S46.1: Development of motor patterns in avian embryos: Control of hatching behaviour
Anne Bekoff
Department of Environmental, Population and Organismic Biology and Center for Neuroscience, University of Colorado, Boulder, Colorado, 80309-0334, USA, fax 1 303 492 8699, e-mail anne.bekoff@colorado.edu
Bekoff, A. 1999. Development of motor patterns in avian embryos: Control of hatching behaviour. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2776-2783. Johannesburg: BirdLife South Africa.One of the most striking features of motor behaviour in avian embryos is the clear discontinuity between early embryonic movements, which Hamburger called Type I embryonic motility, and later appearing embryonic behaviours such as hatching or postnatal walking. Work on embryonic motor behaviours, which has been primarily carried out in chick embryos, shows that the early behaviours are disorganised and uncoordinated in appearance whereas the later behaviours are smooth and coordinated. We have been exploring the extent to which the development of the neural circuitry underlying the production of these behaviours shows parallel discontinuities and instead have found substantial evidence for continuity. That is, it appears that multifunctional neural circuitry is built early in ontogeny and is used and re-used throughout life to produce a wide variety of motor behaviours. We are also interested in the mechanisms by which multifunctional neural circuitry can be modulated to produce different motor behaviours. One of our major interests has been in examining the roles played by environmental and sensory factors in the production of hatching. In particular, we have focused on the role of neck position and the resulting sensory signals in triggering the onset of hatching.
INTRODUCTION
Hatching is one of the distinctive motor behaviours produced by avian embryos. We have the most complete picture of hatching in chickens and so that is the species that will be described in this paper. Nevertheless, because hatching is a very conservative behaviour pattern with nearly identical components in almost every bird examined (Oppenheim 1972; 1973), it seems likely that similar results will apply to other species.
Description of hatching
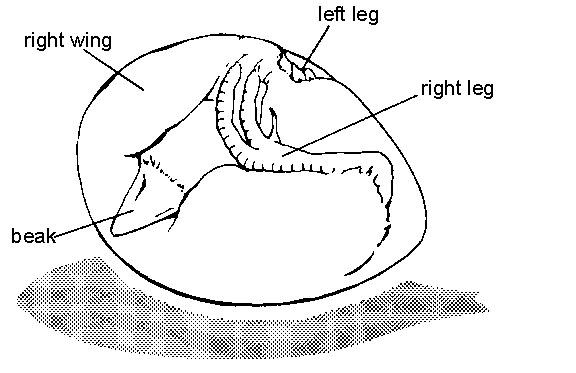
Hatching behaviour occurs at the end of the incubation period, when the embryo is in the characteristic 'hatching position' (Fig. 1; Hamburger & Oppenheim 1967). In the hatching position, the legs are tightly folded, with the ankles extending into the pointed end of the shell and the toes near the head. The head, at the blunt end of the shell near the air space, is tightly bent around to the right so that the beak is oriented over the back pointing upward into the air space. In his classic work on hatchability of chicken eggs, Landauer (1967) points out that embryos that do not attain the normal hatching position show a lower hatching rate. The chick folds itself into the hatching position using an active behaviour called tucking (Hamburger & Oppenheim 1967). Tucking is typically completed on incubation day 17. Some time after attaining the hatching position, and prior to the initiation of hatching, the embryo 'pips'. That is, the beak makes a small hole through the shell using a behaviour called pipping. Pipping is usually completed several hours or even days before the onset of hatching.
Hatching behaviour lasts about an hour in chick embryos, from the time the embryo starts cracking the shell, until it has cracked around the circumference far enough to push off the top and escape from the shell. During that time, it produces episodes of hatching behaviour that last about 1 to 3 seconds, with inter-episode intervals of about 20 to 30 seconds (Bekoff & Kauer 1982; 1984). Bakhuis (1974) has provided a detailed description of the hatching movements, based on filmed records. The most obvious part of the behaviour is the backward thrusting of the head that causes the beak to progressively break the shell in a counterclockwise direction. The wings also extend forward as the beak contacts the shell. A key part of hatching behaviour is the thrusting of the legs against the shell. Thus, the head, neck, wings and legs all extend to enable the beak to crack the shell. Both film (Bakhuis 1974) and electromyogram (EMG) recordings show that the leg movements are synchronous (Fig. 2; Bekoff & Kauer 1984). The leg thrusts apparently provide the force that causes the embryo to rotate in the shell. In the absence of rotation, the beak would hit over and over at the same site, rather than progressing around the shell. Evidence for the importance of the leg movements is provided by studies showing that, in the absence of leg movements, chicks are unable to hatch (Helfenstein & Narayanan 1969; Oppenheim 1975). In contrast 50% were able to hatch when right wing movements were absent (Narayanan & Oppenheim 1968).
