the chick's own immune system are invoked.
Chicks with long incubation periods should have a narrower window of vulnerability because their specific immune system should be mostly developed at the time of hatch and functional prior to the complete loss of maternal Ig. For example, the Helmeted Guinea Fowl (Numida meleagris galeata) has a 27 day incubation period, but only slightly lags behind the Chicken in rate of development and cellularity of the bursa. Consequently it hatches with a more developed and functional bursa, and presumably a more functional repertoire of B-lymphocytes and better protection (Onyeanusi et al. 1993). This scenario is complicated by the fact that maternal Ig inhibits the development of specific immunity by neutralizing antigen before it interacts with lymphocytes (Rose & Orlans 1981). Consequently, the complete elimination of this window of vulnerability may not occur, even in species with very long incubation periods, but it should be shorter in duration.
Pigeons may ameliorate the window of vulnerability by producing crop milk containing IgA. Though IgA is not normally thought to be absorbed from the intestine of Chickens, experiments with hatchling squabs demonstrated absorption into the blood stream (Goudswaard & van der Donk 1979). This strategy would extend the maternal contribution to chick Ig.
Life span:
Species with long life spans are more likely to invest more time and energy into developing a robust specific immune system. This is because long-lived species experience a lower ratio of novel to repeat exposures to pathogens. Presumably most birds have a relatively constant frequency of challenges by pathogens throughout their life (but see below). However, as a bird ages, each pathogen encounter becomes less likely to be novel. Thus, the value of specific immunity in terms of tradeoffs between developmental costs and costs of usage increases with life span. Even though there is a continued expense of maintaining high levels of non-specific immunity as a complementary defence mechanism it is probably necessary because of the rapid evolution of pathogens. But the high costs of using non-specific immunity are less frequently incurred with age.
Pathogen avoidance:
Piersma (1997) suggests that an alternate strategy to investing in a highly functional immune system is to minimise exposure to pathogens by inhabiting low pathogen regions of the earth, such as high arctic tundra or exposed seashores. The principle factors listed above suggest that this strategy would be especially valuable for species with shorter incubation periods (i.e., less specific immunity at hatching) but long growth periods that continue well past the effective life of maternal antibody. Less frequent exposure to novel pathogens should permit faster and nutritionally more efficient growth in species that have a wide window of vulnerability.
Critical stages of life:
The costs of the inflammatory response to novel pathogen challenges must be endured by the hatchling because specific immunity is not sufficiently developed to aid in defence. Thus young birds are obliged to use non-specific immunity due to the lack of redundant mechanisms. Birds with mature immune systems presumably use specific immunity to the extent possible in order to avoid the deleterious costs of the inflammatory response. During periods of reproduction, moult, migration, cold weather, or food shortages the costs of an inflammatory response may be especially deleterious. During these periods, hormonal mechanisms that suppress the inflammatory response are predicted to come into play. The principle factors listed above (7, 8, 9) suggest that these critical periods of life history should be accompanied by a shift towards specific immunity. It is interesting that species which retain bursal function into their adult life often have a resurgence in bursal size at such critical periods.
Pekin Ducks possess a unique truncated form of IgY that does not have an Fc region for interacting with macrophages (Bando & Higgins 1996). Thus, this Ig possesses antigen binding and neutralizing functions but presumably does not activate the inflammatory response. Pekin Ducks also possess a full length IgY that has normal heavy chain functions including fixing complement, antigen precipitation and opsonizination for macrophage clearance. These two forms of IgY are regulated independently. The relationship between expression of the more benign IgY and critical stages of life history remains to be explored.
1
Many of the pathological actions of specific immunity are mediated by macrophages and other cells of the non-specific system activated by inflammatory cytokines produced by responding lymphocytes.REFERENCES
Bando, Y. & Higgins, D.A. 1996. Duck lymphoid organs: Their contribution to the ontogeny of IgM and IgY. Immunology 89: 8-12.
Baracos, V.E., Whitmore, W.T. & Gale, R. 1987. The metabolic cost of fever. Canadian Journal of Physiology and Pharmacology 65: 1248-54.
Betti, F. & Sesso, A. 1989. An allometric study of the development of the cloacal bursa in the domestic fowl. Anatomischer Anzeiger 168: 255-260.
Board, R.G. & Fuller, R. 1974. Non-specific antimicrobial defences of the avian egg, embryo and neonate. Biological Reviews 49: 15-49.
