S45.5: Visual system involvement in magnetoreception in birds
John B. Phillips 1, Wolfgang Wiltschko2 & Ursula Munro3
1Dept. of Biology, Indiana University, Bloomington, IN 47405, USA, fax1 812 855 6705, e-mail phillips@indiana.edu; 2Fachbereich Biologie, Zoologie, der J.W.Goethe-Universitat, Siesmayerstrasse 70, D-60054, Frankfurt a.M., Germany; 3Dept. of Environmental Sciences, University of Technology, Sydney, PO Box 123, Broadway, NSW 2007, Australia
Phillips, J.B., Wiltschko, W. & Munro, U. 1999. Visual system involvement in magnetoreception in birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2759-2774. Johannesburg: BirdLife South Africa.Theoretical models of the mechanism of magnetoreception in birds have implicated specialized photoreceptors as a possible site of sensitivity to the azimuth of the geomagnetic field (e.g., Schulten 1982. Advan. Solid State Phys. 22:61). Evidence consistent with such photoreceptor-based mechanisms includes: sensitivity to the axis, but not polarity, of the magnetic field; disruption of magnetic compass orientation by small ((20-30%) changes in magnetic field intensity; dependence of magnetic orientation on the wavelength of light; dependence of neurophysiological responses to magnetic stimuli recorded in the visual system on the presence and wavelength of light and on input from the retina. However, photoreceptors responding to changes in the alignment of an earth-strength magnetic field have yet to be identified in birds, and a number of inconsistencies in experimental evidence remains, e.g., different wavelength-dependent effects of light observed in behavioural and neurophysiological studies, and difficulty in obtaining neurophysiological responses. Evidence for and against the involvement of a photoreceptor-based magnetoreception mechanism in birds and the question of whether different biophysical mechanisms underlie the magnetic compasses of birds and other terrestrial organisms will be discussed.
INTRODUCTION
Early studies on magnetic compass orientation in migratory birds
The magnetic compass of birds was first described in a migratory bird, the European Robin Erithacus rubecula. When captive individuals were tested for their directional tendencies indoors in circular cages, they were found to prefer their natural migratory direction, i.e. North to Northeast in spring and South to Southwest in fall. When magnetic North of the ambient field was rotated by means of a coil system, the test birds changed their directional preferences accordingly (W. Wiltschko 1968), thus indicating that the magnetic field provided the crucial orientation cue. Subsequent studies revealed two important properties of the birds' magnetic compass mechanism:
Robins also changed their direction of orientation by 180° when the vertical component of the local magnetic field was reversed (Fig. 1). This means that the bird's magnetic compass is sensitive to the axis, but not the polarity, of the magnetic field lines. Robins obtain unambiguous magnetic information on direction by interpreting the inclination or dip angle of the magnetic field lines with the help of gravity (Wiltschko & Wiltschko 1972). They have an inclination compass that distinguishes poleward, where the field lines are inclined into the ground, from equatorward, where they point upward, instead of north from south as indicated by polarity. More recently the presence of a magnetic inclination compass has been demonstrated in six other species of migratory birds with different distribution ranges and migratory habits (for review see Wiltschko & Wiltschko 1995a). Evidence consistent with an inclination compass has also been obtained in homing pigeons (Walcott & Green 1974; Visalberghi & Alleva 1979). This type of magnetic compass seems to be a rather widespread mechanism among birds.
A second important property of the birds' magnetic compass is its tuning to a narrow range of magnetic intensities. Robins captured and kept in a local magnetic field of 46,000 nT were able to orient only within a narrow intensity window that included the ambient intensity + 20 to 30%. Exposure to stronger or weaker fields resulted in disorientation. After three days of acclimatization, however, the birds were also able to orient in fields outside the normal range, e.g. 16,000 nT or 150,000 nT. Moreover, after acclimatization to the much stronger field of 150,000 nT, Robins were able to orient in both the stronger field and the local field, but not in a field that was intermediate in intensity (Wiltschko 1978).
The non-polar sensitivity and the tuning of the magnetic compass response of migratory birds described above played a central role in the development of theoretical models of the mechanism of magnetoreception (Leask 1977; Schulten & Windemuth 1986; Edmonds 1996). The importance of these findings is that they appeared to be incompatible with mechanisms involving electrical induction and permanent magnetic material, the mechanisms most familiar to investigators. In contrast to the magnetic compass orientation of migratory birds, both the electrical fields resulting by movement through a magnetic field and the torque on a permanent magnet (like in a technical magnetic compass) depend on the polarity of the magnetic field and increase linearly as the intensity of the magnetic field increases. Instead, researchers began looking for mechanisms that would show axial sensitivity and would offer an explanation for the disorientation observed in stronger fields before acclimatization, and for acclimatization itself.
