S46.2: Light experience and hormone levels in chick embryo affect posthatching behaviour
Lesley J. Rogers
Neuroscience and Animal Behaviour, School of Biological Sciences, University of New England, Armidale, NSW 2331, Australia, fax 61 267 733 452, e-mail lrogers@metz.une.edu.au
Rogers, L.J. Light experience and hormone levels in chick embryo affect posthatching behaviour. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2784-2806. Johannesburg: BirdLife South Africa.Exposure of the avian embryo to light influences the development of the visual pathways and behaviour after hatching, as revealed by studies using Gallus gallus domesticus. The effect of light is limited to the last few days before hatching when the visual system is becoming functional. From day E17 until hatching the embryo occludes its left eye by its body and its right eye is positioned next to the air sac where it can be stimulated by light. This causes some of the visual neurones that receive input from the right eye to develop in advance of others. Thereby asymmetry develops in the visual pathways and this affects some aspects of behaviour after hatching. Chicks hatched from light-exposed eggs use visual inputs from the right eye to categorise grain from pebbles and visual inputs from the left eye to control attack and copulation responses. These behavioural lateralisations do not occur in chicks hatched from eggs incubated in the dark, and nor does the lateralisation in the visual pathways. Also, artificial exposure of the embryo’s left eye to light reverses both the structural and behavioural lateralisations. The levels of circulating steroid hormones (testosterone, oestrogen and corticosterone) also alter lateralisation both before and after hatching. Before hatching these hormones interact with the effect of light, causing both sex and individual differences in the visual pathways and behaviour. The relevance of this research to other avian species and its potential significance in natural populations is discussed.
INTRODUCTION
The behaviour of birds is influenced by experience in the early posthatching period. It is during the early posthatching stages of development that social behaviours are learnt and other behaviours essential for survival are shaped by experience. The time-frame during which this crucial learning occurs is relatively short in precocial species compared to the more slowly developing, altricial species, and we know for the chick, Gallus gallus, that behavioural development during the first two weeks posthatching passes through a sequence of precisely timed phases during which different aspects of learning occur and different types of behaviour are expressed. The most studied of these sensitive periods for learning is that of imprinting, which occurs optimally towards the end of the first day posthatching (reviewed by Bolhuis 1991) and cannot take place beyond day 4 posthatching, except under very exceptional circumstances which involve pharmacological manipulation of brain function (Parsons & Rogers 1997). In addition to imprinting, there are sensitive periods for learning to feed (Hogan 1973; Hale & Green 1988) and to recognise conspecifics (Bateson 1990; Vallortigara & Andrew 1991). There are also precise ages at which a number of other behaviours appear for the first time (Workman & Andrew 1989). For example, on day 10 posthatching, chicks first begin to move out of sight of the hen for short periods of time and engage in frolicking behaviour (Workman & Andrew 1989).
Lateralisation in chicks
Many of the behaviours expressed by chicks in the early posthatching period depend on lateralisation of brain function. Lateralisation refers to the fact that each side of the brain controls a different set of functions and one way of revealing this is to test chicks monocularly with a patch applied to one or the other eye. The chick performs differently when the left or right eye is occluded. One form of lateralisation is clearly evident in the monocular performance of male chicks tested on the ‘pebble-floor’ task, in which the chick searches for grains scattered on a background or small pebbles adhered to the floor. Binocular chicks begin pecking randomly at grains and pebbles but they soon begin to avoid pecking at the pebbles. This shift away from pecking at pebbles occurs in chicks using the right eye only but not in those using the left eye (Mench & Andrew 1986; Zappia & Rogers 1987). There is also a sex difference in this lateralisation, females having either no lateralisation (Zappia & Rogers 1987) or a lesser degree of lateralisation (Mench & Andrew 1986) than males, possibly depending on the age at which they are tested. Another form of lateralisation has been revealed by testing the sexual and aggressive responses of monocular chicks. Following treatment of young chicks with testosterone, sexual and aggressive behaviours increase provided that the chicks are tested binocularly or monocularly using the left eye, but not if the right eye is used (Rogers et al. 1985). A leftward bias for aggressive responses has been demonstrated to occur in adult hens also (Rogers 1991).
Lateralisation is also apparent in the responses of untreated chicks to stimuli presented in the left or right lateral visual fields. For example, chicks show more fear responses to novel stimuli presented in the left, lateral visual field that to those in the right, lateral visual field (Andrew & Brennan 1983). When tested binocularly, the chicks choose to view different visual stimuli with the left or right lateral field depending on the nature of the stimulus, whether it is novel or familiar and how conspicuous it is (Dharmaretnam & Andrew 1994). These lateralities extend into adulthood: when adult hens hear an alarm call made by another chick signalling the presence of an aerial predator, they use the left, lateral visual field to scan overhead (Evans et al. 1993). Adults hens also show a significant bias to view their partners with the left eye (McKenzie et al. 1998).
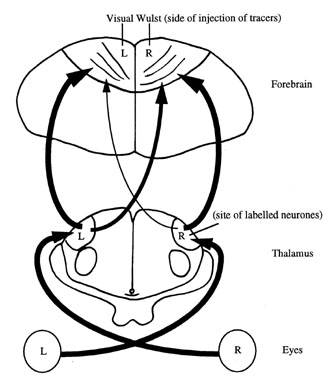
There is now a considerable amount of evidence showing that inputs from the right eye are involved in categorising stimuli according to obvious features, while those from the left eye attend to the details of each stimulus and are used to process spatial or topographic information (Andrew 1988; Rashid & Andrew 1989). These differences between the left and right eyes depend on differential processing of information by the left and right hemispheres of the chick’s brain. The left hemisphere of the forebrain has an essential role in controlling the chick’s ability to categorise grain from pebbles and thus direct pecking away from pebbles towards grain (Howard et al. 1980). In fact, the region involved has now been located quite precisely in the left hemisphere and the same region is also involved in controlling sexual behaviour (Deng & Rogers, 1997; see Fig. 1). This result was achieved by injecting small amounts of glutamate into different regions of the left and right hemispheres and then testing the chicks on a number of tasks. Following injection of the glutamate into the left visual Wulst, the forebrain region that receives visual inputs from the thalamus (Fig. 1), the ability of chicks tested binocularly to avoid pecking at pebbles was impaired and sexual and aggressive behaviour was enhanced. The latter occurs because the glutamate treatment removes inhibition of sexual and aggressive behaviour by the left hemisphere (Bullock & Rogers 1992). No effects followed injection of the right visual Wulst or other regions of either hemisphere (the neostriatum or ectostriatum). This demonstrates clearly that the functions of the hemispheres are lateralised.
There are two sets of visual inputs to the avian forebrain, one from the thalamus to the Wulst and the other from the optic tectum to the ectostriatum. Only the thalamo-Wulst projections are involved in the forms of lateralisation discussed here but it is possible that lateralisation occurs at other levels of neural processing also.
Each hemisphere also has a different role in memory formation and recall of imprinting (Cipolla-Neto et al. 1982; Johnston et al. 1993), and learning to avoid pecking at a bitter-tasting bead (Patterson & Rose 1992; Andrew 1996). Recall of the memory trace follows different cycles in the left and right hemispheres (Andrew 1997). Memories are encoded at different sites in the left and right hemispheres and the neurochemical changes that occur are different in equivalent locations in the left and right hemispheres (Rose 1991).
As a result of lateralisation of the hemispheres for processing information and for consolidation and recalling memories, preferred use of the lateral visual field of the left or right eye to view different stimuli reflects not only the use of different ways of processing but also different ways of encoding the memory of those stimuli. As a result, dominance of one or other of the hemispheres at any particular time may determine the behavioural responses given at that time, as experiments to follow will show. Although the chicken appears to be able to choose which lateral visual field it will use to view specific stimuli, and therefore which types of central processing will be used, dominance of the left hemisphere may confer better performance in pecking at food versus nonfood (e.g. pebbles) but less attention to novel stimuli and lower levels of aggression and sexual behaviour, whereas dominance of the right hemisphere may lead to the opposite and, as Rashid and Andrew (1989) have shown, also to better use of spatial information based on assessing more distant topographical cues.