Cohn, M. & Langman, R.E. 1990. The protection: the unit of humoral immunity selected by evolution. Immunological Reviews 115: 11-147.
Connors, V.A. & Nickol, B.B. 1991. Effects of Plagiorhynchus cylindraceus (Acanthocephala) on the energy metabolism of adult starlings, Sturnus vulgaris. Parasitology 103: 395-402.
Cooper, M.D., Cain, W.A., Van Alten, P.J. & Good, R.A. 1969. Development and function of the immunoglobulin producing system. International Archives of Allergy and Applied Immunolgoy 35: 242-252.
Dantzer, R., Bluth, R.M., Kent, S. & Goodall, G. 1993. Behavioral effects of cytokines: An insight into mechanisms of sickness behavior. In: De Souza, E.B. (ed.) Neurobiology of Cytokines. San Diego, CA; Academic Press: 130-143.
Del Cacho, E., Gallego, M., Galmes, M., Lloret, E. & Bascuas, J.A. 1993a. Immunocytochemical and immunoelectron microscopy study of dendritic cells in the caecal tonsils of chickens infected with Eimeria tenella. Veterinary Immunology and Immunopathology 38: 123-137.
Del Cacho, E., Gallego, M., Marcotegui, M.A. & Bascuas, J.A. 1993b. Follicular dendritic cell activation in the Harderian gland of the Chicken. Veterinary Immunology and Immunopathology 35: 339-351.
Elgert, K.D. 1996. Immunology. New York, Wiley-Liss.
Elsasser, T.H., Steele, N.C. & Fayer, R. 1995. Cytokines, stress, and growth modulation. In: Myers M.J., & Murtaugh, M.P. (eds.) Cytokines in Animal Health and Disease. New York; Marcel Dekker, Inc.: 261-290.
Funk, P.E. & Tompson, C.B. 1996. Current concepts in chicken B cell development. Current Topics in Microbiology 212: 17-28.
Gabrielsen A.E., Pickering R.J, Linna T.J. & Good, R.A. 1973. Haemolysis in chicken serum. II. Ontogenetic development. Immunology 25: 179-184.
Glick, B. 1994. The bursa of Fabricius: the evolution of a discovery. Poultry Science 73: 979-83.
Glick, B. 1995. Embryogenesis of the bursa of Fabricius: stem cell, microenvironment, and receptor-paracrine pathways. Poultry Science 74: 419-26.
Glick, B. & Olah, I. 1982. The morphology of the Starling (Sturnus vulgaris) bursa of Fabricius: A scanning and light microscope study. Anatomical Record 204: 341-348.
Gobel, T.W.F. 1996. The T-dependent system. In: Davison, T.F. (ed.)Avian Immunology. Abingdon; Carfax Publishing Co.: 327-339.
Gomez Del Moral, M., Fonfria, J., Varas, A., Jimenez, E., Moreno, J. & Zapata, A.G. 1998. Appearance and development of lymphoid cells in the chicken (Gallus gallus) caecal tonsil. Anatomical Record 250: 182-189.
Goodman, G.J. 1996. Metabolic disorders. In: Rosskopf, W. & Woerpel, R. (eds) Diseases of Cage and Aviary Birds. Baltimore; Willians & Wilkins: 218-234.
Goudswaard, J. & van der Donk, J.A. 1979. Peculiar IgA transfer in the pigeon from mother to squab. Developmental and Comparative Immunology 3: 307-319.
Graczyk, T.K., Cranfield, M.R., Shaw, M.L. & Craig, L.E. 1994. Maternal antibodies against Plasmodium spp. in African black-footed penguin (Spheniscus demersus) chicks. Journal of Wildlife Diseases 30: 365-371.
Hashimoto, Y. & Sugimura, M. 1976. Histological and quantitative studies on the postnatal growth of the thymus and bursa of Fabricius of White Pekin Ducks. Japanese Journal of Veterinary Science 24: 65-76.
Higgins, D.A. 1996. Comparative Immunology of Avian Species. In: Davison, T.F. (ed.) Avian Immunology. Abingdon, Carfax Publishing Co: 149-208.
Higgins, D.A. & Warr, G.W. 1993. Duck immunoglobulins structure functions and molecular genetics. Avian Pathology 22: 211-236.
