S46.3: Maternal hormonal effects on postnatal development
Hubert Schwabl
Centre of Reproductive Biology and Department of Zoology, Washington State University, Pullman, WA 99164-4236, USA., fax 1 509 335 3181, e-mail Huschwabl@wsu.edu; internet: http://www.crb.wsu/3FacultyPages/Schwabl.html
Schwabl, H. 1999. Maternal hormonal effects on postnatal development. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2807-2816. Johannesburg: BirdLife South Africa.The contents of maternal sex steroid hormones in eggs vary within clutches, among females, and among species. This relates to egg sequence, environmental conditions during egg formation, and reproductive strategies. Variation within a clutch modifies competition in a brood and together with the timing of the onset of incubation may allow females of altricial birds to influence the fitness of specific offspring. This hormonal influence is mediated by increased begging vigour of the chicks. Variation among species in the egg content of sex steroids and in patterns of allocation to the eggs of a clutch may be related to reproductive strategies. Species with brood reduction may show higher allocation of steroids to the core brood, while species without brood reduction may show patterns of androgen allocation in which the levels increase from egg to egg. Variation in concentrations among the eggs of females within a species can result from social interactions. Since the exposure of the embryo to steroid hormones can permanently modify the behaviour of offspring the latter influence may function to produce offspring phenotypes that are prepared for the prevailing conditions in the parental population. The mechanisms of transfer of maternal steroids into the eggs and their action on the offspring are not clear but our studies suggest that maternal hormones can modify offspring behaviour at the nestling and adult stage. Therefore the endocrine control mechanisms of female reproduction may have been shaped, at least in part, by transgenerational effects of steroid hormones.
Maternal hormones and growth factors in the egg
The avian egg is a repository for maternal hormones and growth factors. Various hormones of maternal origin have been detected in the albumin and in the yolk of freshly laid eggs. For example, insulin and insulin-like growth factor I activity has been demonstrated in unfertilised chicken Gallus domesticus eggs (e.g. De Pablo et al. 1982; Scavo et al. 1989). The hormones of this family act as growth and differentiation factors and may be essential for the induction and normal progress of muscle and nervous tissue development during early embryogenesis (De Pablo & de la Rosa 1995). Several studies found maternal thyroid hormones in the egg yolks of gallinaceous birds (Mitchell et al. 1985; Sechman & Bobek 1988; Pratt et al. 1992). Exogenous thyroid hormones, applied to female Japanese quail Coturnix japonica during laying, are deposited in the egg yolk, disappear from the yolk during embryogenesis, and accelerate the differentiation and growth of pelvic cartilage (Wilson & McNabb 1997). Thyroid hormones are essential for the early development of vertebrates but we do not know if the presence of maternal thyroid hormones - before the embryonic thyroid glands start to secrete these hormones - is necessary for proper development or if maternal thyroid hormones act as modifying factors.
This review focuses on the role of maternal steroid hormones in avian development. The levels of maternal steroid hormones, in particular of androgens in the yolk, vary among the eggs of a clutch, among the eggs of different females, and among the eggs of different species (Schwabl 1997b). Because of this variation it is likely that these hormones are not essential for normal embryonic growth and differentiation but rather modify development. In the following I address potential mechanisms of steroid deposition in the yolk, ovarian steroidogenesis in relation to hormone deposition, effects of steroids on embryonic and postnatal development, and differences in steroid concentrations in relation to clutch size, egg size, and developmental mode.
Transfer of exogenous steroid hormones into the egg yolk
It has long been known that exogenous steroid hormones, when applied to females during egg formation, are deposited in the egg yolk. These early studies focused on estrogens. Riddle & Dunham (1942), for example, showed that a single intramuscular injection of milligram doses of 17b -estradiol and dehydroandrosterone into laying ring doves Streptopelia risoria led to the feminisation of the testes of male chicks. They concluded that estradiol is transported into the egg and that it interferes with the sexual differentiation of the gonads. Later studies showed that daily injections or implants of estradiol-benzoate result in elevated yolk estradiol concentrations in the eggs of Japanese quail Coturnix japonica and a higher incidence of right oviducts in females hatching from these eggs (Adkins-Regan et al. 1997). When tritiated 17b -estradiol was infused into laying hens Gallus domesticus radioactivity was accumulated in the egg yolk and labelled 'free' estrone and estradiol and a polar conjugate of estrone could be isolated and identified (Arcos 1971). Although these studies demonstrate that exogenous estrogens can be transported from the mother into the egg yolk and influences sexual differentiation we cannot conclude that maternal estrogens are also deposited in the yolk in significant amounts.