Shifts in lateralisation during development
Shifts in hemispheric dominance during the first two weeks posthatching explain some of the sharply timed changes in behaviour occurring over this period of development. There are ages at which one or the other hemisphere takes charge of behaviour and these shifts in hemispheric dominance follow a precise time-course. The left hemisphere is in charge of controlling behaviour between days 2 and 5 and again on day 8, whereas the right hemisphere is in charge on days 10 and 11 (Andrew 1991; Rogers 1991). These shifts in hemispheric dominance appear to underlie some of the transitions in behaviour that occur posthatching. For example, learning to find food usually occurs early when the left hemisphere is dominant and, as Workman and Andrew (1989) and Vallortigara et al. (1997) have reported, chicks begin to move out of sight of the hen to explore the environment on day 10 or 11 when the right hemisphere, which processes topographical information, asserts its dominance. The same researchers have also shown that on day 10 chicks show a sudden increase in visually fixating human observers, a behaviour that may reflect the right hemisphere’s interest in novel stimuli.
The time-course of these rather sharp transitions in hemispheric dominance and behaviour may reflect a predetermined program for development. They may be at least partly dependent on changes in the levels of circulating steroid hormones, as suggested by one of the experiments to be reported here, and they may also be influenced by experience during the embryonic stages of development.
Light experience determines some aspects of lateralisation
Relatively little attention has been paid to the effects of experience during the prehatching period on posthatching behaviour even though it is known that the embryos of precocial species respond to tactile, auditory, visual and olfactory stimuli, the onset of responding in each sensory system being in this particular order of development (summarised in Rogers 1995). In fact, it is known that the chick embryo can be conditioned to tactile, auditory and visual stimuli (Gottlieb 1968). Thus sensory experience prior to hatching is likely to leave a memory trace and also to affect brain development. Both of these factors may influence behaviour after hatching.
In particular, it is known that lateralisation of some forms of visual behaviour is determined by light experience of the embryo during the last stages before hatching occurs. From day E17 to E18 of incubation (E referring to embryonic age as distinct from posthatching) the embryo is oriented in the egg so that its head is turned to its left side and the left eye is occluded by the body. The right eye is positioned next to the membranes of the air sac and directed outward so that it can receive stimulation by light that passes through the shell and membranes (Rogers 1990, 1995). This lateralised stimulation of the eyes establishes the lateralisation of performance on the pebble floor and lateralised aggressive and sexual responses. Chicks hatched from eggs incubated in the dark from day E17 until hatching lack lateralisation for these behaviours (Zappia & Rogers 1983; Rogers 1990, 1991, 1997; and see below). It is even possible to reverse the direction of lateralisation by withdrawing the embryo’s head from the egg on day E19 or E20, applying a patch to the right eye and then exposing the left eye to light (Rogers 1990, 1996, 1997): chicks that have received exposure of the left eye to light, instead of the right eye, are able to avoid pecking at pebbles when they use the left eye only but not when they use the right eye only. It is possible that the abnormal exposure of the embryo’s left eye to light also alters the normal program for shifts in hemispheric dominance, and one of the experiments to be reported here examines this.
The effect of light exposure of the embryo has been tied to the thalamo-Wulst (also referred to as the thalamofugal) visual pathway of the young chick. In chicks that have been exposed to light before hatching, the visual projections arising from the left side of the thalamus and projecting to the right visual Wulst develop in advance of those arising from the right side of the thalamus and projecting to the left visual Wulst (Rogers & Sink 1988; Rogers & Deng 1998; see Fig. 1). As each eye projects exclusively to the thalamus on the contralateral side of the brain, it is the right eye, which receives stimulation by light, that relays its inputs via the better developed thalamofugal projections. Stated more simply, the eye stimulated by light before hatching develops more projections to the forebrain than does the occluded eye.
Conclusive evidence for the role of light in establishing lateralisation in the thalamofugal visual projections has come from experiments in which the light exposure of the embryo was manipulated. The thalamofugal projections are symmetrical in chicks hatched from eggs incubated in the dark (Rogers & Bolden 1991) and the direction of the asymmetry is reversed by exposing the embryo’s left eye, instead of the right, to light (Rogers & Sink 1988). Thus the structural asymmetry in these visual projections to the forebrain correlates with the functional asymmetries found for pebble-floor performance, sexual and aggressive behaviour.
There is a sex difference in the degree of asymmetry in these visual projections, females having a lesser degree of asymmetry compared to males (Rajendra & Rogers 1993), which is consistent with the lesser degree of lateralisation of pebble-floor performance in females than in males.
Effect of steroid hormones
The lesser degree of lateralisation in females than in males raised the possibility that the sex steroid hormones may have a role in the differentiation processes by which light stimulation influences the development of the visual projections in the embryo. Previous experimentation has shown that this is the case (Rogers & Rajendra 1993; Schwarz & Rogers 1992). Administration of either testosterone or oestrogen to the egg prevents the development of asymmetry which usually occurs as a consequence of exposing the late embryo to light.
The role of the steroid hormones will be reported in more detail in experiment 4 to follow and with the addition of the adrenal steroid hormone, corticosterone. It was considered to be important to investigate the effects of corticosterone because it is known to influence brain development (Doupe & Patterson 1982; Meyer 1985) by altering synaptic plasticity (García-Segura et al. 1994; McEwen & Sapolsky 1995), neurone survival (Sapolsky et al. 1990; Gould et al. 1991) and receptor expression (Biegon et al. 1985; Sarrieau et al. 1988). In addition, memory formation in chicks after hatching can be enhanced by the administration of corticosterone just prior to training (Sandi & Rose 1994; Sandi et al. 1995) or, of particular interest here, by administration of corticosterone to chick embryos on day E19 or E20 (Sui et al. 1997).
The hypothesis being tested here is that variation in the levels of sex and adrenal steroid hormones from one embryo to another may interact with the critical role of light exposure to bring about both sex differences and individual differences in the degree of structural asymmetry and behavioural lateralisation. In addition to the hormones produced by the embryo itself, steroid hormones from the hen may be deposited in the egg and may influence embryonic development. Schwabl (1993) has shown that eggs of the canary (Serinus canaria) laid at the beginning of the clutch contain lesser amounts of maternal testosterone than those laid later: there is a progressive increase in testosterone level with order of laying, regardless of the genetic sex of the embryo. Adrenal steroid hormones, including corticosterone, may also be deposited in the egg by the hen, although Schwabl (1993) found that corticosterone was undetectable in canary eggs and at low levels in zebra finch eggs. However, the amount of these hormones secreted by the hen’s adrenal glands and deposited in the egg may also vary according to the hen’s state of stress or arousal. Also, it is possible that the embryo itself secretes some adrenal steroids. Sui et al. (1997) cite evidence that plasma corticosterone levels are high on the day of hatching and gradually decline over the next few days of life, indicating that the levels might also be high in the period just prior to hatching. For this reason and because corticosterone affects neurone development and function, it was considered to be important to look at the potential effects of altering the levels of corticosterone in ovo on the development of the visual projections as a first step in understanding the role of stress hormones in avian development.
The experiments to follow will demonstrate different factors that influence the presence and direction of lateralisation in the chick. Some have immediate and direct effects, while others are longer-lasting effects on development.
METHODS
Incubation conditions
Fertilised eggs (Black Australorp x White Leghorn) were incubated in an automatically turning, forced-draught incubator during the first 16 to 17 days. They were candled on day E7 of incubation and any infertile eggs were discarded. On day E16 or E17 of incubation, they were moved to either a dark incubator located in a dark room or to an incubator illuminated with a 40 W light bulb suspended inside the incubator (150-300 lux measured at the level of the eggs).
Experiment 1: Light exposure of the embryo and lateralisation of pebble-floor performance
Chicks hatched from eggs incubated in the dark from day E17 until hatching or exposed to light for 24 hours on day E17 of incubation were tested on the pebble-floor task with grains of chick mash scattered on a background of small pebbles (first described by Rogers et al. 1974, but see also Rogers 1997). The pebbles, which are adhered to the floor, overlap with the grain in their range of sizes, shapes and colours but differ from grain in texture and brightness. On day 8 posthatching the chicks were deprived of food for 3 hours. Ten minutes before testing a conical-shaped piece of adhesive tape was applied to either the left or right eye. There were also separate groups of chicks tested binocularly. The first 60 pecks at grain and pebbles were scored. The number of pecks at pebbles in the last block of 20 pecks was used to indicate the ability of the chick to perform the task by categorising grain from pebbles. Sex of the chicks was determined after testing by inspection of the gonads. There were 8 to 11 chicks in each group.