Light-dependent magnetoreception: radical pair reactions
Theoretical models of the mechanism(s) of magnetoreception in terrestrial organisms are as diverse as the backgrounds of the scientists who have proposed them, involving everything from super-conduction (Cope 1973), to optical detection of magnetic resonance (Leask 1977), to interacting arrays of single-domain or super-paramagnetic particles of magnetite (Kirschvink & Gould 1981; Kirschvink & Walker 1985), to radical pair reactions (Schulten & Windemuth 1982) to magnetite-containing liquid crystals (Edmonds 1996), as well as a variety of combinations thereof. Our focus here will be mechanisms that implicate specialized photoreceptors in magnetoreception.
Kirschvink & Gould (1981) (for review see Kirschvink & Walker 1985) were the first to point out that axial sensitivity could be exhibited by a receptor involving single-domain particles of magnetite (small particles with a stable magnetization) that were free to rotate, as well as mechanisms involving still smaller super-paramagnetic particles in which the alignments of the magnetic moments of the particles could track an external magnetic field without rotation of the particles. Edmonds (1996) combined the idea of freely rotating single domain particles with the ordering of light-absorbing molecules in solution (i.e. a liquid crystal) by the strong magnetic fields that occur in the vicinity of single domain particles. He demonstrated that rotation of the magnetite particles as they track the alignment of an external field can produce a corresponding changes in the alignment of the light-absorbing molecules, producing a change in spectral transmission properties (Fig. 2). If such a mechanism were to occur in the oil droplets present in the inner segments of photoreceptors in the retinas of many vertebrates, the resulting modulation of light intensity reaching the photopigment-containing outer segment would exhibit a dependence on the alignment of the axis, but not the polarity, of an earth-strength magnetic field.
Photoreceptor-based mechanisms involving direct magnetic effects on the visual transduction process have been proposed by Leask (1977) and Schulten & Windemuth (1982). Both mechanisms have been important in stimulating research on the visual system's role in magnetoreception. They both assume, as a first step, that certain macromolecules are elevated to excited states by absorption of a photon. Inspired by properties observed in physical systems Leask (1977) proposed that macromolecules in excited triplet states would be subject to a resonance process in the radio frequency range. Since the required radio frequency does not seem to be available in living systems, interest has shifted to a model proposed by Schulten that will be the focus here. Schulten (Schulten 1982; Schulten and Windemuth 1986) hypothesizes that the magnetic field affects a biochemical reaction involving radical pair intermediates. In the case of a radical pair formed by photoexcitation, absorption of a photon of light causes a molecule (A) to accept an electron from a donor molecule (D), resulting in a radical pair (1A* + 1D ---> 2A- + 2D+). If the unpaired electrons associated with the members of the radical pair retain the antiparallel spin alignments that existed prior to the electron transfer, transfer of an electron from molecule A back to molecule D will result in both members of the pair returning to a singlet excited state. Because the unpaired electrons associated with the two radical pairs are no longer constrained by sharing an orbital with another electron, one of the electrons may flip so that the two electrons are in parallel spin alignments. In this case, transfer of an electron from A back to D will yield a triplet excited state in which the excited electron and the remaining unpaired electron in the ground state have parallel spin alignments. In this case, the magnetic moments of the two electrons no longer cancel out. Certain alignments of a static external field may increase the probability that one of the unpaired electrons in the radical pair will flip, so that back transfer of an electron will result in a triplet, rather than a single, excited state. Such an effect, however, is independent of the polarity of the magnetic field. In an ordered array of molecules, therefore, the triplet state yield may show a dependence on the axial alignment of the external field.
A molecule in a triplet excited state exhibits distinctly different properties from the same molecule in a singlet excited state. For example, the singlet excited state of rhodopsin is responsible for initiating the cascade of events that result in visual transduction. Specific alignments of the magnetic field could increase the probability that a photopigment will end up in a triplet excited state and, therefore, change the efficiency of the visual transduction process with a corresponding change in the sensitivity of the photoreceptor to light. Although magnetic field effects on radical pair reactions are well established, they have only been observed at field strengths that are 10-50 times stronger than the earth's field. Yet on theoretical grounds there is no reason to rule out the possibility that, in a molecule with suitable properties, similar effects could be produced by earth-strength field.