Honjo, K., Hagiwara, T., Itoh, K., Takahashi, E. & Hirota, Y. 1993. Immunohistochemical analysis of tissue distribution of B and T cells in germfree and conventional chickens. Journal of Veterinary Medical Science 55: 1031-1034.
Itchon, C.R.S. 1994. Developmental Microanatomy of the Primary Lymphoid Organs and Selected Hemopoietic Organs of the Cockatiel (Nymphicus hollandicus). MS Thesis, University of California, Davis.
Jeurissen, S.H., Claassen, E. & Janse, E.M. 1992. Histological and functional differentiation of non-lymphoid cells in the chicken spleen. Immunology 77: 75-80.
Jeurissen, S.H.M., Verveld L. & Janse, E.M. 1994. Structure and function of lymphoid tissues of the chicken. Poultry Science Review 5: 183-207.
Johnson, R.W. 1997. Inhibition of growth by pro-inflammatory cytokines: An integrated view. Journal of Animal Science 75: 1244-1255.
Johnson, R.W., Curtis, S.E., Dantzer, R., Bahr, J.M. & Kelley, K.W. 1993. Sickness behavior in birds caused by peripheral or central injection of endotoxin. Physiology and Behavior 53: 343-8.
Kaspers, B., Bondl, H. & Goebel, T.W.F. 1996. Transfer of IgA from albumen into the yolk sac during embryonic development in the chicken. Journal of Veterinary Medicine Series A 43: 225-231.
Kaufman, J. & Salomonsen, J. 1993. What the dickens is with these chickens? Research in Immunology 144: 495-502.
Kent, R. 1961. The development of the phagocytic activity of the reticulo-endothelial system in the chick. Journal of Embryology and Experimental Morphology 9: 128-136.
Klasing, K.C. 1994. Avian leukocytic cytokines. Poultry Science 73: 1035-1043.
Klasing, K.C. 1997. Interactions between nutrition and infectious disease. In: Calnek B.W. (ed.) Diseases of Poultry. Ames; Iowa State University Press: 73-80.
Klasing, K.C. 1998a. Avian Macrophages: Regulators of local and systemic immune responses. Poultry Science 77, in press.
Klasing, K.C. 1998b. Nutritional modulation of resistance to infectious diseases. Poultry Science 77, in press.
Klasing, K.C. & Austic, R.E. 1984. Changes in protein synthesis due to an inflammatory challenge. Procedings Society Experimental Biology and Medicine 176: 285-291.
Klasing, K.C. & Korver, D.R. 1997. Leukocytic cytokines regulate growth rate and composition following activation of the immune system. Journal of Animal Science 75 (Supplement 2): 58-68.
Klasing, K.C., Laurin, D.E., Peng, R.K. & Fry, D.M. 1987. Immunologically mediated growth deptression in chicks: Influence of feed intake, corticosterone, and interleukin-1. Journal of Nutrition 117: 1629-1637.
Klasing, K.C. & Roura, E. 1991. Interactions between nutrition and immunity in chickens. Cornell Nutrition Conference Proceedings: 94-101.
Kluger, M.J. 1979. Fever: its Biology, Evolution, and Function. Princeton; Princeton University Press.
Korver, D.R., Wakenell, P. & Klasing, K.C. 1997. Dietary fish oil or Lofrin, a 5-lipoxygenase inhibitor, decrease the growth-suppressing effects of coccidiosis in broiler chicks. Poultry Science 76: 1355-1363.
Kowalczyk, K., Daiss, J., Halpern, J. & Roth, T.F. 1985a. Quantitation of maternal-fetal IgG transport in the chicken. Immunol. 54: 755-762.
Kowalczyk, K., Daiss, J., Halpern, J. & Roth, T.F. 1985b. Quantitation of maternal-fetal IgG transport in the chicken. Immunology 54: 755-762.
Langman, R.E. & Cohn, M. 1993. A theory of the ontogeny of the chicken humoral immune system: The consequences of diversification by gene hyperconversion and its extension to rabbit. Research in Immunology 144: 422-446.
Larsson, A., Balow, R.M., Lindahl, T.L. & Forsberg, P.O. 1993. Chicken antibodies taking advantage of evolution a review. Poultry Science 72: 1807-1812.
Lassila, O. 1989. Emigration of B cells from chicken bursa of Fabricius. European Journal of Immunology 19: 955-958.