Experiment 2: Reversal of lateralisation of pebble-floor performance
A separate procedure was carried out to test performance on the pebble-floor task of chicks incubated in conditions that reverse the direction of lateralisation (Rogers 1990). On day E19/20 of incubation (the tucking stage of embryonic development, when the embryo begins to breathe air), the shell and membranes over the air sac of the egg were removed and the embryo’s head was withdrawn carefully from the egg. A patch was applied to the left or right eye and the embryos were exposed to light until hatching 24 hours later. After hatching the eye patches were removed. There were 84 chicks and these were subdivided into groups to be tested monocularly on the pebble floor on day 3, 5, 8 or 12 of life posthatching. Each chick was tested once only on one of these ages. Both male and female chicks were tested.
Experiment 3: Acute effects steroid hormones on pebble-floor performance
The effects of administering steroid sex hormones on binocular and monocular performance on the pebble floor on day 12 was tested. The aim was to see whether these hormones had any influence on lateralisation revealed as a difference in performance between the left and right eyes. The steroids were dissolved in enthanol: arachis oil (1:10) and each chick received a single 10 µl subcutaneous injection 10 min. prior to testing on the pebble floor on day 14 posthatching. Controls received 10 µl of the vehicle only. The steroid treatments tested were 100 µg testosterone, 100 µg of 5alpha-dihydrotestosterone and 10 µg or 100 µg of oestradiol (the lower dose of oestradiol being chosen on the basis of reported relative potencies of these steroids). Males only were used in these experiments. All had been exposed to light during the last days of incubation. Performance on the pebble-floor task was tested as described above. In each group tested monocularly or binocularly there were 8 to 12 chicks.
Experiment 4: Effects of steroid hormones on development of the thalamofugal visual projections
On day 16 of incubation, eggs were removed from a dark incubator and injected with either testosterone (12.5 mg of testosterone oenanthate), oestradiol (1.5 or 2.5 mg oestradiol oenanthate), corticosterone (0.06 mg) or vehicle. The doses of these slow-release forms of testosterone and oestrogen were chosen on the basis of previous research on effective doses posthatching (Andrew 1975; Astiningsih & Rogers 1996). The dose of corticosterone was chosen to match previous studies of this steroid’s effect on memory (Sui et al. 1997). Note that the oenanthate forms of the steroids release the steroid slowly over a longer period of time than do the steroids used in experiment 2. In the case of testosterone the vehicle was arachis oil, of oestradiol it was 10% ethanol in olive oil and of corticosterone it was 1% ethanol in physiological saline. Matched controls received the appropriate vehicle only. There were 6 to 10 chicks in each group.
On day 16 of incubation, a small hole was drilled through the shell of the egg above the position of the embryo (determined by candling). The needle (30G) of a syringe was inserted perpendicularly to the shell surface into the egg, the solution injected and, after removal of the needle, the hole sealed by adhesive tape. Despite the proximity of the site of injection to the embryo, it is unlikely that the needle penetrated the embryo because the latter moves aside as the needle is inserted into the egg. After being injected, the eggs were then transferred to an incubator in which they were exposed to light continuously until hatching (see above).
The organisation of the visual projections from the thalamus to the visual Wulst regions in each hemisphere was determined by injection fluorescent tracer-dyes into the Wulst regions on day 2 posthatching, followed by counting the labelled cell bodies in the thalamus. All of the neurones projecting to the site of the injection are labelled by tracer because they take the tracer up through their end terminals and transport it back along the nerve cell axons to label the cell bodies.
On day 2 posthatching, the chicks were anaesthetised by intramuscular injection of 50 mg/Kg ketamine and 5 mg/Kg xylazine and then they were placed in a stereotaxic apparatus. Each chick received an injection of 0.3 µl of 4% solution of Fluorogold (FG, Fluorochrome Inc., Englewood, Colorado) into the visual Wulst on one side of the forebrain and 0.3 µl of 2% solution of Rhodamine B Isothiocyanate (RITC, Sigma R1755, St. Louis, Montana) into the visual Wulst on the other side of the forebrain, the side and tracer dye injected being randomised. FG was dissolved in sterile, pyrogen-free water and RITC was dissolved in 1% dimethyl sulfoxide (DMSO) made up in sterile, pyrogen free water. The injections were made using a 1 µl Hamilton glass microsyringe attached with a 26 gauge needle. To avoid leakage from the needle tip during advancement of the needle through the brain, the plunger was raised to withdraw the column of fluid by 0.1µl before inserting the needle into the brain. The tracers were injected slowly over a 2 minute period. Before withdrawing, the needle was kept in place for 10 minutes to minimise the diffusion of the tracer into the needle track.
After injection of the tracers, the chicks were allowed to survive for 4 days. Then each chick was injected with a lethal dose of sodium pentobarbitone and perfused transcardially with physiological saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brain was removed and post-fixed in the same fixative for up to 2 weeks before sectioning.
The brains were sectioned into 40 µm coronal sections using a freezing microtome and the sections were collected in 0.1 M phosphate buffer. All of the sections were mounted on gelatin-coated slides and dried in air. Alternate sections were placed on different slides, thus giving two series of sections. One series was coverslipped with Fluoromount and it was used to examine fluorescent labelling of the injection site and the neuronal cell bodies labelled by FG or RITC using a Nikon episcopic-fluorescence microscope (for details see Deng and Rogers 1998). The number of each kind of labelled neurone in the visual regions of the thalamus was counted. The other series of sections was stained with cresyl violet and used to determine the exact location of the labelled neurones and the injection site.
For each injection site, two scores for the number of labelled cells were obtained: the number labelled in the thalamus on the same side as the injection site (ipsilateral) and the number labelled on the side of the thalamus opposite to the injection site (contralateral). As it is impossible to control the exact amount of tracer injected each time (FG, in particular, tends to precipitate out of solution in the syringe), the ipsilateral and contralateral cell counts could not be compared directly. Instead, the ratio of the contralateral cell count to the ipsilateral cell count was calculated (c/i ratio) and used in comparisons. This procedure controls for variations in the amount of tracer injected (Adret & Rogers 1989; Rogers & Rajendra 1993).
As Schwabl (1993) found that testosterone levels in the egg yolk correlated with the social rank of the young canaries, based on competition and aggressive interactions, the attack and copulation responses of a cohort of 5 males and 5 females treated with 12.5 mg of testosterone in ovo and 8 control males and 5 control females were assessed from day 6 to 12 posthatching. The responses were scored using standard hand-thrust tests, originally designed by Andrew (1966) and used frequently to assess aggressive and sexual behaviour in young chicks (for details see Zappia & Rogers 1983). The responses of the chick to the human hand are ranked on a scale from 0 to 10. A maximal attack score involves, active sparring, pecking at the hand and attack leaping. A maximal copulation score involves mounting the flattened hand held close to the floor of the cage, crouching, grasping the hand with the beak, treading and pelvic thrusting.
RESULTS
Experiment 1: Light exposure of the embryo and lateralisation of pebble-floor performance
Figure 2 presents the mean scores for the number of pecks at pebbles made in the last 20 pecks of the pebble-floor task (pecks 41 to 60). The full set of results for the entire 60 pecks has been reported previously (Rogers 1997). Here it is noted that no effects of sex or incubation condition are present in the groups of chicks tested in the binocular condition. Chicks in all of the binocular groups were able to categorise grains as different from pebbles and adopt a strategy of pecking predominantly at grains. Significant effects of incubation condition and sex emerged when the chicks were tested using either the left eye (LE) or right eye (RE). Males exposed to light during incubation made few pecks at pebbles in the last 20 pecks of the task provided that the were using the RE: their performance did not differ from that of the binocular groups. Light-exposed males using the LE continued to peck almost at random throughout the task, making a mean of 12 pecks at pebbles in the last 20 pecks. (Note that, in this experiment, there were approximately four pebbles to every grain on the floor and therefore random pecking gave a mean of about 16 pecks at pebbles in a block of 20 pecks.)
There was a marked difference between the performance in the last 20 pecks of LE and RE males exposed to light (t-test, P<0.001). In other words, the male, light-exposed chicks exhibited lateralisation. This lateralisation was not present in males incubated in the dark: performance of both the LE and RE groups was identical and pecking close to random. Females were not lateralised in either the dark-incubated or light-exposed condition: dark- and light-females pecked almost at random and thus pecked many more pebbles than the binocular females (P<0.001 in each case). In summary, lateralised performance of this task occurs only in males exposed to light before hatching.