The light-dependent magnetoreception mechanisms proposed by Leask (1977) and Schulten & Windemuth (1986) would generate complex, three-dimensional patterns of response. For example, the pattern might appear as two rings of brighter or dimmer light intensity aligned orthogonally around each end of the magnetic field axis. Provided that the field was not horizontal, this type of pattern would provide an unambiguous source of directional information. Changes in the intensity of the magnetic field would produce qualitative, rather than quantitative, changes in the pattern of response (Schulten 1982; Schulten & Windemuth 1986). For example, if the intensity of the magnetic field increased, the two rings of response might diminish in size until they formed two points at either end of the magnetic axis. In contrast, if the intensity decreased, the rings might expand and move closer to the mid line until they fused into a single large ring orthogonal to and centered on magnetic field lines. This suggests that the disorientation of Robins when they were first exposed to changes in the intensity of the ambient magnetic field might have resulted from such a qualitative change in pattern - the birds were disoriented until they became familiar with the novel pattern. Familiarity with the novel pattern generated by stronger fields would not prevent them orienting to the familiar pattern generated by the local field. The pattern generated at the intermediate intensity, however, might be sufficiently different from the patterns generated in both the local and strong field intensities, that it would still be unfamiliar and undecipherable for the birds. Thus the light- dependent mechanisms described above are not only compatible with the non-polar response of the birds' inclination compass, but also with the effects of intensity on the magnetic compass orientation of migratory birds.
Light-dependent effects on magnetic compass orientation.
The models by Leask (1977) and Schulten & Windemuth (1982), postulating the primary processes of magnetoreception to involve photopigments, led to the prediction that magnetoreception requires light. A first attempt to test for a light-dependency in magnetoreception was based on the observation that very young, inexperienced pigeons determine their home course by magnetic information obtained during the outward journey. When such birds are displaced without meaningful magnetic information and released away from their loft, their initial departure directions are randomly distributed. Apparently, inexperienced pigeons obtain crucial information for homeward orientation by recording the net direction of displacement with their magnetic compass (R. Wiltschko & Wiltschko 1978). Displacing young, inexperienced pigeons in total darkness had the same effect as depriving them of magnetic information; it caused the pigeons departure directions to be disoriented (Fig. 3; W. Wiltschko & Wiltschko 1981). These first findings were in accordance with a light-dependent mechanism of magnetoreception.
Studies with caged migrants attempted to analyze the wavelength-dependency of magnetic compass orientation. The spectral sensitivity of birds is slightly different from our own. In contrast to humans, most birds have at least four types of colour receptors, one in the red, one in the green, one in the blue and one in the violet or ultraviolet. In passerines, behavioural studies as well as the data on pigment absorption, oil droplets transmission and the absorbance of the ocular media indicate peak sensitivities at around 620 nm, 530 nm, 460 nm and 370 nm. As a result, the spectral sensitivity of passerines extend from approximately 320 nm in the ultraviolet to approximately 680 nm in the far red (Maier 1992; Maier & Bowmaker 1993). Orientation tests were carried out in the local geomagnetic field under near monochromatic light adjusted to be of equal quantal flux. Blue light with a peak at 443 nm (half maximum bandwidth 402 to 472 nm) was produced by a incandescent lamp and a glass filter, green with a peak at 565 nm (half maximum bandwidth 550 to 583 nm) and red with a peak at 630 nm (half maximum bandwidth 613 to 656 nm) were produced by LEDs. Under 'white' light, which served as a control, Australian Silvereyes Zosterops lateralis and European Robins exhibited well-oriented behaviour in the seasonally appropriate direction. Under 433 nm blue and 565 nm green light, the birds were similarly well oriented in their migratory direction. Under 633 nm red light, however, both species failed to show a consistent orientation relative to the magnetic field (Fig. 4; W. Wiltschko et al. 1993; W. Wiltschko & Wiltschko 1995b, submitted). The responses of the birds thus suggested that light in the shorter wavelength end of the spectrum was crucial for normal magnetic compass orientation.
Tests with Silvereyes revealed no difference in response to the various wavelengths between young birds on their first migration and their old, experienced conspecifics on later migrations (Munro et al. 1997a). This points to an effect on the magnetic compass which is used by both naive and experienced migrants, in contrast to the navigational map which is thought to be used only by experienced migrants (Perdeck 1958). To test this in a different behavioural context, inexperienced young pigeons were transported under 565 nm green and 630 nm red light to their release site. The results coincide with those obtained with migrants: the birds transported under green light were well homeward oriented, those transported under red were disoriented (R. Wiltschko & Wiltschko 1998).
In order to narrow down the limit of orientation at the long-wavelength end of the spectrum, European Robins were also tested under yellow-orange light with a peak at 590 nm (produced by LEDs, half maximum bandwidth 572 to 609 nm); like under red, the Robins were disoriented (W. Wiltschko & Wiltschko, submitted). So there is rapid change in response over a rather narrow wavelength range of only about 25 nm, from 565 nm green, where excellent orientation is observed, to 590 nm yellow, where the birds are no longer oriented. It is intriguing that this sharp decline roughly coincides the long-wavelength flank of the absorbance spectrum of rhodopsin (peak absorption around 500 nm) found in passerines (Maier & Bowmaker 1993).