Control of hatching
Timing
Hatching is particularly interesting because it must be so tightly controlled. There is only a narrow window in which successful hatching can occur. Hatching must take place after the lungs and other organs are mature enough to support postnatal survival, and before yolk supplies are exhausted or extraembryonic membranes become desiccated. Effectively, this limits the window for hatching to a period lasting about 24 to 36 hours, which occurs on day 20 or 21 in chickens. In most birds there are additional constraints, such as that the first hatched often have a significant survival advantage or that the young must hatch at similar times in order to leave the nest all together with the mother (e.g., Vince 1969; Mock & Parker 1997).
Mechanisms
A number of mechanisms have been suggested as likely to be involved in the initiation of hatching. These include changes in O2/CO2 tension, vocalisations of other embryos, hormones, and sensory signals. Although O2/CO2 tension may play a role in stimulating pipping, it does not appear to affect the time of hatching (Visschedijk 1968). Vocalisations of neighbouring embryos have been shown to be an effective mechanism for either advancing or delaying the time of hatching. This work has been well reviewed by Vince (1969). Vocalisations, or other environmental signals, must work through intrinsic mechanisms and these will be the focus of the remainder of this paper.
Neck bending
In a series of studies, we have shown that the bending of the chick embryo's neck (see Fig. 1) is a specific signal that turns on hatching. If the head is released from the egg so that the neck straightens, hatching ceases (Bekoff & Kauer 1982; Bekoff 1992). In contrast, if the legs are released, but the neck remains bent, hatching movements continue. In another study, Provine (1972) showed that if the egg shell were taped so that the chick could not push the top off and get its head out, it would continue to rotate around, completing as many as three rotations before stopping. These results suggest that the termination signal for hatching is the straightening of the neck that results from the release of the head from the egg.
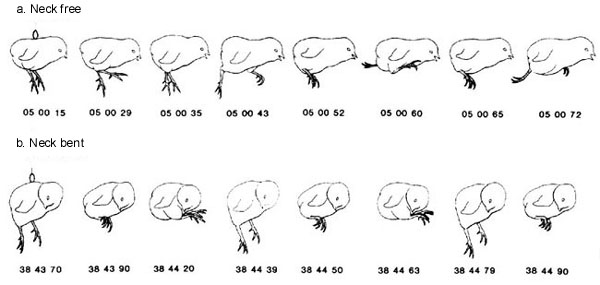
The most convincing study showing that the bent neck is a specific signal for the initiation of hatching is one in which each chick was suspended from a ring glued to its back, leaving all body parts free to move (Bekoff & Kauer 1982). Then selected body parts: wings, legs or neck, were restrained in the position they would normally be in during hatching. Only restraint of the neck by bending it to the side and taping it firmly resulted in the initiation of hatching movements (Fig. 3). Restraining the neck in other positions, for example, by bending it downward toward the breast or immobilising it in a straight position, failed to elicit hatching movements. Interestingly bending the neck either to the right or to the left can elicit hatching despite the fact that in the normal hatching position the head and neck are bent to the right. In fact, bending of the head and neck to the left results in Malposition III, which has been associated with lower hatching success (Landauer 1967). Our results suggest that the lowered hatching success is not due to a failure of the neck signal to initiate hatching movements, but to some other factor associated with turning the neck to the left.
To further investigate the role played by the bent neck, we examined the source of the sensory signal within the neck. Using injections of the local anesthetic, lidocaine, we were able to show that the signal was not due to cutaneous input from the neck region, but to proprioceptive input (Bekoff & Sabichi 1987). Taken together, our results led us to the hypothesis that the hatching initiation signal results from the asymmetric stretching of the muscles on the two sides of the chick embryo's neck.
Recently, in our lab Edmiston and her collaborators have been examining the specific role played by one of the neck muscles, the musculus complexus, often called the 'hatching muscle' (Edmiston et al. 1997). She has shown that selective injections of local anesthetic into the hatching muscle are more effective in preventing hatching than are injections into other neck muscles, such as the spinalis cervicus. Edmiston has also confirmed and extended previous studies by Fisher (1958) and Ashmore et al. (1973) showing that the hatching muscle increases in size from incubation day 17 to hatching on day 21 and then rapidly decreases in size after hatching. It has been shown that this hypertrophy is not due to an increase in size or number of muscle fibers (Ashmore et al. 1973). Instead it has been suggested, though not confirmed, that the increase is due to an infiltration of lymph fluid (Fisher 1958). In any case, the muscle increases in wet weight by about six times.