Experiment 2: Reversal of lateralisation of pebble-floor performance
Further evidence for the critical role of light in establishing lateralisation of monocular performance on the pebble floor was obtained from the experiment in which patches were applied to the left or right eye of the embryo during the last stages of incubation. At each age, the scores for the number of pecks at pebbles in the last 20 pecks at pebbles were analysed initially by ANOVA (sex x eye used in testing x eye exposed to light during incubation). There were no significant main effects but there were significant interactions between the eye exposed to light during incubation and the eye used in testing on day 3 (F1,18=16.4, p<0.0008), day 8 (F1,17=3.20, P=0.09) and day 12 (F1,17=12.8, P=0.002). These results are presented in Figure 3; the sexes were not separated because there was no main effect of sex and no interaction between sex and any other factor tested by the ANOVA.
As can be seen in Figure 3, applying the patch to the right eye reversed the direction of lateralisation that normally occurs (cf. Fig. 2). Full details of these results will be published elsewhere (Rogers et al., unpublished). Here it is noted that at all ages tested, apart from day 8, chicks that had received the abnormal condition of exposure of the LE to light (RE patched) prior to hatching shifted to pecking predominantly at grain when they used the LE but not when they used the RE (t-tests of LE versus RE, P<0.001 on all days except day 8). The opposite direction of lateralisation occurred in the chicks that had had the left eye patched (mimicking the natural condition). The latter shifted to peck predominantly at grain in the last 20 pecks of the task provided that they were using the RE and not the LE, this occurring at all ages except day 5 (as before P<0.001 for LE versus RE comparisons, except on day 5). Despite the aberrant days 5 and 8, following embryonic exposure of the RE to light, LE performance at all ages tested involved failure to shift away from pebbles to peck at grain and, following embryonic exposure of the LE to light, the RE performance showed clear shifting to pecking predominantly at grain at all ages tested.
Note that, in this experiment and the one to follow, there were slightly more grains scattered on the floor than in experiment 1 and therefore the mean scores for the groups that were not successful in shifting to peck at grain are lower.
Experiment 3: Acute effects of steroid hormones on pebble-floor performance
All of the steroid hormones administered had marked effects on the lateralisation of performance revealed in the pebble-floor task (Fig. 4). The data for all but the high dose of oestradiol were analysed by 2-way ANOVA (treatment by eye system), the high dose being analysed separately because it was administered to a different batch of chicks. The ANOVA revealed a significant main effect of eye system (F2,151=15.6, P<0.0001) and a significant interaction between eye system and treatment (F6,151=15.5, P<0.0001). Only one set of control data are presented in Figure 4 but the control group matching the high dose of oestradiol gave similar results; viz., high numbers of pecks at pebbles in chicks tested using the LE and low numbers of pecks at pebbles in those tested using the RE. It will be recalled that all of these chicks were male and that they had received exposure to light before hatching. These control results match those of light-exposed males in Figure 2. Both testosterone and 5alpha-dihydrotestosterone reversed the direction of lateralisation, pecks at pebbles being high in groups using the RE and low in those using the LE. The effect of testosterone was further confirmed by repeating this treatment two times and the same results was obtained with each repeat. Treatment with the lower (10 µg) of oestradiol had no significant effect; as in the control group, LE had high scores for pecking at pebbles and RE had low scores. Increasing the dose of oestradiol to 100 µg lowered the error score in the LE group but it was still significantly above the binocular and RE groups. In addition, the higher dose of oestradiol had no effect on the performance of the RE group. Thus, testosterone and 5alpha-dihydrotestosterone reverse the direction of lateralisation but oestradiol has no such effect.
Experiment 4: Effect steroid hormones on development of the thalamofugal visual projections
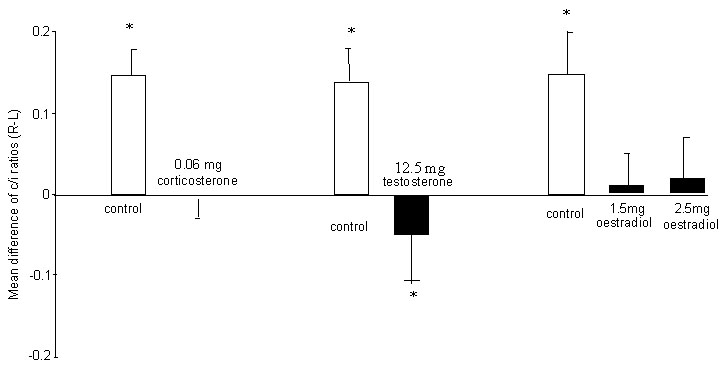
The c/i ratios for individual chicks treated with corticosterone, or its vehicle (controls), are presented in Figure 5. They were analysed first by ANOVA (treatment x side of injection of tracer): treatment of saline versus corticosterone, F1,28=3.35, P=0.078; side of injection of tracer, F1,28=11.52, P=0.002; interaction between treatment and side of injection, F1,28=12.73, P=0.003.
It will be noted that there is significant lateralisation in the control group, the values for injections of the right Wulst being higher than those for injections in the left Wulst (paired t-test, t=-4.05, df=5, P=0.010). There was no lateralisation in the group treated with corticosterone (paired t-test, t=0.15, df=9, P=0.89). While the corticosterone treatment caused some individuals to have a lesser degree of lateralisation, others had no lateralisation or, particularly in the case of one individual, reversed lateralisation, the net effect being absence of lateralisation at a group level. Comparison between the control and the treated groups revealed no significant difference between treated and control scores following injection of the left Wulst (unpaired t-test, t=1.21, df=14, P=0.25) but a significant difference between the treated and control scores following injection of the right Wulst (unpaired t-test, t=-3.87, df=14, P=0.007). Therefore, the effect of corticosterone appears to be limited to the projections to the right Wulst.
The results for chicks treated as embryos with testosterone and oestradiol have been reported in detail previously (Schwarz & Rogers 1992; Rogers & Rajendra 1993). Figure 6 presents these in summarised form. Note that both of these hormones, like corticosterone, prevent the development of lateralisation in the thalamofugal projections to the visual Wulst regions in each hemisphere. There was a marginal reversal of the lateralisation following testosterone treatment and no lateralisation following oestradiol treatment.
The attack responses of male chicks treated with testosterone in ovo were elevated above control levels from day 9 posthatching to day 12. The mean attack score for treated males from day 9 to 12 was 6.5 + 1.0 (S.E.) compared to a mean of 2.9 + 0.7 for controls (1-tailed U-test, P<0.01). Copulation scores were elevated also: mean of 4.6 + 1.0 for chicks treated in ovo compared to 0.8 + 0.7 for controls (1-tailed U-test, P<0.01). In treated females there was no elevation of attack responses and only a minor elevation of copulation responses.
DISCUSSION
The results reported here demonstrate that light exposure before hatching and also the levels of circulating steroid hormones before and after hatching influence lateralisation in the chick. These particular factors are not likely to have effects on all forms of lateralisation but they have definite and differing effects on performance of the pebble-floor task and lateralisation of the visual projections from the thalamus to the forebrain.
Lateralisation determined by light exposure of the embryo
The first experiment demonstrates that the LE versus RE differences in performance by male chicks on the pebble floor are determined by light exposure of the embryo during the stage of development at which visual connections to the forebrain become functional. Male chicks hatched from eggs exposed to light for 24 hours on day E17 make very few pecks at pebbles in the last 20 pecks of the task provided that they are using the RE, but not if they are using the LE. Thus light exposure of the embryo has a delayed effect on lateralised performance on the pebble floor after hatching.