Leslie, G.A. & Clem, L.W. 1970. Chicken immunoglobulins: biological half-lives and normal adult serum concentrations of IgM and IgY. Proceedings of the Society of Experimental Biology and Medicine 134: 195-198.
Ling, Y.S., Guo, Y.J., Li, J.D., Yang, L.K., Luo, Y.X., Yu, S.X., Zhen, L.Q., Qiu, S.B. & Zhu, G.F. 1998. Serum and egg yolk IgG antibody titers from laying chickens vaccinated with Pasteurella multocida. Avian Diseases 42: 186-189.
Loeken, M.R. & Roth, T.F. 1983. Analysis of maternal IgG subpopulations which are transported into the chicken oocyte. Immunology 49: 21-28.
Lung, N.P., Thompson, J.P., Kollias, G.V., Jr. & Klein, P.A. 1996a. Development of monoclonal antibodies for measurement of immunoglobulin G antibody responses in blue and gold macaws (Ara ararauna). American Journal of Veterinary Research 57: 1157-1161.
Lung, N.P., Thompson, J.P., Kollias, G.V., Jr., Olsen, J.H., Zdziarski, J.M. & Klein, P.A. 1996b. Maternal immunoglobulin G antibody transfer and development of immunoglobulin G antibody responses in blue and gold macaw (Ara ararauna) chicks. American Journal of Veterinary Research 57: 1162-1167.
Lydyard, P.M., Grossi, C.E. & Cooper, M.D. 1976. Ontogeny of B cells in the chicken. Journal of Experimental Medicine 144: 79-97.
McCormack, W.T. & Thompson, C.B. 1993. Special features of the development of the chicken humoral immune system. Research in Immunology 144: 467-476.
Medway, W. & Kare, M.R. 1959. Blood and plasma volume hematocrit blood specific gravity and serum protein of the chicken. Poultry Science 38: 624-630.
Mercer-Oltjen, S.L. & Woodard, A.E. 1987. Development of the bursa of Fabricius in the Partridge and Pheasant. Poultry Science 66: 418-421.
Noor, S.M., Husband, A.J. & Widders, P.R. 1993. Prenatal oral vaccination establishes early development of intestinal immunity in chickens. Journal of Leukocyte Biology: 111.
Noor, S.M., Husband, A.J. & Widders, P.R. 1995. In ovo oral vaccination with Campylobacter jejuni establishes early development of intestinal immunity in chickens. British Poultry Science 36: 563-573.
Onyeanusi, B.I., Ezeokoli, C.D., Onyeanusi, J.C. & Ema, A.N. 1993. The anatomy of the cloacal bursa (bursa of Fabricius) in the helmeted Guinea fowl (Numida meleagis galeata). Anatomia Histologia Embryologia 22: 212-221.
Paramithiotis, E., Jacobsen, K.A. & Ratcliffe, M.J. 1995. Loss of surface immunoglobulin expression precedes B cell death by apoptosis in the bursa of Fabricius. Journal of Experimental Medicine 181: 105-13.
Paramithiotis, E. & Ratcliffe, M.J. 1994. Survivors of bursal B cell production and emigration. Poultry Science 73: 991-7.
Paramithiotis, E. & Ratcliffe, M.J. 1996a. Evidence for phenotypic heterogeneity among B cells emigrating from the bursa of Fabricius: a reflection of functional diversity? Current Topics in Microbiology and Immunology 212: 29-36.
Paramithiotis, E. & Ratcliffe, M.J.H. 1996b. Evidence of phenotypic heterogeneity among B cells emigrating from the bursa of Fabricius: A reflection of functional diversity. Current Topics in Immunnology 212: 29-36.
Phalen, D.N., Wilson, V.G. & Graham, D.L. 1995. Failure of maternally derived yolk IgG to reach detectable concentrations in the sera of nestling budgerigars (Melopsittacus undulatus). Avian Diseases 39: 700-708.
Piersma, T. 1997. Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos 80: 623-631.
Pope, C.R. 1991. Pathology of lymphoid organs with emphasis on immunosuppression. Veterinary Immunology and Immunopathology 30: 31-44.
Ratcliffe, M.J. & Jacobsen, K.A. 1994. Rearrangement of immunoglobulin genes in chicken B cell development. Seminars in Immunology 6: 175-184.
Reynaud, C.A. & Weill, J.C. 1996. Postrearrangement diversification processes in gut-associated lymphoid tissues. Current Topics in Immunnology 212: 7-16.