Previous work has shown that the enlarged hatching muscle is still capable of contraction (Bock & Hikeda 1968). Recently, Edmiston et al. (1997) have used EMG recordings to show that it contracts in coordination with leg muscles during hatching. Current work by Edmiston focuses on the relative roles of motor and sensory activity in the hatching muscle. For example, EMG results suggest that contractions of the hatching muscle, along with contractions of other neck muscles such as the spinalis cervicus, are used during hatching to thrust the beak against the shell (Edmiston, et al. 1997). Since the swelling of the hatching muscle is due to fluid retention rather than an increase in number or size of muscle fibers, it seems unlikely that the hypertrophy increases its effectiveness in moving the head. However, hypertrophy may result in stretching the muscle sensory receptors and may thereby increase the gain of the signal from the bent neck. This issue is currently under investigation
.Hormones
Although it is clear that a sensory signal from the bent neck can turn on hatching behaviour, it is also clear that another level of control must be involved. We reach this conclusion because the chick embryo does not begin hatching as soon as it attains the hatching position. In fact, the embryo may be in the final hatching position many hours, or even days, prior to the initiation of hatching. Thus, the signal from the bent neck is either inhibited, or not activated, until the actual time of hatching. Furthermore, several other processes must be completed immediately prior to the onset of hatching behaviour. These include internalisation of the yolk sac and clamping off of the extraembryonic circulation. If these processes are not complete by the time the chick embryo begins to rotate in the eggshell, the yolk sac and/or extraembryonic blood vessels will be damaged and the embryo is unlikely to survive.
Presumably whatever signal initiates the internalisation of the yolk sac and the clamping off of the extraembryonic blood vessels also either removes the inhibition from, or directly activates, the neck proprioceptive signal for the onset of hatching behaviour. The most likely candidate for a signal that could coordinate such disparate processes as these is a neurochemical, possibly a hormonal, signal. Previous work, reviewed by Oppenheim (1973), showed that thyroxin could advance and thiourea could delay the time of hatching if given on day 17 of incubation, but not at later times. Thus, while thyroxin may play a role, it is not the proximate signal for the initiation of hatching. Recent work by Cornwall et al. (1998) in our lab provides evidence that endogenous corticosterone levels peak just prior to hatching and that exogenous corticosterone, injected on day 18, but not earlier, may advance the time of hatching. However, it is still not clear whether corticosterone is the proximate signal. Further work is needed to determine what neurochemical signal(s) might be operating immediately prior to the initiation of hatching.
Circuitry
As soon as hatching is completed and the chick has emerged from the egg, hatching behaviour ceases and the newly hatched chick's behaviour takes on characteristics more appropriate to postnatal life. For example, the leg movements immediately switch from the synchronous pattern of coordination that is typical of hatching to alternation, which is characteristic of postnatal walking (Bekoff & Nicholl 1992). This switch is triggered by the unbending of the neck since re-bending the neck and placing chicks in artificial glass eggs will re-elicit hatching in chicks up until at least 61 days after hatching (Bekoff & Kauer 1984).
The glass egg experiments show clearly that the circuitry underlying the initiation and production of hatching behaviour is still present and functional long after hatching behaviour is normally completed. Thus the hatching circuitry remains intact in the adult chicken. Based on similarities in the EMG patterns underlying the leg movements of hatching and walking, we have suggested that the same, or at least elements of the same, circuitry is used for both behaviours (Bekoff et al. 1987).
Furthermore, both hatching and walking appear to share elements of the so-called 'basic circuit' used in the production of the leg movements of embryonic motility at 9 days of incubation (Bradley & Bekoff 1990; Bekoff 1992). Therefore, at least some of the circuitry used for hatching develops very early in embryonic life. It is reconfigured or modulated to produce the characteristic hatching behaviour by a hormonal trigger and sensory signal from the neck right at the end of the incubation period.
REFERENCES
Ashmore, C.R., Addis, P.B., Doerr, L. & Stokes, H. 1973. Development of muscle fibers in the complexus muscle of normal and dystrophic chicks. Journal of Histochemistry and Cytochemistry 21: 266-278.
Bakhuis, W.L. 1974. Observations on hatching movements in the chick (Gallus domesticus). Journal of Comparative and Physiological Psychology 87: 997-1003.
Bekoff, A. 1992. Neuroethological approaches to the study of motor development in chicks: Achievements and challenges. Journal of Neurobiology 23: 1486-1505.
Bekoff, A. & Kauer, J.A. 1982. Neuronal control of hatching: Role of neck position in turning on hatching leg movements in post-hatching chicks. Journal of Comparative Physiology 145: 497-504.
Bekoff, A. & Kauer, J.A. 1984. Neural control of hatching: Fate of the pattern generator for the leg movements of hatching in post-hatching chicks. Journal of Neuroscience 4: 2659-2666.