This leads to the question of how the absence of orientation under yellow and red light is to be interpreted. Since nocturnal migrants typically become inactive when the light level is below the visual threshold (Gwinner 1974) and the Robins did not show a decrease in activity under 590 yellow and 633 nm red light, it is unlikely that the disorientation resulted from an inability to see. This indicates that the visual range of migrants exceeds that where they can use their magnetic compass at the long-wavelength end of the spectrum. The data are, therefore, consistent with a photoreceptor-based magnetoreception system that allows magnetic compass orientation in a wavelength range from below 443 nm blue to 565 nm green, but not at the long-wavelength end of the spectrum beyond 590 nm yellow.
However, when disorientation is observed, as in tests carried out in total darkness and under yellow and red light, non-specific effects can never be excluded. In young pigeons, the observation that initial orientation was disrupted if they were transported in total darkness, but not if they were held in total darkness after arrival at the release site, argues against a non-specific (e.g., stress-related) effect of the treatment itself (W. Wiltschko & Wiltschko 1981). In funnel experiments with migrants, yellow and red light eliminates the clustering of activity in the migratory direction. The corresponding findings with red light in pigeons involve a different type of orientation behaviour. The use of magnetic compass orientation to keep track of the direction of displacement by inexperienced pigeons does not require an oriented response (and pigeons typically sit quietly in transport containers), but only that the birds pay attention to the directional information during displacement. It is unlikely, therefore that the same type of non-specific effect could explain the failure to utilize directional information from the magnetic field in both the homing pigeons during displacement and the migratory birds in their test cages. Together, the findings that long-wavelength light interferes with the use of magnetic compass information by birds of different taxonomic orders and using the magnetic compass in different behavioural contexts is consistent with birds obtaining information for magnetic compass orientation by a light-dependent mechanism of magnetoreception.
Neurophysiological evidence for the involvement of the visual system in magnetoreception.
Neurophysiological studies have also provide evidence that is consistent with photoreceptor-based mechanisms of magnetoreception, as electrophysiological responses to magnetic stimuli were recorded in a number of centers in the pigeon's brain that receive visual input. Responses to gradual changes in the direction of an earth strength magnetic field were recorded from the nucleus of the basal optic root (nBOR), part of the accessory optic system, and from the tectum opticum. The proportion of cells responding to magnetic stimulation was about 70% in both the nBOR and the tectum (Semm et al. 1984; Semm & Demaine 1986). The responses in the nBOR and the tectum opticum were found to depend on the presence of light and an intact retina (Semm & Demain 1986; Semm & Schneider 1991). This is in accordance with the behavioural studies and with light-dependent processes of magnetoreception taking place in photoreceptors in the eyes.
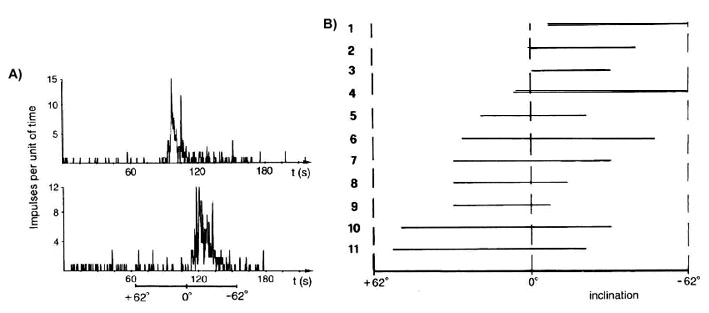
On the basis of their visual responses, units in the nBOR and the tectum may be classified as movement-sensitive cells or as direction-selective cells (Britto et al. 1981). The latter are excited by movements in a preferred direction and inhibited by motion in the opposite direction; only cells of this type responded to magnetic stimuli. Semm and Schneider (1991, p. 10) speculate that 'the clear correlation between the direction selectivity to both photic and magnetic stimuli observed in two parts of the visual system (i.e. nBOR and tectum) suggests that the directional component of magnetic responses may already be present in the retina itself, since it appears that directionality in the transfer of photic information depends on excitatory retinal photoreceptor 'units' having the same preferred direction as the tectal cells with which they make contact. This in turn implies that the magnetic detection system of the pigeon may function by exploiting the sensory organization which allows processing of visually related information'. Single units or small groups of units in the nBOR and tectum responded over a small range of directions of the magnetic field, which varied between units (Fig. 5). These findings suggest that the entire array of possible magnetic bearings may be represented in these visual centers and, therefore, provide the type of information required for magnetic compass orientation (Semm et al. 1984).
Magnetic sensitivity in nBOR units also appeared to be influenced by the wavelength of light. The wavelengths tested had peaks at 400 nm, 468 nm, 503 nm, 582 nm and 674 nm and were produced by narrow band filters. Individual units showed peak responses at either 500 nm blue or 582 nm green-yellow. Many units in the nBOR responded to the magnetic stimuli with moderate levels of activity also under 674 nm light (Semm and Demaine 1986). This means that an equivalent to the sharp drop off between 565 nm and 590 nm and the disorientation beyond 590 nm that had been observed in the behavioural studies were not found in the electrophysiological recordings. The reason for this discrepancy is unclear. It may be the result of differences in light intensity used in the behavioural and neurophysiological experiments or of differences between the input at the receptor level and the information processed by the higher centers. Further investigations will have to answer these questions.