Presence of maternal steroid hormones in the egg
Because estrogen injections into laying females or directly into eggs caused intersexuality (e.g. Domm 1939; Riddle & Dunham 1942) earlier studies aimed to identify maternal estrogens in the egg yolk. Most of these studies failed to conclusively demonstrate estrogens in yolk (reviewed in Hertelendy & Common 1965). Recent studies found also very little, if any, estradiol in the yolk, confirming these earlier reports. However, substantial quantities of maternal androgens were detected in the egg yolks of the canary Serinus canaria (Schwabl 1993), cattle egret Bubulcus ibis (Schwabl et al. 1997), Snowy egret Egretta thula (H. Schwabl & D. W. Mock, unpublished data), red-winged blackbird Agelaius phoeniceus (Lipar et al. 1997), house sparrow Passer domesticus (Schwabl 1997a), European starling Sturnus vulgaris (Schwabl 1997b), Brown-headed cowbird Molothrus ater (Schwabl 1997b), common tern Sterna hirundo (J. French, I. Nisbet, & H. Schwabl, unpublished data), black-headed gull Larus melanocephalus (T. Groothuis & H. Schwabl, unpublished data), and American kestrel Falco sparverius (K. Sockman & H. Schwabl, unpublished data). The androgens that were measured in the yolk are androstenedione, testosterone, and 5a -dihydrotestosterone and their allocation to individual eggs and their absolute concentrations vary substantially within and among species.
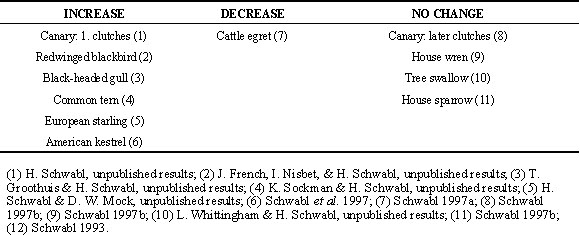
Variation in androgen levels of eggs
a) Within clutches:
In some species, such as the canary (Schwabl 1993) and the redwinged blackbird (Lipar et al. 1997), the concentrations of testosterone in the yolk increase from egg to egg in a clutch (Table 1). In other species, such as the facultatively siblicidal Cattle egret, they may decrease in the later laid eggs of a clutch (Schwabl et al. 1997). Therefore these authors proposed the hypothesis that the patterns of androgen allocation to the eggs of a clutch function to modify the competitive ability of nestlings. This hormonal influence, combined with egg-laying intervals and the timing of the onset of incubation of a clutch, may allow females to fine-tune (Schwabl 1993; Winkler 1993; Schwabl et al. 1997) the fitness of individual nestlings in a brood. Studies are in progress to test this hypothesis of hormonal parental favouritism.
b) Variation among clutches:
In addition to these different yolk concentrations of androgens in the eggs of a clutch there is also considerable variation in yolk androgen concentrations among clutches. For example, in the canary the yolk concentrations of testosterone vary between the eggs of subsequently laid clutches in a single breeding season. Eggs laid early in the breeding season contain more testosterone than eggs laid later in the season and concentrations increase from egg to egg in early but not in later clutches (Schwabl 1996a). The onset of incubation of a clutch changes also seasonally, from late to early during laying, in this species (H. Schwabl, unpublished results). Since testosterone and the onset of incubation work in opposite directions it is possible that the differential allocation of steroids in combination with the timing of the onset of incubation functions to favour specific offspring in a brood. High testosterone levels in last laid eggs combined with a late onset of incubation (resulting in synchronous hatching) favour the hatchling from the last eggs via hormones; low testosterone levels in last laid eggs combined with an early onset of incubation (resulting in asynchronous hatching) favour the hatchlings from first eggs via their seniority (Schwabl 1996a).