Bekoff, A. & Nicholl, D.L. 1992. Timing vs. pattern in cyclic motor patterns in chicks. Society for Neuroscience Abstracts 18: 1278.
Bekoff, A., Nusbaum, M.P., Sabichi, A.L. & Clifford, M. 1987. Neural control of limb coordination. I. Comparison of hatching and walking leg motor output patterns in normal and deafferented chicks. Journal of Neuroscience 7: 2320-2330.
Bekoff, A. & Sabichi A.L. 1987. Sensory control of the initiation of hatching in chicks: Effects of a local anesthetic injected into the neck. Developmental Psychobiology 20: 489-49.
Bock, W.J. & Hikeda, R.S. 1968. An analysis of twitch and tonus fibers in the hatching muscle. Condor 70: 211-222.
Bradley, N.S. & Bekoff, A. 1990. Development of coordinated movement in chicks: I. Temporal analysis of hindlimb synergies at embryonic days 9 and 10. Developmental Psychobiology 23: 763-782.
Cornwall, G., White, E., Spencer, R. & Bekoff, A. 1998. Fetal corticosterone appears to be involved in the initiation of hatching in the domestic chicken. Society for Neuroscience Abstracts 24: (in press).
Edmiston, A.S., Hernandez, T.D. & Bekoff, A. 1997. What is the role of neck muscles (complexus and spinalis cervicus) in hatching of domestic chick? Society for Neuroscience Abstracts 23: 2136.
Fisher, H.I. 1958. The "hatching muscle" in the chick. Auk 75: 391-399.
Hamburger, V. & Oppenheim, R. 1967. Prehatching motility and hatching behavior in the chick. Journal of Experimental Zoology 166: 171-203.
Helfenstein, M. & Narayanan, C.H. 1969. Effects of bilateral limb-bud extirpation on motility and prehatching behavior in chicks. Journal of Experimental Zoology 172: 233-244.
Landauer, W. 1967. The hatchability of chicken eggs as influenced by environment and heredity. Storrs Agricultural Bulletin 1.
Mock, D.W. & Parker, G.A. 1997. The Evolution of Sibling Rivalry. Oxford, New York.
Narayanan, C.H. & Oppenheim, R.W. 1968. Experimental studies on hatching behaviour in the chick. 2. Extirpation of the right wing. Journal of Experimental Zoology 168: 387-394.
Oppenheim, R.W. 1973. Prehatching and hatching behavior: A comparative and physiological consideration. In: Gottlieb, G. (ed.) Behavioral embryology. New York, Academic Press: 163-244.
Oppenheim, R.W. 1975. The role of supraspinal input in embryonic motility: A re-examination in the chick. Journal of Comparative Neurology 160: 37-50.
Provine, R.R. 1972. Hatching behavior of the chick (Gallus domesticus): Plasticity of the rotatory component. Psychonomic Science 2: 27-28.
Vince, M.A. 1969. Embryonic communication, respiration and the synchronisation of hatching. In: Bird Vocalisations. Hinde, R.A. (ed.) Cambridge University Press: 233-260.
Visschedijk, A.H.J. 1968. The airspace and embryonic respiration. 2. The times of pipping and hatching as influenced by artificially changed permeability of the shell over the airspace. British Poultry Science 9: 185-196.
Fig. 1. Diagram of a 21-day old chick embryo in the hatching position.

Fig. 2. EMG record from right and left ankle extensor muscles (GL, gastrocnemius lateralis) during two representative hatching episodes. The top record shows an episode consisting of two leg thrusts; the bottom shows an episode with one leg thrust. In both cases, right and left GL are activated synchronously. (modified from Bekoff and Kauer, 1982).
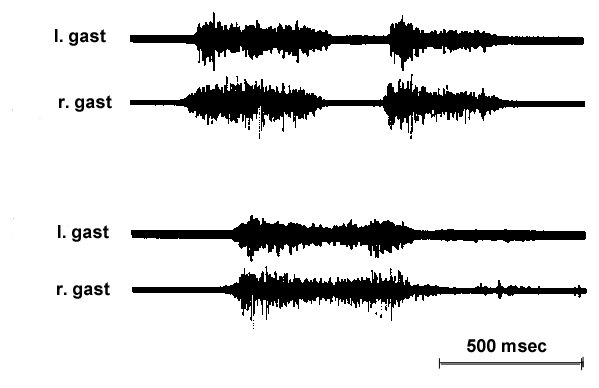
Fig. 3. Drawings made from single fields of videotaped records. Numbers indicate real time in minutes, seconds and hundredths, read from a digital stopwatch in the field of view. (a) Suspended chick with head and neck free. (b) Suspended.