In pigeons, Demaine and Semm (1985) also reported responses to magnetic stimuli in pineal cells. These responses persisted after all neural input had been eliminated, suggesting that the pineal itself is sensitive to magnetic stimuli. The pineal, representing the ancient third eye of vertebrates, is photosensitive in birds (Semm and Demaine 1983). One class of avian pinealocytes resembles rudimentary photoreceptors and contains a photoreceptive opsin protein (Okano et. al. 1994). Therefore, the data indicating magnetic sensitivity of pinealocytes are also in accordance with a photoreceptor-based mechanism for magnetoreception. The biological significance of this sensitivity is unclear, however. It does not seem to contribute to magnetic compass orientation, since pinealectomy did not eliminate the ability of birds to oriented in appropriate directions with the magnetic field as only orientation compass cue (Maffei et al. 1983; Semm et al. 1987; Schneider et al. 1994). A possible role in the organization of circadian and circannual rhythms has been discussed (see Reiter 1993a,b).
A non-light-dependent magnetoreception mechanism in birds?
Photoreceptor-based mechanisms are not the only models of magnetoreception currently discussed. A popular alternative proposes that particles of permanent magnetic material, such as magnetite, may be the transducer of magnetic information (see Kirschvink & Gould 1981; Kirschvink & Walker 1985; Kirschvink et al. 1985). In birds, as in other vertebrates, single-domain particles of magnetite (that possess stable magnetization) have been found in the ethmoidal region above the upper mandible (Beason & Nichols 1984; Beason 1989; for other animals, see Kirschvink et al. 1985).
In order to test a possible involvement of magnetite in magnetoreception, birds were treated with a magnetic pulse designed to alter the magnetization of single domain particles of the kind found in birds (Kalmijn 1981). The pulse used in the avian studies was short (typically 4 to 5 ms) with an intensity (typically around 0.5 T) that is greater than the coercivity of single domains. This treatment will alter the response of magnetoreceptors that involve single domain particles, provided these particles are not free to rotate with respect to the surrounding tissue (Kirschvink & Walker 1985; Phillips & Deutschlander 1997), while it should not have a lasting effect on other types of magnetoreceptors.
Adult Australian Silvereyes tested during their northward migration responded to pulse treatment with a change in direction of approximately 90° toward East. This deflection was most pronounced during the first two days after treatment; after that, the birds slowly returned to their original headings in the course of about 10 days (W. Wiltschko et al. 1994). Similar deflections after pulse treatment were observed in adult Bobolinks Dolichonyx orizivorus. Pulses applied in different directions led to different deflections, indicating that the direction of induced deflection depends on the direction of the pulse (Beason et al. 1995). In homing pigeons, pulse treatment also led to similar deflections from the headings of controls, with the direction of the response depending on the type of pulse treatment. Significant differences between pulse-treated pigeons and controls were only observed at distances greater than 80 km from the loft, suggesting that pulse treatment may only affect magnetic information used by pigeons for long-distance homing (Beason et al. 1997).
The behavioural evidence for a magnetite-based magnetoreception system is complemented by electrophysiological evidence from the opthalamic branch of the trigeminal nerve that innervates the ethmoidal region where particles of single-domain magnetite have been found (see Beason 1989). In this nerve, changes in direction as well as changes in intensity of as little as 50 to 100 nT were reported to produce changes in the firing rate of individual units (Beason and Semm 1987, Semm and Beason 1990). The absolute sensitivity of these responses, as well as the anatomical proximity of the nerve to magnetite, suggest that magnetite may be the transducer in this system, an assumption supported by the finding that blocking the trigeminal nerve with a local anesthetic eliminated the effect of the pulse remagnetization (Beason and Semm 1997).
This leads to the question of what kind of magnetic information the magnetite-based system might provide. It is important to note that the effects of pulse magnetization have been observed only in experienced birds, i.e., in experienced adult homing pigeons and migrants. Juvenile migrants that had been captured shortly after fledging and thus lacked migratory experience, in contrast, were not affected by the pulse, but continued in their normal migratory direction (Munro et al. 1997b). The difference between experienced and naive birds suggest that pulse remagnetization is not affecting an innate system like the magnetic compass. This suggests that a magnetite-based receptor provides birds with magnetic information about geographic position, which can only be interpreted by birds with sufficient experience to have developed a navigational map (see Perdeck 1958, 1967). The observation that the young birds used magnetic compass information to continue in their normal migratory direction after pulse treatment (Munro et al. 1997b), as well as the behaviour of the birds in the nerve-blocking experiment who also continued in their migratory direction after pulse treatment (Beason & Semm 1997), strongly suggest that the magnetic pulse did not affect the magnetic compass.