Social conditions can also modify the testosterone concentrations in the eggs. This was initially discovered in canaries in which the housing of several females together resulted in higher yolk testosterone concentrations compared to females that were housed with their mates in visual isolation from other pairs (Schwabl 1997b). Subsequently it was shown that the yolk concentrations of testosterone in the eggs of free-living house sparrows are positively correlated with nesting density (Schwabl 1997a). These social influences on the androgen exposure of embryos, which may function to modify offspring development in response to the prevailing social conditions in the population, warrant further studies.
c) Variation among species:
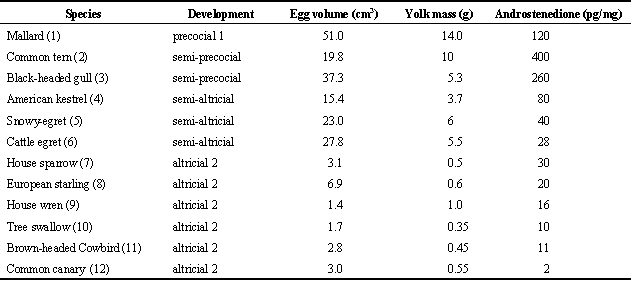
In addition to species differences in patterns of androgen allocation to the eggs of a clutch the absolute concentration of androgens vary also among species. For example, maternal androstenedione varies more than 400 fold. This could be related to factors such as egg size, clutch size, and developmental mode. A preliminary comparison of a very limited data set suggests the following: Highest levels occur in the large eggs of precocial and semi-precocial species, intermediate levels occur in the eggs of semi-altricial species, and lowest levels occur in the small eggs of altricial species (Table 2). While this might point towards a correlation with egg or yolk size - i.e., duration of vitellogenesis - it is also possible that it hints at a correlation with developmental mode. It is intriguing to speculate that the deposition of maternal steroids in the yolk and modes of development may be linked and may have co-evolved. A thorough analysis of the eggs of species with different developmental modes, which takes phylogenetic relationships into account, is necessary to test these ideas (Rickleffs 1993).
Effects on offspring development
1) Uptake and metabolism of maternal androgens by embryos.
Presently we do not understand how maternal androgens are assimilated from the yolk, how and where they are metabolised, and when, where, and how they act in the embryo. We know, however, that exogenous testosterone can be metabolised by embryonic cartilage tissue in vitro (Murota & Tamaoki 1967) - indicating the presence of enzymes for steroid metabolism - and that aromatase, which catalyses the conversion of testosterone into estradiol, is active in embryonic gonads (Yoshida et al. 1996) and hatchling brains (Vanson et al. 1996). We also know that exogenous androgens can be enriched in cells of the embryonic spinal chord and the glycogen body of chickens (Reid et al. 1981) - indicating the presence of intracellular androgen receptors at an early embryonic age in a precocial bird. Moreover, recent studies demonstrated the expression of androgen receptor mRNA in both sexes of altricial canaries before hatching (Gahr et al. 1996) - indicating the production of receptors in the embryo. While these studies suggest that various embryonic tissues possess enzymes for steroid metabolism and androgen receptors we do not know if maternal androgens obtained from the yolk are their substrates and ligands. More studies are needed to explore these questions. Despite this lack of information on molecular and biochemical mechanisms of maternal hormone action in the egg organismal studies clearly suggest developmental effects in both sexes.
2) Developmental effects.
a) Embryo: There is no evidence that androgens enhance the overall rate of embryonic development (i.e., shorten the incubation period). Canary and house sparrow eggs that were injected with testosterone and/or 5a -dihydrotestosterone hatched at the same time as control-injected eggs (Schwabl 1996b, H. Schwabl, unpublished results). On the other hand, the later laid eggs of mallards Anas plathyrhynchos (MacClusky et. al. 1997) and black kites Milvus migrans (Viñuela 1997) appear to have shortened developmental periods. Several intrinsic differences between sequentially laid eggs might cause earlier hatching, including different contents of maternal hormones such as thyroid hormones or growth factors but not androgens.