Performance of the pebble-floor task is known to depend on inputs to the forebrain via the thalamofugal visual projections, which relay information to the visual Wulst. As discussed in the Introduction, administration of glutamate to the left visual Wulst, not the right visual Wulst or other visual regions in the forebrain, impairs performance of the pebble- floor task (Deng & Rogers 1997). It is known that the glutamate treatment promotes growth of extra projections from the right side of the thalamus (fed by the left, occluded eye) to the left visual Wulst and thereby removes the normal asymmetry in the thalamofugal projections (Khyentse & Rogers 1997). Thus, symmetry of the visual projections correlates with impaired performance of chicks tested binocularly on the pebble-floor task (i.e. more pecking at pebbles in the last 20 pecks). Incubation in the dark also leads to the development of symmetry in the thalamofugal visual projections but, in this case, the symmetry is caused by the development of fewer projections from the left side of the thalamus to the right visual Wulst (Rogers & Bolden 1991) and, as shown in experiment 1, it does not cause impaired binocular performance on the pebble floor. Thus it would appear to be the exuberant growth of visual projections to the left Wulst, caused by glutamate, that impairs binocular performance possibly because the extra connections interfere with normal processing of inputs.
The first experiment also revealed a sex difference: females tested on the pebble floor on day 8 posthatching, unlike males, did not show lateralisation. Irrespective of whether they were using the LE or RE, females pecked almost at random. This result is, largely, consistent with the lesser degree of asymmetry in the thalamofugal visual projections of females compared to males (Rajendra & Rogers 1993). It also confirms an earlier report of a similar sex difference in chicks tested in the second week of life (Zappia & Rogers 1987) but it remains possible that the sex difference is age-dependent because no overall effect of sex was found in experiment 2, which investigated reversal of the lateralisation by exposing the embryo’s left eye to light followed by testing at various ages posthatching.
Despite the sex difference in the data reported in experiment 1, and despite almost random pecking by both LE and RE females and males incubated in the dark, binocular performance permitted the chicks to peck predominantly at grain. Neither sex or incubation condition affected binocular performance. This suggests that the inability of RE females and RE-dark incubated males to peck predominantly at grain may be due to interference from regions of the forebrain that receive input from the non-seeing eye, an effect suggested for other behaviours by Andrew (1991). The same may be the case in dark-incubated males using the right eye, although further experimentation will be needed to determine the exact mechanism(s) involved.
The considerable influence of light on lateralisation of performance on the pebble-floor task was demonstrated further by the reversal of lateralisation by occluding the embryo’s right eye and exposing the left eye to light. After occluding the RE and exposing the LE of the embryo to light, chicks tested on the pebble-floor task using the LE peck mainly at grain at all ages on which they were tested (days 3, 5, 8 or 12 posthatching), while chicks tested using the RE deliver high numbers of pecks at pebbles. In other words the direction of the lateralisation is reversed compared to the normal situation and compared to their matched controls in which the embryo’s head was withdrawn from the eggs and the LE was occluded. There were, however, two ages on which the results differed from this overall pattern: day 8 for the chicks that had received light stimulation of the LE and day 5 for those that had received light stimulation of the RE. On these days there was no lateralisation of performance, probably as a consequence of the known shifts in hemispheric dominance during development.
Importance of the lateralisation determined by light exposure of the embryo
Previous studies have shown that light exposure before hatching affects lateralisation of sexual and aggressive responses in young chicks in the same way as it does for pebble-floor performance (Rogers 1982; Zappia & Rogers 1983; Rogers 1991, 1995). Since all of these behaviours are important for survival, we may deduce that the conditions in which the egg is incubated must be rather critical for normal development. In a natural setting it is known that the hen responds to the vocalisations made by the chick embryos just prior to hatching and her response is to stand up when the embryo makes distress calls and to turn the eggs (Tuculescu and Griswold 1983). Such embryo-hen interactions occur just prior to hatching during the sensitive period for neural and behavioural development of the visual system. Their potential effects on the embryo persist into early posthatching life and possibly even into adulthood.
Light exposure of the embryo does not determine the presence of all forms of lateralisation in the chick. In fact, the influence of light on visual lateralisation in the chick might be limited to visual behaviours that rely predominantly on the thalamofugal visual system, as this is the pathway in which lateralisation is determined by light exposure of the embryo. The other visual pathway, the tectofugal pathway, of the chick has only a minor degree of lateralisation, if any at all, and it is not influenced by light-exposure prior to hatching (Rogers & Deng 1998). Thus, in the chick, visual behaviours that rely on the tectofugal system are unlikely to be lateralised (e.g. detection of moving stimuli, visual acuity and pattern discrimination).
From the now extensive information on lateralised behaviour in the chick, we can deduce that lateralisation occurs also at higher levels of visual processing and integration, not just at the level of visual input to the forebrain. These forms of lateralisation may be less dependent on light exposure of the embryo, if they are influenced by it at all. In fact, chicks using the left eye display superior ability to make decisions to approach familiar versus unfamiliar stimuli (Vallortigara & Andrew 1991) and recent evidence shows that this form of lateralisation is not influenced by light exposure of the embryo (Andrew et al., unpublished). Lateralisation of responding to sensory inputs other than visual is also unaffected by light exposure of the embryo. For example, lateralisation of olfactory responses, demonstrated by greater responsiveness to odours by chicks using the right nostril than the left, is not influenced by light exposure (Rogers et al. 1998) and nor is lateralisation of auditory responding (Rogers, unpublished data). This does not detract, however, from the important role of light in establishing the lateralisation of the behaviours of feeding, sex and aggression. Contrary to the statement by McKenzie et al. (1998) that light exposure "is not necessary to produce the usual asymmetries in visually evoked behaviour", the lateralised behaviours affected by light exposure of the embryo are not only "usual" but also critical for survival.
In contrast to the chick, the pigeon has lateralisation of the tectofugal system, also determined by light exposure of the embryo (Güntürkün 1993). We might deduce that pigeons will show lateralisation of behaviours that are not lateralised in the young chick. The organisation of the tectofugal visual system of the pigeon also differs from that of the chick in other ways (Deng & Rogers 1998) and this too indicates that there will be species differences in laterality. Güntürkün et al. (1989) have reported some evidence suggesting that the thalamofugal system of the pigeon transmits information only from the lateral visual field and that this system is "blind" in the frontal field. This is not the case in the chick: the thalamofugal visual system receives input from the frontal as well as the lateral fields (Deng & Rogers 1998). Another major difference between these two species is that the entire optic tectum of the chick projects to the nucleus rotundus (tectofugal system), whereas only the ventral optic tectum does so in the pigeon (Hellman & Güntürkün 1996). These species differences caution against unqualified extrapolation of details of lateralised performance across species but, nevertheless, light exposure plays equally important roles in establishing lateralisation in both species and is likely to do so in other avian species.
Acute effects of steroid hormones on lateralisation
The effect of the steroid hormones on the lateralised aspects of these behaviours must now be discussed. The results of experiment 2 show that acute elevation of the circulating levels of testosterone or its 5alpha reduced metabolite, 5alpha-dihydrotestosterone, causes a rapid reversal in the lateralisation of pebble-floor performance, although oestradiol has no such effect. The higher dose of oestradiol did reduce pebble pecking by LE chicks but it did not reverse the lateralisation. Therefore there is specificity in the action of steroid hormones on lateralisation of this task. Although many of testosterone’s central effects require its metabolism to oestrogen, in this case it appears that the 5alpha-reductase pathway to 5alpha-dihydrotestosterone may be more important. The latter is widely distributed in birds (Schlinger 1997).
The rapidity of the effect of the androgenic steroid hormones indicates that this is not developmental but rather a temporary shift in the interaction between the hemispheres. It also shows that both the LE and RE systems can direct pecking away from pebbles if they are called upon to do so but, in any context, this role is adopted by one eye system and not the other. It is possible that the strategies used by the LE and the RE to direct pecking away from pebbles differ although they have the same overall effect on behaviour. Chicks using the left eye might be attending to the detailed features of the food and pebbles, while those using the RE may attending to a more limited range of features (or even a single feature) that distinguish(es) grain from pebbles. Either strategy may be lead to avoidance of pecking pebbles but the memories formed would differ and may have different outcomes on behaviour in other contexts. It is important to note that the results reported here show that, once one eye system assumes dominance for this task, use of the other eye system is unable to shift pecking away from random. The steroid hormones modulate this interaction between the eye systems/hemispheres and may therefore switch the performance of the chick from one strategy to another.