The response of birds to variations in the light regime and to pulse treatment suggests two independent mechanisms of magnetoreception in birds, namely a light-dependent mechanism providing birds with compass information and a second mechanism involving permanent magnetic material, probably magnetite, that provides birds with information on position. Interestingly, in Bobolinks, responses to magnetic stimuli have been recorded from both the tectum opticum and from the ophthalmic nerve, indicating the presence of two independent magnetoreception mechanisms in the same species (Beason & Semm 1987).
One, or more than one, magnetic compass mechanism among vertebrates?
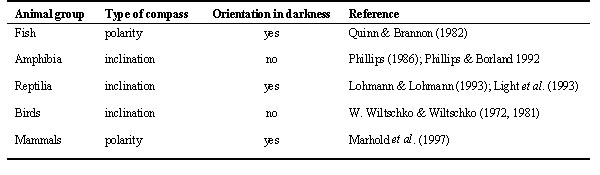
Studies of the magnetic compass mechanisms of birds have so far revealed a consistent set of properties: All species tested were found to use an inclination compass; behavioural evidence from the few species studied also implicates light-dependency. This mechanism is by no mean universal in vertebrates, however. A summary of the functional properties of the magnetic compass responses in vertebrates is shown in Table 1. There are important differences between birds and other taxonomic groups. For example, the only fish and the only mammals that have been studied intensively, the Sockeye Salmon Oncorhynchus nerka (Salmonidae), and the African Mole Rat Cryptomys (Bathyergidae), use a polarity compass. Moreover, both the salmon and the Mole Rats are able to orient with respect to the magnetic field in total darkness (Quinn & Brannon 1982; Marhold et al. 1997). The two species of reptiles tested, the Loggerhead Sea Turtle Caretta caretta (Cheloniidae), and the Leatherback Sea Turtle Dermochelys coriacea (Dermochelidae), are also able to orient in total darkness (Lohmann 1991; Lohmann & Lohmann 1993), but use an inclination compass (Light et al. 1993).
Amphibians appear to be the only group similar to birds in that they also use an inclination compass (Phillips 1986) and fail to show magnetic compass orientation in the absence of visible light (Phillips & Borland 1992). However, studies of the wavelength- dependence of shoreward magnetic compass orientation by the only species intensively studied so far, the Eastern Red-Spotted Newt Notophthalmus viridescens (Salamandridae), have revealed striking differences from the findings in birds. Tested under various wavelength of light, the newts exhibited normal orientation under full spectrum, 400 nm violet and 450 nm blue light. Under 500 nm blue-green, 550 nm green and 600 nm orange light, however, their orientation was rotated counterclockwise by approximately 90° Comparison of the responses of newts after training under full spectrum and light of wavelength longer than 500 nm indicated that the wavelength-dependent 90° shift results from a direct effect of light on the underlying magnetoreception mechanism (Phillips & Borland 1992). In short, the light-dependent magnetic inclination compass of newts differs from that of birds in two important details: (1) Newts show a normal response only in the rather narrow range up to 450 nm blue, whereas birds show normal orientation over a much wider wavelength range up to 565 nm green. (2) At the long-wavelength end of the spectrum from 500 nm onward, newts show a 90° counterclockwise deflection, whereas no such shift has been observed in birds; birds are no longer oriented beyond 590 nm.
The overall impression that one gets from a comparison of different types of vertebrates thus is the absence of a consistent set of properties. Even if the effects of the presence/absence and the wavelength of light are the only properties considered, the findings on the different groups of vertebrates (Table 1) are consistent with possibility of multiple light-dependent and light-independent mechanisms. However, the ability to orient in the absence of light need not indicate a totally different mechanisms of magnetoreception, as long as the animal uses an inclination compass. Magnetic sensitivity could be mediated by a biochemical reaction involving radical-pair-intermediates that does not require photoexcitation (Canfield et al. 1994, 1995), and may or may not occur in a photoreceptor (Phillips & Deutschlander 1997). The advantages of a photoreceptor include access to a ready supply of photons (for radical pair reactions that require photooxidation), an organized array of molecules, and associated neural processing mechanisms that would be well suited to extract directional information from the complex three dimensional pattern of response generated by this type of magnetoreception mechanism (Schulten & Windemuth 1986). On the other hand, magnetic field sensitivity may be derived from a radical pair reaction that does not require photooxidation, e.g., in animals that live in poorly or variably illuminated environments.