b) Post-hatching: Exposure to maternal androgens does influence post-hatching development. Injections of testosterone into the yolk of freshly first-laid canary eggs in dosages that were equivalent to the high maternal dosages of later laid eggs in a clutch resulted in faster hatchling growth compared to synchronously hatching control eggs (Schwabl 1996b). Moreover, in unmanipulated broods chicks that had hatched from eggs with a higher concentration of maternal testosterone grew faster compared to broodmates that had hatched synchronously from eggs with less maternal testosterone (Schwabl 1996b). The enhanced growth rate became apparent within the first 24 hours after hatching and was correlated with a higher frequency of food begging just after hatching. Apparently these hatchlings were able to obtain more food from their parents. There are several possibilities by which testosterone could enhance begging. Testosterone exposed chicks may have a higher metabolism, greater muscular strength, and better developed motor control or sensory systems (Schwabl 1996b). Although more studies are necessary to distinguish between these possibilities, these results furnish evidence that maternal testosterone influences the development of altrical birds before or shortly after hatching thereby intensifying the hatchling’s capability to compete for food with nest mates. Whether such effects occur also in precocial birds remains unknown.
c) Adulthood: Maternal testosterone appears to have long-lasting influences on offspring behaviour similar to the permanent organisational effects of steroid hormones on sex differences in brain function and behaviour (Balthazart & Ball 1995). First, the aggressive behaviour of both sexes of canaries (after they were fully grown and independent from their parents) was positively correlated with the dose of maternal testosterone they were exposed to in the egg (Schwabl 1993). Second, testosterone injections into freshly laid eggs resulted in higher rates of aggression in the sexually mature adults (H. Schwabl, unpublished results). Future studies will have to show if such long-lasting modification of aggressive behaviour by maternal steroids are analogous to the organisational effects of embryonic and hatchling steroids during sexual differentiation and if and how maternal steroids interact or interfere with the hormonal mechanisms of sexual differentiation. Also, studies of other behavioural traits such as courtship and parental behaviour are necessary to fully understand the impact of maternal hormone transmission on offspring fitness. Overall, there is good evidence that the differential deposition of maternal testosterone influences offspring behaviour during the nestling stage and has also effects that last into adulthood.
Ovarian endocrine function, ONSET OF incubation, and hormone deposition in the yolk
1) Ovarian steroidogenesis
Studies of gallinaceous birds show that the theca and granulosa cell layers of the follicles are the sites of ovarian steroid hormone production . In vitro measurements of steroidogenesis of intact follicles (Yu & Robinson 1992), isolated follicular tissue (Shahabi et al. 1975), and isolated theca or granulosa cells (Bahr et al. 1983; Etches & Duke 1984; Huang & Nalbandov 1979; Porter et al. 1989; Robinson & Etches 1986; Tilly et al. 1991) show that the outer, theca cell layer produces mainly estrogens and androgens while the inner, granulosa cell layer produces mainly androgens and progestins. The production of these steroids changes as a follicle is recruited into the follicular hierarchy, grows, and sequesters yolk. The theca cells of large preovulatory follicles cease to produce estrogens and androgens, while the granulosa cells of these follicles begin to produce large amounts of progesterone (Bahr et al. 1983). This is accompanied by a shift between the follicular wall compartments of the activities of key enzymes which catalyse the conversion of cholesterol to pregnenolone and progesterone, and androgens or estrogens (Kato et a. 1995; Nitta et al. 1993). The steroid hormones that were commonly measured in these studies were 17b -estradiol, testosterone, and progesterone. Androstenedione contents, measured in some studies, change in parallel with those of estradiol (Etches & Duke 1984). It is important to notice that the follicular contents and outputs of androstenedione and testosterone are much higher than those of estradiol (Etches & Duke 1984; Robinson & Etches 1986; Shahabi et al. 1975; Yu & Robinson 1992). These changes in androgen production during follicular growth may be important for the transmission of maternal steroid hormones into the egg yolk. Unfortunately we know very little about follicular steroid production in species other than poultry (Etches & Pettite 1990) and we have to rely on measurements of blood or excreted levels of hormones which reflect the overall steroidogenic activity of the entire ovary.