Ritchie, B.W., Niagro, F.D., Latimer, K.S. & Steffens, W.L. 1992. Antibody response to and maternal immunity from an experimental psittacine beak and feather disease vaccine. American Journal Veterinary Research 53: 1512-1518.
Rose, M.E. & Orlans, E. 1981. Immunoglobulins in the egg, embryo and young chick. Developmental and Comparative Immunology 5: 15-20.
Roura, E., Homedes, J. & Klasing, K.C. 1992. Prevention of immunologic stress contributes to the growth-permitting ability of dietary antibiotics in chicks. Journal of Nutrition 122: 2383-90.
Selvaraj, P. & Pitchappan, R.M. 1988. Post-hatching development of the immune system of the pigeon, Columba livia. Developmental and Comparative Immunology 12: 879-884.
Seto, F. 1981. Early development of the avian immune system. Poultry Science 60: 1981-1995.
Sharma, J.M. 1997. The structure and function of the avian immune system. Acta Veterinaria Hungarica 45: 229-238.
Shawky, S.A., Saif, Y.M. & McCormick, J. 1994. Transfer of maternal anti-rotavirus IgG to the mucosal surfaces and bile of turkey poults. Avian Diseases 38: 409-417.
Shawky, S.A., Saif, Y.M. & Swayne, D.E. 1993. Role of circulating maternal anti-rotavirus IgG in protection of intestinal mucosal surface in turkey poults. Avian Diseases 37: 1041-1050.
Sheldon, B.C. & Verhulst, S. 1996. Ecological immunology: Costly parasite defenses and trade-offs in evolutionary ecology. Trends in Ecology and Evolution 11: 317-321.
Shields, J.W., Dickson, J.R., Abbott, W. & Delvin, J. 1979. Thymic, bursal and lymphoreticular evolution. Developmental and Comparative Immunology. 3: 5-22.
Siegel, H.S., Henken, A.M., Verstegen, M.W.A. & van der Hel, W. 1982. Heat production during the induction of an immune response to sheep red blood cells in growing pullets. Poultry Science 61: 2296-2300.
Smith, N.C., Wallach, M., Petracca, M., Braun, R. & Eckert, J. 1994. Maternal transfer of antibodies induced by infection with Eimeria maxima partially protects chickens against challenge with Eimeria tenella. Parasitology 109: 551-557.
Sugita-Konishi, Y., Shibata, K., Yun, S.S., Hara-Kudo, Y., Yamaguchi, K. & Kumagai, S. 1996. Immune functions of immunoglobulin Y isolated from egg yolk of hens immunized with various infectious bacteria. Bioscience Biotechnology and Biochemistry 60: 886-888.
Szakal, A.K. 1989. Microanatomy of lymphoid tissue during humoral immune responses. Annual Review Immunology 7: 91-109.
Vainio, O. & Imhof, B.A. 1995. The immunology and developmental biology of the chicken. Immunology Today 16: 365-370.
Von Rautenfeld, D.B. & Budras, K.D. 1981. The bursa cloacae (Fabricii) of Struthioniformes in comparison with the bursa of other birds. Journal of Morphology 172: 123-188.
Table 1. Relationship between types and costs of immune responses.

Fig. 1. The relationship between chronological age and important developmental events for specific and non-specific immunity in the chicken.
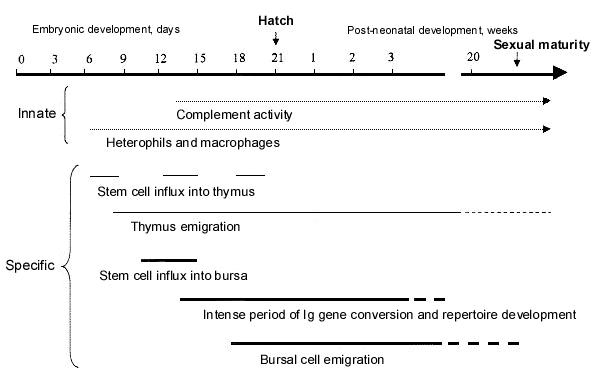
Fig. 2. Interactions between vaccination of hens with lipopolysaccharide (LPS) and the inflammatory response induced by LPS in Japanese Quail chicks during the first week post-hatch. LPS was injected into chicks on days 2, 4, and 6. Serum interleukin-1 activity was determined by bioassay.