Polar sensitivity, in contrast, as seen in Sockeye Salmon (Quinn & Brannon 1982) and African Mole Rats (Marhold et al. 1991), is incompatible with the models of photoreceptor-based magnetoreception proposed to date (Leask 1977, Schulten 1986, Edmonds 1997), because these provide only axial sensitivity. Polar sensitivity appears to require different primary processes and a different receptor type, for example, one involving magnetite (Kirschvink & Gould 1981; Phillips & Deutschlander 1997). Axial sensitivity, on the other hand, is not restricted to the radical pair mechanism, but is compatible with many of different types of magnetoreception mechanisms discussed above, including mechanisms based on magnetite (see Kirschvink & Gould 1981). As long as light appears to be involved in magnetoreception, however, mechanisms involving specialized photoreceptors are likely candidates.
Outlook
This is an exciting time to be involved in research on the possible role of the visual system in magnetoreception. On the one hand, the findings from behavioural and neurophysiological studies in birds indicate that light is involved, and specific models of light-dependent mechanisms of magnetoreception have been proposed. On the other hand, current knowledge on details of the primary processes, their dependency on wavelengths and intensity, the exact site where they take place and the further processing of magnetic information is by far too limited to allow us to tell how magnetoreception works and what mechanisms are involved. However, we are optimistic and believe that the research over the next decade will provide the relevant answers.
REFERENCES
Beason, R.C. 1989 Use of an inclination compass during migratory orientation by the bobolink (Dolichonyx oryzivorus). Ethology 81: 291-299.
Beason, R.C. & Nichols, J.E. 1984. Magnetic orientation and magnetically sensitive material in a transequatorial migratory bird. Nature 309: 151-153.
Beason, R.C., Drussourd, N. & Deutschlander. M. 1995. Behavioural evidence for the use of magnetic material in magnetoreception by a migratory bird. Journal of Experimental Biology 198: 141-146.
Beason, R.C. & Semm, P. 1987. Magnetic responses of the trigeminal nerve system of the bobolink (Dolichonyx oryzivorus). Neuroscience Letters 80: 229-234.
Beason, R.C. & Semm, P. 1997. Does the avian ophthalmic nerve carry magnetic navigational information? Journal of Experimental Biology 199: 1241-1244.
Beason, R.C., Wiltschko, R. & Wiltschko, W. 1997. Pigeon homing: Effects of magnetic pulses on initial orientation. Auk 114: 405-415.
Britto, L.R.G., Natal, C.L. & Marcondes, A.M. 1981. The accessory optic system in pigeons: receptive field properties of identified neurons. Brain Research 206: 149-154.
Canfield, J.M., Belford, R.L., Debrunner, P.G. & Schulten, K.J. 1994. A perturbation theory treatment of oscillating magnetic fields in the radical pair mechanism. Chemical Physics 182: 1-18.
Canfield, J.M., Belford, R.L., Debrunner, P.G. & Schulten, K.J. 1995. A perturbation theory treatment of oscillating magnetic fields in the radical pair mechanism using the Liouville equation. Chemical Physics 195: 59-69.
Cope, F.W. 1973. Biological sensitivity to weak magnetic fields due to biological superconductive Josepheson Junctions? Physiological Chemistry and Physics 5: 173-176.
Demaine, C. & Semm, P. 1985. The avian pineal as an independent magnetic sensor. Neuroscience Letters 62: 119-122.
Edmonds, D.T. 1996. A sensitive optically-detected magnetic compass for animals. Proceedings Royal Society B. 263: 295-298.
Gwinner, E. 1974. Endogenous temporal control of migratory restlessness in warblers. Naturwissenschaften 61: 405.
Kalmijn, Ad.J. & Blakemore, R.P. 1977. Geomagnetic orientation in marine mud bacteria. Proceedings International Union of Physiological Sciences 13, 377-379.
Kalmijn, Ad.J. & Blakemore, R.P. 1978. The magnetic behaviour of mud bacteria. In: Schmidt-Koenig, K., Keeton, W.T. (eds) Animal Migration, Navigation and Homing. Berlin; Springer Verlag: 354-355.
Kirschvink, J.L. & Gould, J.L. 1981. Biogenic magnetite as a basis for magnetic field detection in animals. BioSystems 13: 181-201.
Kirschvink, J.L. & Walker, M.M. 1985. Particle-size considerations for magnetite-based magnetoreceptors. In: Kirschvink, J.L., Jones, D.S. & McFadden, B.J. (eds), Magnetite Biomineralization and Magnetoreception in Animals. New York; Plenum Press: 243-254.
Kirschvink, J.L., Jones, D.S. & McFadden, B.J. (eds) 1985. Magnetite Biomineralization and Magnetoreception in Animals. New York; Plenum Press.
Leask, M.J.M. 1977. A physico-chemical mechanism for magnetic field detection by migratory birds and homing pigeons. Nature 267: 144-145.
Light, P., Salmon, M. & Lohmann, K.J. 1993. Geomagnetic orientation of loggerhead sea turtles: evidence for an inclination compass. Journal of Experimental Biology 182: 1-10.