Taken together, these data suggest that there are two ways in which lateralisation revealed by the pebble-floor task is malleable. The first is an effect on development involving light experience prehatching. The second is a direct activational effect on the balance between the hemispheres involving androgenic steroid hormones acting posthatching. This affect of androgens on lateralisation is acute and it must be able to override the functional lateralisation predetermined by light exposure of the embryo, at least temporarily. We might consider that there is a baseline lateralisation of structure and function determined by light exposure of the embryo and that androgens superimpose their effects on this. It is not yet known how circulating androgenic steroids might act in chicks hatched from eggs incubated in darkness and therefore with no lateralisation of the visual projections and no baseline lateralisation of performance of the pebble-floor task. However, in chicks that have been exposed to light before hatching fluctuating levels of androgenic steroids posthatching may underlie at least some of the shifts in hemispheric dominance that occur during the first two weeks posthatching. Changing androgen levels might therefore underlie key phases in posthatching behavioural development.
Developmental effects of steroids on lateralisation of the visual projections
In addition to their acute affects on lateralisation, the androgenic steroids, as well as oestradiol and corticosterone, have developmental effects on the development of lateralisation in the thalamofugal visual projections. When administered to the embryo so that they act during the period when light exposure determines visual lateralisation, they prevent the development of that lateralisation. It is possible that the mechanisms by which the various steroid prevent the development of asymmetry in the thalamofugal visual projections may vary. Previous evidence suggests that oestradiol removes the asymmetry by promoting growth of the projections from both sides of the thalamus and thereby overriding any effects of light stimulation of the RE only (Rogers & Rajendra 1993). The data reported here for corticosterone indicate that this hormone prevents the development of lateralisation by suppressing the light-induced growth of projections for the left side of the thalamus to the right Wulst because corticosterone has a significant effect on the c/i ratios obtained by injecting tracer into the right Wulst but not the left. Thus raised corticosterone levels may suppress light-dependent growth of the projections or decrease the survival of neurones (Gould et al. 1991).
The effects of these steroid hormones on the development of the visual projections are likely to have long-lasting effects on processing of visual information and behaviour. The details of their effects have yet to be tested behaviourally. Here the attack and copulation responses of male chicks treated with testosterone in ovo were found to be elevated, but this was not the case in females. The posthatching effects on aggression in male chicks are consistent with Schwabl’s (1993) finding a correlation between testosterone levels in the egg and social aggression but the absence of effect in female chicks suggests that genetic sex plays a role in the chick, if not in the canary. There is also the possibility that the long-acting steroids injected in these experiments are present in the chick's circulation after hatching and that the elevation of attack and copulation in males has nothing to do with the effect of testosterone on lateralisation. It is recalled, however, that attack and copulation responses in the chick are lateralised (Rogers et al. 1985; Bullock & Rogers 1992).
Lateralisation and development in the natural environment
These somewhat disparate, yet interactive, ways in which it has been possible to influence the expression of lateralisation show that (1) development of the chick brain is influenced by both experience and hormone levels, (2) modulation of brain lateralisation is a mechanism by which these factors have long-lasting consequences on behaviour, and (3) acute hormonal influences can override, at least temporarily, the lateral bias determined during embryonic development. They also demonstrate that behaviour after hatching is influenced by the internal and external environments of the embryo.
The intensity of light necessary to establish lateralisation is likely to occur in most conditions of natural incubation. In the laboratory intensities as low as 100 to 200 lux have been found to be sufficient to establish lateralisation and the effective period of exposure can be as brief as 2 hours (Rogers 1982). Given that the required 2 hours might be achieved cumulatively from even shorter periods during which the hen leaves the nest, these conditions would be met by most natural environments in which birds incubate eggs, apart from in species that nest in caves or, as in the megapodes of Australia, which incubate their eggs by burying them in mounds of earth and rotting material. It is of interest to note that embryos of the latter species are the only ones that do not turn their heads against one side of the body during the final stages of incubation. Apart from these unusual species, as far as known, the embryos of all avian species turn their heads so that they occlude the left eye. Given that it has been found that light exposure of the pigeon embryo, an altricial species, establishes visual lateralisation (Güntürkün 1993), as it does in the precocial chick, it would appear that the role of light in establishing visual lateralisation is wide spread in avian species. It is, however, possible that environments of very high light intensity mask or inhibit the development of these lateralisations because, at such high intensities, sufficient light may reach the retina of the left eye by passing through the retina and/or cranium and thereby entering through the back of the eye. In such cases, growth of the visual projections from both sides of the thalamus may be promoted and no lateralisation would be present. The colour and structure of the egg shell would add another variable. The shell and membranes of domestic hen eggs transmit roughly 10 percent of ambient light intensity but there have been no measurements of transmission by the egg shells of other species. The wavelength of light transmitted would vary between species also but this is less likely to be important because there is little specificity in the effectiveness of different wavelengths in establishing lateralisation in the chick (Rogers & Krebs 1996).
Steroid hormone levels in the egg and embryo modulate the effectiveness of light. As shown here, abnormally high levels prevent the development of lateralisation in the visual projections of the chick. One assumes that this would be a graded effect dependent on the level of circulating steroid hormones during the sensitive period when light exposure establishes lateralisation. As far as we know, in male embryos developing normally, there is a trough of testosterone levels just at the stage when light has its effect (Rogers 1995). This might be permissive of the lateralising effect of light. Sex differences in the level of the steroid hormones circulating in the embryo might determine the known sex difference in the degree of structural and functional lateralisation after hatching. In addition, variations in steroid hormone levels between individuals, determined partly by in ovo deposits from the mother (Schwabl 1993), may lead to individual differences in the degree of lateralisation.
Corticosterone levels in the embryo also have an important influence on the degree of light-dependent lateralisation. These might be modulated by stress experienced by the embryo itself (e.g. heat or cold stress, or even some forms of toxic chemical stress) and also, as in the case of testosterone, by levels circulating in the hen at the time the egg is being formed. Stress experienced by the hen at the time the egg is forming might carry over to the environment of the egg and thus influence visual pathway development and lateralisation of the embryo. We now need to pay more attention to the possible effects of environmental and social stress on avian embryo development and its potential long-lasting consequences. Even though many aspects of lateralisation may remain unaffected by light exposure of the eggs and/or the hormonal condition of the embryo, these factors do modulate important forms of lateralisation evident in feeding behaviour, as well as aggressive and sexual behaviour.
ACKNOWLEDGEMENTS
The author is grateful for research assistance from G. Krebs, A.N.B. Johnston, J.V. Zappia and C. Deng, who collected the data presented in each of the experiments in the order listed. The research was funded by an Australian Research Council grant to L.J. Rogers.
REFERENCES
Adret, P. & Rogers, L.J. 1989. Sex difference in the visual projections of young chicks: A quantitative study of the thalamofugal pathway. Brain Research 478: 59-73.
Andrew, R.J. 1966. Precocious adult behaviour in the young chick. Animal Behaviour 14: 485-500.
Andrew, R.J. 1975. Effects of testosterone on the behaviour of the domestic chick. II. Effects present in both sexes. Animal Behaviour 23: 156-168.
Andrew, R.J. 1988. The development of visual lateralization in the domestic chick. Behavioural Brain Research 29: 201-209.
Andrew, R.J. 1991. The nature of behavioural lateralization. In: R.J. Andrew (ed.) Neural and behavioural plasticity: The use of the domestic chick as a model. Oxford; Oxford University Press: 536-554.
Andrew, R.J. 1996. Brief retention deficits associated with cyclically recurring left hemisphere events. Physiology & Behavior 60: 1323-1329.
Andrew, R.J. 1997. Left and right hemisphere memory traces: Their formation and fate. Evidence from events during memory formation in the chick. Laterality 2: 179-198.
Andrew, R.J. & Brennan, A. 1983. Sex differences in lateralization in the domestic chick. Neuropsychologia 22: 503-509.
Astiningsih, K. & Rogers, L.J. 1996. Sensitivity to testosterone varies with strain, sex, and site of injection in chickens. Physiology & Behavior 59: 1085-1091.
Bateson, P.P.G. 1990. Is imprinting such a special case? Philosophical Transactions of the Royal Society of London 329: 125-131.
Biegon, A., Rainbow, T.C. & McEwen, B.S. 1985. Corticosterone modulation of neurotransmitter receptors in rat hippocampus: a quantitative autoradiographic study. Brain Research 332: 309-314.
Bolhuis, J.J. 1991. Mechanisms of avian imprinting; a review. Biological Reviews 66: 303-345.
Bullock, S.P. & Rogers, L.J. 1992. Hemispheric specialization for the control of copulation in the young chick and effects of 5alpha-dihydrotesosterone and 17ß-oestradiol. Behavioural Brain Research 48: 9-14.