2) Female and yolk androgen levels
In the canary the faecal testosterone and estradiol contents, which probably reflect their ovarian production and rates of excretion, increase a few days before egg laying commences, peak one day before or on the day when the first egg is laid, and decrease at different times during the completion of a clutch (Schwabl 1996a; Sockman & Schwabl, unpublished manuscript). Faecal testosterone levels are higher during the formation of first than second clutches (Schwabl 1997b). The yolk testosterone concentrations of eggs are positively correlated with the concentrations of faecal testosterone during the putative time of maximum yolk deposition of each egg (Schwabl 1996a). Despite this correlation, which suggests that the differences in yolk steroid concentrations reflect changes in the steroidogenic activity of the entire ovary during clutch formation, other mechanisms for the differential incorporation of steroids into the yolk of different eggs are possible. For example, testosterone may be actively transported from the follicular walls into the yolk of a follicle without ever reaching the circulation. The shift of enzyme activities between follicular cell layers during follicular growth may provide such a mechanism by which transmission of steroids into the yolk or into the maternal systemic circulation are regulated. Or, specific androgen binding proteins may be present in the yolky follicles. Further investigations, in vivo and in vitro, are required to resolve this issue. However, even if the yolk steroid concentrations simply reflect the overall steroid production of all ovarian follicles and hence the blood concentrations, the variability among and within species in patterns of circulating blood levels of androgens during vitellogenesis will result in differential concentrations of these hormones in the yolk.
3) Variation in female androgen levels among and within species
There is great variation in concentrations and patterns of plasma gonadal steroid levels before and during egg laying. Circulating estradiol levels are generally low compared to androgen levels and show similar patterns among species (Etches 1990; Etches & Cheng 1981; Wingfield & Farner 1993; but see Groscolas et al. 1986; Hector et al. 1986a, 1986b; Mauget et al. 1994). In contrast, circulating androgen levels vary tremendously among and also within species. For example, female albatrosses and penguins have very high testosterone levels during egg formation and laying (Fowler et al. 1994, Hector et al. 1986a, 1986b; Mauget et al. 1994) while females of most passerine birds have comparatively low levels (Wingfield & Farner 1993). Female western gulls Larus occipitalis show low plasma testosterone levels (0.2ng/ml) during ovulation (Wingfield et al. 1980) while closely related black-headed gulls Larus melanocephalus show levels of more than 1ng/ml during egg laying (Malickiené et al. 1997). These differences may result from differences in life histories, i.e., social behaviour, and predict different yolk concentrations of testosterone in the eggs. Plasma androgen concentrations during egg-formation and laying vary also within species, for example with the progress of the breeding season. Female house sparrows (Hegner & Wingfield 1986), white-crowned sparrows Zonotrichia pugetensis (Wingfield & Farner 1978), and song sparrows Melospiza melodia (Wingfield 1985) have higher testosterone levels when they produce their first clutches than when they produce their second clutches. Apparently blood androgen levels, reflecting their production by the ovary, can change in response to environmental conditions while follicle maturation, ovulation, and laying proceed normally. This diversity in circulating steroid hormone blood levels prior and during laying, while certainly related to behavioural changes (Wingfield 1994), will result in the deposition of different doses of maternal steroids in the eggs, which in turn will influence offspring development.
4. Onset of incubation and ovarian steroidogenesis
Incubation and ovarian steroid production seem to be physiologically coupled as ovarian steroid production ceases as incubation commences. For example, the faecal testosterone and estradiol levels of canaries peak with the ovulation of the first egg of a clutch and plummet with the onset of incubation, suggesting that the mechanism that triggers incubation causes a cessation of ovarian steroid production (Schwabl 1996a; K. Sockman & H. Schwabl, unpublished manuscript). If incubation and steroid production are indeed incompatible, then we have here a mechanism that will result in low levels of steroids in later laid eggs, when incubation starts early during laying, and in higher levels of steroids in later laid eggs, when incubation starts after the last egg has been ovulated. This has been observed with regard to yolk testosterone levels in canary eggs (Schwabl 1996a) but experiments are needed to establish this causal link. Functionally, the physiological coupling of these two reproductive functions will allow for 'hormonal favouritism' of last-egg chicks and for 'asynchronous hatching favouritism' of first-egg chicks. Whether species differences in steroid hormone proliferation of eggs are also based on such a physiological link between ovarian steroidogenesis and onset of incubation remains to be studied.
CONCLUSIONS
The presence of maternal peptide, thyroid, and steroid hormones, and growth factors in the eggs suggest a hormonal continuum between the female and her offspring. While some of these maternal factors may be essential for early embryogenesis others might act as developmental modifiers. The variation in steroid hormone contents among and within species of birds suggests modifying influences on intra-clutch offspring competition, long-term effects on the behavioural phenotype of the offspring, and relationships with egg size or developmental mode. Future studies will have to show whether transgenerational influences by steroid and other hormones are a common feature of vertebrate reproduction and development. If so, then the hypothalamo-adenohypophysio-ovarian axis may have been shaped, at least in part, by the role of ovarian cells to deliver steroid hormones to the offspring.
ACKNOWLEDGEMENTS
The author’ s work reported in this article was supported by grant MH 49877-01 from the National Institute of Mental Health, by grant IBN-9604370 from the National Science Foundation, and by a grant from the Harry Franck Guggenheim Foundation.
REFERNCES
Adkins-Regan, E., Ottinger, M.A. & Park, J. 1995. Maternal transfer of estradiol to egg yolks alters sexual differentiation of avian offspring. Journal of Experimental Zoology 271: 466-470.
Arcos, M. 1971. Steroids in egg yolk. Steroids 19: 25-34.
Bahr J.M., Wang, S.-C., Huang, M.Y. & Calvo, F.O. 1983 Steroid concentrations in isolated theca and granulosa layers of preovulatory follicles during the ovulatory cycle of the domestic hen. Biology of Reproduction 29: 326-334.
Balthazart, J. & Ball, G.F. 1995. Sexual differentiation of brain and behavior in birds. Trends in Endocrinology and Metabolism 6: 21-29.
De Pablo, F. & de la Rosa, J. 1995. The developing CNS: a scenario for the action of proinsulin, insulin, and insulin-like growth factors. Trends in Neuroscience 18: 143-150.
De Pablo, F., Roth, J., Hernandez. E. & Pruss, R.M. 1982. Insulin is present in chicken eggs and early chick embryos. Endocrinology 111: 1909-1916.
Domm, L.V. 1939. Intersexuality in adult Brown leghorn male as a result of estrogenic treatment during early embryonic life. Proceedings of the Society Experimental Biology 42: 310-312.
Etches, R.J. 1990. The ovulatory cycle of the hen. CRC Critical Reviews of Poultry Biology 2: 293-318
Etches, R.J. & Cheng, K.W. 1981. Changes in the plasma concentrations of luteinizing hormone, progesterone, oestradiol and testosterone and in the binding of follicle-stimulating hormone to the theca of follicles during the ovulation cycle of the hen (Gallus domesticus). Journal of Endocrinology 91: 11-22
Etches, R.J. & Duke, C.E. 1984. Progesterone, androstenedione and oestradiol content of theca and granulosa tissue of the four largest ovarian follicles during the ovulatory cycle of the hen (Gallus domesticus). Journal of Endocrinology 103: 71-76.
Etches, R.J. & Petitte, J.N. 1990. Reptilian and avian follicular hierarchies: Models for the study of ovarian development. Journal of Experimental Zoology, Supplement 4: 112-122.
Fowler, G.S., Wingfield J.C., Boersma, P.D. & Sosa, R.A. 1994. Reproductive endocrinology and weight change in relation to reproductive success in the Magellanic penguin (Sphenicis magellanicus). General and Comparative Endocrinology 94: 305-315.
Gahr, M., Metzdorf, R. & Aschenbrennner, S. 1996. The ontogeny of the canary HVC revealed by the expression of androgen and estrogen receptors. NeuroReport 8: 311-315.
Groscolas, R., Jallageas, M., Goldsmith, A. & Assenmacher, I. 1986. The endocrine control of reproduction and molt in male and female Emperor (Aptenodytes forsteri) and Adelie (Pygoscelis adeliae) penguins. General and Comparative Endocrinology 62: 43-53.
Hector, J.A.L., Croxall, J.P. & Follett, B.K. 1986a. Reproductive endocrinology of the Wandering albatross, Diomedea exulans, in relation to biennial breeding and deferred sexual maturity. Ibis 128: 9-22.
Hector, J.A.L., Follett, B.K. & Prince, P.A. 1986b. Reproductive endocrinology of the Black-browed albatross Diomedea melanophrys and the Grey-headed albatross Diomedia chrysostoma. Journal of Zoology, London 208: 237-253.
Hegner, R.E. & Wingfield, J.C. 1986. Behavioral and endocrine correlates of multiple brooding in the semicolonial house sparrow Passer domesticus. II. Females. Hormones and Behavior 20: 313-326.
Hertelendy, F. & Common, R.H. 1965. A chromatographic investigation of egg yolk for the presence of steroid estrogens. Poultry Science 44: 1205-1209.