Cipolla-Neto, J., Horn, G. & McCabe, B.J. 1982. Hemispheric asymmetry and imprinting: the effect of sequential lesions of the hyperstriatum ventrale. Experimental Brain Research 48: 22-27.
Dharmaretnam, M. & Andrew, R.J. 1994. Age- and stimulus-specific use of right and left eyes by the domestic chick. Animal Behaviour 48: 1395-1406.
Deng, C. & Rogers, L.J. 1997. Differential contributions of the two visual pathways to functional lateralization in chicks. Behavioural Brain Research 87: 173-182.
Deng, C. & Rogers, L.J. 1998. Organisation of the tectorotundal and SP/IPS-rotundal projections in the chick. The Journal of Comparative Neurology 394: 171-185.
Doupe, A.J. & Patterson, P.H. 1982. Glucocorticoids and the developing nervous system. In Ganten, D. & Pfaff, D. (eds) Adrenal actions on the brain. New York, Springer: 23-43.
Evans, C.S, Evans, L. & Marler, P. 1993. On the meaning of alarm calls: functional references in an avian vocal system. Animal Behaviour 46: 23-28.
García-Segura, Chowen, J.A., Párducz, A. & Naftolin, F. 1994. Gonadal hormones as promoters of structural synaptic plasticity: Cellular mechanisms. Progress in Neurobiology 44: 279-307.
Gottlieb, G. 1968. Prenatal behavior in birds. Quarterly Review of Biology 43: 148-174.
Gould, E., Woolley, C.S., Cameron, H.A., Daniels, D.C. & McEwen, B.S. 1991. Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell death. Journal of Comparative Neurology 313: 486-493.
Güntürkün, O. 1993. The ontogeny of visual lateralization in pigeons. German Journal of Psychology 17: 276-287.
Güntürkün, O., Emmerton, J. & Delius, J.D. 1989. Neural asymmetries and visual behavior in birds. In: Lüttgau, H.C. & Necker, R. (eds) Biological signal processing. Weinheim, FRG Deutsche Forschungsgemeinschaft, VCH Verlagsgesellschaft: 122-145.
Hale, C. & Green, L. 1988. Effects of early ingestional experiences on the acquisition of appropriate food selection by young chicks. Animal Behaviour 102: 211-224.
Hellmann, B & Güntürkün, O. 1996. Diversity of the tectofugal connections in the pigeon: A reinvestigation of the tecto-rotundal projection. Abstracts of the Avian Brain and Behaviour meeting. Tihany, Hungary: Hungarian Academy of the Sciences.
Hogan, J. 1973. The development of food recognition in young chicks: I. Maturation and nutrition. Journal of Comparative and Physiological Psychology 83: 355-366.
Howard, K.J., Rogers, L.J. & Boura, A.L.A. 1980. Functional lateralization of the chicken forebrain revealed by use of intracranial glutamate. Brain Research 188: 369-382.
Johnston, A.N., Johnston, G.A.R. & Rogers, L.J. 1993. Glutamate and imprinting memory: The role of glutamate receptors in encoding imprinting memory. Behavioural Brain Research 54: 137-143.
Khyentse, M. D. & Rogers, L.J. 1997. Glutamate affects the development of the thalamofugal visual projection of the chick. Neuroscience Letters 230: 65-68.
McEwen, B.S. & Sapolsky, R.M. 1995. Stress and cognitive function. Current Opinion in Neurobiology 5: 205-216.
McKenzie, R., Andrew, R.J. & Jones, R.B. 1998. Lateralization in chicks and hens: new evidence for control of response by the right eye system. Neuropsychologia 36: 51-58.
Mench, J. & Andrew, R.J. 1986. Lateralization of a food search task in the domestic chick. Behavioral and Neural Biology 46: 107-114.
Meyer, J.S. 1985. Biochemical effects of corticosterone on neural tissue. Physiological Reviews. 65: 946-1020.
Parsons, C.H. & Rogers, L.J. 1997. Pharmacological extension of the sensitive period for imprinting in Gallus domesticus. Physiology and Behavior 62: 1303-1310.
Patterson, T.A. & Rose, S.P.R. 1992. Memory in the chick: Multiple cues, distinct brain locations. Behavioural Neuroscience 106: 465-470.
Rajendra, S. & Rogers, L.J. 1993. Asymmetry is present in the thalamofugal projections of female chicks. Experimental Brain Research 92: 542-544
Rashid, N. & Andrew, R.J. 1989. Right hemisphere advantage for topographical orientation in the domestic chick. Neuropsychologia 27: 937-948
Rogers, L.J. 1982. Light experience and asymmetry of brain function in chickens. Nature, 297, 223-225.
Rogers, L.J. 1990. Light input and the reversal of functional lateralization in the chicken brain. Behavioural Brain Research 38: 211-221.
Rogers, L.J. 1991. Development of lateralization. In: R.J. Andrew, R.J. (3ed) Neural and Behavioural Plasticity: The Use of the Domestic Chick as a Model. Oxford; Oxford University Press: 507-535.
Rogers, L.J. 1995. The development of brain and behaviour in the chicken. Oxon: CAB International: 273pp.
Rogers, L.J. 1996. Behavioral, structural and neurochemical asymmetries in the avian brain: A model system for studying visual development and processing. Neuroscience and Biobehavioral Reviews 20: 487-503.
Rogers, L.J. 1997. Early experiential effects on laterality: Research on chicks has relevance to other species. Laterality 2, 199-219.
Rogers, L.J. & Bolden, S.W. 1991. Light-dependent development and asymmetry of visual projections. Neurosicience Letters 121: 63-67.
Rogers, L.J. & Deng, C. 1998. Light experience and lateralisation of the two visual pathways in the chick. Behavioural Brain Research, in press.
Rogers, L.J. & Krebs, G.A. 1996. Exposure to different wavelengths of light and the development of structural and functional asymmetries in the chicken. Behavioural Brain Research 80: 65-73.
Rogers, L.J. & Rajendra, S. 1993. Modulation of the development of light-induced asymmetry in chick thalamofugal visual projections by estradiol. Experimental Brain Research 93: 89-94.
Rogers, L.J. & Sink, H.S. 1988. Transient asymmetry in the projections of the rostral thalamus to the visual hyperstriatum of the chicken, and reversal of its direction by light exposure. Experimental Brain Research 70: 378-384.
Rogers, L.J., Andrew, R.J. & Burne, T.H.J. 1998. Light exposure of the embryo and development of behavioural lateralisation in chicks: I. Olfactory responses. Behavioural Brain Research: 97: 195-200.
Rogers, L.J., Drennen, H.D. & Mark, R.F. 1974. Inhibition of memory formation in the imprinting period: irreversible action of cycloheximide in young chickens. Brain Research 79: 213-253.
Rogers, L.J., Zappia, J.V. & Bullock, S.P. 1985. Testosterone and eye-brain asymmetry for copulation in chickens. Experientia 1, 1447-1449.
Rose, S.P.R. 1991. How chicks make memories, the cellular cascade from c-fos to dendritic modelling. Trends in Neurosciences 14: 390-397.
Sandi, C. & Rose S.P.R. 1994. Corticosterone enhances long-term retention in one-day-old chicks trained in a weak passive avoidance paradigm. Brain Research 647: 106-112.
Sandi, C., Rose S.P.R., Mileusnic, R. & Lancashire, C. 1995. Corticosterone facilitates long-term memory formation via enhanced glyocoprotein synthesis. Neuroscience 69: 1087-1093.
Salpolsky, R.M., Uno, H, Rebert, C.S. & Finch, C. 1990. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. Journal of Neuroscience 10: 2897-2902.
Sarrieau, A., Sharma, S. & Meaney, M.J. 1988. Postnatal development and environmental regulation of hippocampal glucocorticoid and mineralcorticoid receptors. Developmental Brain Research 43: 158-162.
Schlinger, B.A. 1997. Sex steroids and their actions on the birdsong system. Journal of Neurobiology 33: 619-631.
Schwabl, H. 1993. Yolk is a source of maternal testosterone in developing birds. Proceedings of the National Academy of the Sciences USA 90: 1446-11450.
Schwarz, I.M. & Rogers, L.J. 1992. Testosterone: A role in the development of brain asymmetry in the chick. Neuroscience Letters 146: 167-170.
Sui, N., Sandi, C. & Rose S.P.R. 1997. Interactions of corticosterone and embryonic light deprivation on memory retention in day-old chicks. Developmental Brain Research 101: 269-272.
Tuculescu, R.A. & Griswold, J.G. 1983. Prehatching interactions in domestic chicks. Animal Behaviour 31: 1-10.
Vallortigara, G. & Andrew, R.J. 1991. Lateralization of response by chicks to change in a model partner. Animal Behaviour 41, 187-194.
Vallortigara, G., Andrew, R.J., Sertori, L. & Regolin, L. 1997. Sharply timed behavioral changes during the first 5 weeks of life in the domestic chick (Gallus gallus). Bird Behavior 12: 29-40.
Workman, L. & Andrew, R.J. 1989. Simultaneous changes in behaviour and lateralization during the development of male and female domestic chicks. Animal Behaviour 38: 596-605.
Zappia, J.V. & Rogers, L.J. 1983. Light experience during development affects asymmetry of forebrain function in chickens. Developmental Brain Research 11: 93-106
Zappia, J.V. & Rogers, L.J. 1987 Sex differences and reversal of brain asymmetry by testosterone in chickens. Behavioural Brain Research 23: 261-267.
Fig. 1. Diagrammatic representation of the thalamofugal visual system. Note that projections from each eye cross over completely and terminate in the side of the thalamus opposite to the eye from which they arise. Projections from each side of the thalamus go to the visual Wulst (also known as they visual hyperstriatum) regions in each hemisphere of the forebrain. There are projections to both the ipsilateral and contralateral visual Wulst from each side of the thalamus. Asymmetry is located in the contralateral projections, as shown. (See Deng & Rogers (1998) for more detail on the structure of the visual regions of the thalamus of the chick.)

Fig. 2. Experiment 1: Effect of light exposure of the embryo on lateralisation of performance on the pebble floor task. The chicks have been tested on day 8 posthatching with a choice of grains and small pebbles, the latter adhered to the floor. The mean number of pecks at pebbles in the last 20 pecks (pecks 41 to 60) of the task (with standard error score indicated) has been plotted for groups of male and female chicks incubated with exposure to light for the last 4 or 5 days before hatching or incubated in the dark during the same period. BI (grey bars) indicates the groups tested binocularly, LE (black bars) chicks tested using the left eye only and RE (white bars) chicks tested using the right eye only. Asterisks indicate that the scores are significantly different from the control, BI group (p<0.001). Note the lateralisation in males exposed to light (LE scores differ from RE scores) but not in males incubated in the dark. Lateralisation is not evident in the females, irrespective of incubation conditions.
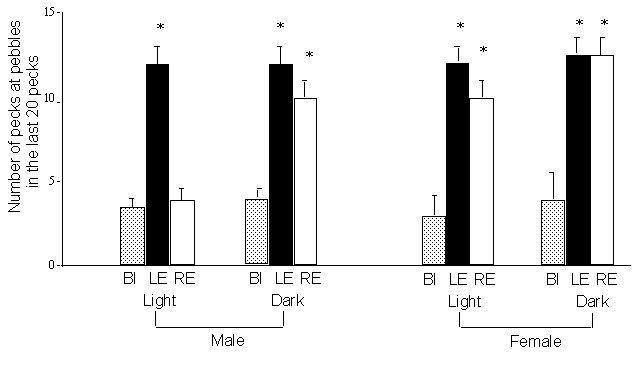
Fig. 3. Experiment 2: Reversal of lateralisation by exposure of the embryo’s left eye to light. The embryo’s head was withdrawn from the egg on day E19/20 and a patch applied to the left or right eye for 24 hours. The data presented are for performance on the pebble-floor task at various ages posthatching (each chick being tested at only one age). The presentation is as in Figure 2. Black bars represent chicks tested using the LE. White bars represent chicks tested using the RE. Asterisks indicate the presence of lateralisation. A, results for chicks which had the LE exposed to light before hatching (right eye occluded). B, chicks which had the RE exposed to light before hatching. Scores for males and females have not been separated as there was no significant effect of sex in this experiment. N= 10 to 11 per group.
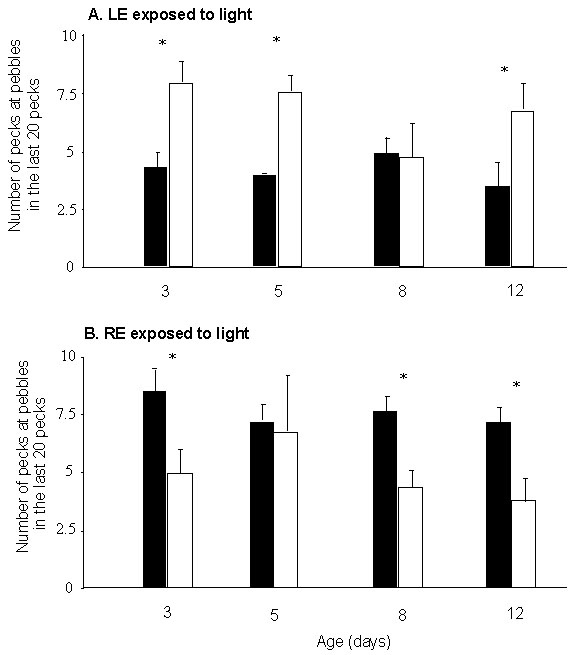
Fig. 4. Experiment 3: Acute effects of steroid hormones on lateralisation of performance on the pebble floor. The data are presented as in Figure 2. The steroid hormones were administered subcutaneously to male chicks 10 min. before testing. Note that both testosterone and 5alpha- dihydrotestosterone reverse the direction of the lateralisation. The low dose of oestradiol has no effect on the normal lateralisation and, although the high dose appears to have some effect, the chicks using the LE still direct more of their pecks at pebbles than the BI group.
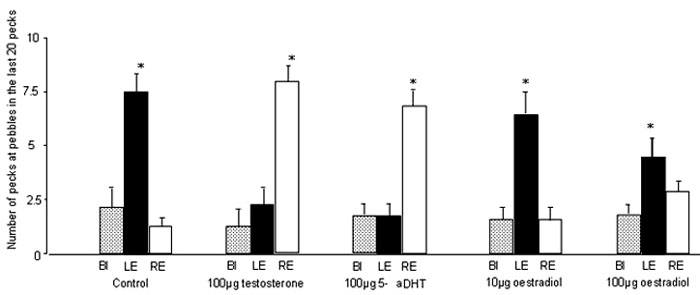
Fig. 5. Experiment 4: Organisation of the thalamofugal visual projections in chicks exposed to light before hatching. The c/i ratio is calculated for each site of injection of tracer into the visual Wulst by dividing the number of labelled cells in the contralateral side of the thalamus by the number of labelled cells in the ipsilateral side of the thalamus. ‘Left’ and ‘Right’ on the X-axis indicates the side of the Wulst into which the tracer is injected. Each pair of connected dots represents the c/i ratios (with respect to tracer injection into the left and right Wulst) for one chick. The arrows indicate the means. Note that all of the controls have a lower c/i ratio following injection of tracer into the left Wulst than they do following injection into the right Wulst. This asymmetry in controls matches that represented in Figure 1. Corticosterone treatment removes this asymmetry.
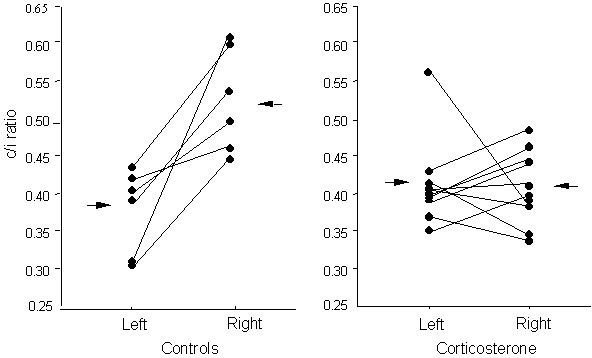
Fig. 6. Summary of the effects of steroid hormones on the asymmetry of the visual projections. The mean difference between the c/i ratios (right minus left) has been plotted. Asterisks indicate significant divergence from zero. For all control groups the scores are biased to the right side, meaning that the c/i ratio is higher following injection of the right Wulst than the left. Corticosterone and oestrogen remove the bias and testosterone marginally reverses it.