Huang, E.S.-R. & Nalbandov, A.V. 1979. Steroidogenesis of chicken granulosa and theca cells: in vitro incubation system. Biology of Reproduction 20: 442-453.
Kato, M., Shimada, K., Saito, N., Noda, K. & Ohta, M. 1995. Expression of P45017a -hydroxylase and P450 aromatase genes in isolated granulosa, theca interna, and theca externa layers of chicken ovarian follicles during follicular growth. Biology of Reproduction 52: 405-410.
Lipar, J.L., Ketterson, E.D. & Nolan, V., Jr. 1995. Steroid hormones in the yolk of Red-winged blackbird eggs. Poultry and Avian Biology Reviews 6: 329.
Mac Cluskie, M.C., Flint, P.L. & Sedlinger, J.S. 1997. Variation in incubation periods and egg metabolism in mallards: Intrinsic mechanisms to promote hatch synchrony. The Condor 99: 224-228.
Malickiené, D., Jurkevic, D. & Zurba, M. 1997. Gonadal steroids, luteinizing hormone and prolactin secretion in black-headed gulls in relation to egg laying, incubation and rearing of youngsters. Biologija 1: 117- 119.
Mauget, R., Jouventin P., Lacroix, A. & Ishii, S. 1994. Plasma LH and steroid hormones in King penguin (Aptenodytes patagonicus) during the onset of the breeding cycle. General and Comparative Endocrinology 93: 36-43.
Mitchell, M.A., Raza, A. & Uglow, P. 1985. Transfer of maternal thyroid hormones to the embryo in the domestic fowl. Journal of Physiology 360: 74.
Murota, S.-I. & Tamaoki, B.-I. 1967. Metabolism of progesterone and testosterone by chick embryo cartilage in vitro. Biochimica and Biophysica Acta 137: 347-355.
Nitta, H., Mason, J.I. & Bahr, J. M. 1993. Localization of 3b -Hydroxysteroid dehydrogenase in the chicken ovarian follicle shifts from the theca layer to granulosa layer with follicular maturation. Biology of Reproduction 48: 110-116.
Porter, T. E., Hargis, B.M., Silsby, J.L & El Halawani, M. E. 1989. Differential steroid production betweeen theca interna and theca externa cells: a three-cell model for follicular steroidogenesis in avian species. Endocrinology 125: 109-116.
Pratt, M., Calvo, R. & Morreale De Escobar, G. 1992. L-thyroxine and 3,5,3’ - triiodothyronine concentrations in the chicken egg and the embryo before and after the onset of thyroid function. Endocrinology 130: 2651-2659.
Reid, F.A., Gasc J.-M., Stumpf, W.E. & Sar, M. 1981. Androgen target cells in spinal cord, spinal ganglia, and glycogen body of chick embryos. Experimental Brain Research 44: 243-248.
Ricklefs, R.E. 1993. Sibling competition, hatching asynchrony, incubation period, and lifespan in altricial birds. In: Powers, D. M. (ed) Current Ornithology Vol. 11. New York, London; Plenum Press: 199-276.
Riddle, O. & Dunham, H.H. 1942. Transformation of males to intersexes by estrogen passed from blood of ring doves to their ovarian eggs. Endocrinology 30: 959-968.
Robinson, F.E. & Etches, R.J. 1986. Ovarian steroidogenesis during follicular maturation in the domestic fowl (Gallus domesticus). Biology of Reproduction 35: 1096-1105.
Scavo, L., Alemany, J., Roth J. & De Pablo, F. 1989. Insulin-like growth factor I activity is stored in the yolk of the avian egg. Biochemical and Biophysical Research Communications 162: 1167-1173.
Schwabl, H. 1993. Yolk is a source of maternal testosterone for developing birds. Proceedings of the National Academy of Sciences USA 90: 11466-11450.
Schwabl, H. 1996a. Environment modifies the testosterone levels of a female bird and its eggs. Journal of Experimental Zoology 276: 157-163.
Schwabl, H. 1996b. Maternal testosterone in the avian egg enhances postnatal growth. Comparative Biochemistry and Physiology 114A: 271-276
Schwabl, H. 1997a. The contents of maternal testosterone in house sparrow Passer domesticus eggs vary with breeding conditions. Naturwissenschaften 84: 1-3.
Schwabl, H. 1997b. Maternal Steroid Hormones in the Egg. In: Etches. R. & Harvey S. (eds) Perspectives in Avian Endocrinology. Bristol; Journal of Endocrinology Limited: 3-13.
Schwabl, H., Mock, D.W. & Gieg, J.A. 1997. A hormonal mechanism of parental favouritism. Nature 386: 231.
Sechman, A. & Bobek, S. 1988. Presence of iodothyronines in the yolk of the hen’s egg. General and Comparative Endocrinology 69: 99-105.
Shahabi, N.A., Norton, H.W. & Nalbandov, A.V. 1975. Steroid levels in follicles and the plasma of hens during the ovulatory cycle. Endocrinology 96: 962-968.
Starck, J.M. 1993. The evolution of avian ontogenies. In: Powers, D. M. (ed) Current Ornithology Vol. 10. New York, London; Plenum Press: 275-366.
Tilly, J.L., Kowalsky, K.I. & Johnson, A.L. 1991. Stages of ovarian follicular development associated with the initiation of steroidogenic competence in avian granulosa cells. Biology of Reproduction 44: 305-314.
Vanson, A., Arnold, A.P. & Schlinger, B.A. 1996. 3b -Hydroxysteroid dehydrogenase/isomerase activity in primary cultures of developing zebra finch telencephalon: dihydroepiandrosterone as substrate for synthesis of androstenedione and estrogens. General and Comparative Endocrinology 102: 342-350.
Viñuela, J. 1997. Laying order affects incubation duration in the Black kite (Milvus migrans): Counteracting hatching asynchrony? The Auk 114: 192-199.
Wilson, C.M. & McNabb, F.M.A. 1997. Maternal thyroid hormones in Japanese quail eggs and their influence on embryonic development. General and Comparative Endocrinology 107: 153-165.
Wingfield, J.C. 1985. Influences of weather on reproductive function in female song sparrows Melospiza melodia. Journal of the Zoology, London, 205: 545-558.
Wingfield, J.C. 1994. Hormone-behavior interactions and mating systems in male and female birds. In: Short, R. V. & Balaban E. (eds) The differences between the sexes. Cambridge; University Press: 303-330.
Wingfield, J.C. & Farner D.S. 1978. The endocrinology of a natural breeding population of the White-crowned sparrow (Zonotrichia leucophrys pugetensis) Physiological Zoology 51: 188-205.
Wingfield, J.C. & Farner, D.S. 1993. Endocrinology of reproduction in wild species. In: Farner, D. S., King, J.R. & Parkes K.C. (eds) Avian Biology. Vol. 9. New York; Academic Press: 163-327.
Wingfield,. J.C., Newman, A., Hunt, G.L., Jr. & Farner, D.S. 1980. Androgen in high concentrations in the blood of female Western gulls, Larus occidentalis. Naturwissenschaften 67: 514-515.
Winkler, D.W. 1993. Testosterone in egg yolks: an ornithologist’s perspective. Proceedings of the National Academy of Sciences, USA, 90: 11439-11441
Yoshida, K., Shimada, K. & Saito, N. 1996. Expression of P450 17a and P450 aromatase genes in the chicken gonad before and after sexual differentiation. General and Comparative Endocrinology 102: 233-240.
Yu, M.W. & Robinson, F.E. 1992. Quantification of ovarian steroidogenesis in the domestic fowl by incubation of intact large follicles. Poultry Science 71: 346-351.
Table 1. Species in which the yolk testosterone concentrations increase, decrease, or do not change from egg to egg in a clutch. (1) Schwabl, 1993; (2) Lipar et al. 1995; (3) Groothuis, T. & Schwabl H. unpublished results; (4) French, J., Nisbet, I. & Schwabl, H. unpublished results; (5) Schwabl, H. unpublished results; (6) Sockman, K. & Schwabl, H. unpublished results; (7) Schwabl et al. 1997; (8) Schwabl 1996a; (9) Schwabl, H. unpublished results; (10) Whittingham, L. & Schwabl, H. unpublished results; (11) Schwabl, H. unpublished results.

Table 2. Yolk androstenedione concentrations in relation to egg mass, clutch size, colonial versus solitary breeding, and developmental mode. Ontogenies are classified according to Starck (1993).