Lohmann, K.J. 1991. Magnetic orientation by hatchling loggerhead sea turtles (Caretta caretta). Journal of Experimental Biology 155: 37-49.
Lohmann, K.J. & Lohmann, C.M. 1993. A light-independent magnetic compass in the leatherback sea turtle. Biological Bulletin Marine Biology Laboratory, Woods Hole 185: 149-151.
Maffei, L., Meschini, E. & Papi, F. 1983. Pineal body and magnetic sensitivity: homing in pinealectomized pigeons under overcast skies. Zeitschrift fur Tierpsychologie 60: 151-156.
Maier, E.J. 1992. Spectral sensitivities including the ultraviolet of the passerine bird Leiothrix lutea. Journal of Comparative Physiology A 170: 709-714.
Maier, E.J. & Bowmaker, J.K. 1993. Colour vision in the passerine bird Leiothrix lutea: correlation of visual pigment absorbance and oil droplet transmission with spectral sensitivity. Journal of Comparative Physiology. A 172: 295-301.
Marhold, S., Wiltschko, W. & Burda, H. 1997. A magnetic polarity compass for direction finding in a subterranean mammal. Naturwissenschaften 84: 421-423.
Munro, U., Munro, J.A., Phillips, J.B, Wiltschko. R. & Wiltschko, W. 1997a. Evidence for a magnetite-based navigational 'map' in birds. Naturwissenschaften 84: 26-28.
Munro, U., Munro, J.A., Phillips, J.B. & Wiltschko, W. 1997b. Effect of wavelength of light and pulse magnetization on different magnetoreception systems in a migratory bird. Australian Journal of Zoology 45: 189-198.
Okano, T., Yoshizawa, T. & Fukada, Y. 1994. Pinopsin is a chicken pineal photoreceptive molecule. Nature 372: 94-97.
Perdeck, A.C. 1958. Two types of orientation in migrating starlings Sturnus vulgaris L., and Chaffinches, Fringilla coelebs L., as revealed by displacement experiments. Ardea 46: 1-37.
Phillips, J.B. 1986. Two magnetoreception pathways in a migratory salamander. Science 233: 765-767.
Phillips, J.B. & Borland, S.C. 1992. Behavioural evidence for the use of a light-dependent magnetoreception mechanism by a vertebrate. Nature 359: 142-144.
Phillips, J.B. & Deutschlander, M.E. 1997. Magnetoreception in terrestrial vertebrates: Implications for possible mechanisms of EMF interaction with biological systems. In: Stevens, R.G., Andrews, L.E. & Wilson, B.W. (eds) The Melatonin Hypothesis: Electric Power andthe Risk of Breast Cancer. Columbus, OH; Battelle Press: 111-172.
Quinn, T.P. & Brannon, E.L. 1982. The use of celestial and magnetic cues by orienting sockeye salmon smolts. Journal of Comparative Physiology 147: 547-552.
Reiter, R.J. 1993a. Static and extremely low frequency electromagnetic field exposure: reported effects on the circadian production of melatonin. Journal of Cellular Biochemistry 51: 394-403.
Reiter, R.L. 1993b. A review of neuroendocrine and neurochemical changes associated with static and extremely low frequency electromagnetic fields. Integrative Physiological and Behavioural Science 28: 57-75.
Schneider, T., Thalau, H.P., Semm, P. & Wiltschko, W. 1994. Melatonin is crucial for the migratory orientation of Pied Flycatchers (Ficedula hypoleuca Pallas). Journal of Experimental Biology 194: 255-262.
Schulten, K. 1982. Magnetic field effects in chemistry and biology. In: Advances in Solid State Physics 22: 61-83.
Schulten, K. & Windemuth, A. 1986. Model for a physiological magnetic compass. In: Maret, G. (ed.) Biophysical Effects of Steady Magnetic Fields. Berlin; Springer Verlag: 99-106.
Semm, P. 1983. Neurobiological investigations on the magnetic sensitivity of the pineal gland in rodents and pigeons. Comparative Biochemistry and Physiology 76: 683-689.
Semm, P. & Beason, R.C. 1990. Responses to small magnetic variations by the trigeminal system of the bobolink. Brain Research Bulletin 25: 735-740.
Semm, P. & Demaine, C. 1983. Electrical responses to direct and indirect photic stimulation of the pineal gland of the pigeon. Journal of Neural Transmission 58: 281 - 289.
Semm, P., Brettschneider, H., Dolla, K. & Wiltschko, W. 1987. Interaction between magnetic stimuli and annual activity in birds. In: Pevet (ed.), Comparative Physiology of Environmental Adaptations, Vol. 3; Basel; Karger: 171-182.
Semm, P. & Demaine, C. 1986. Neurophysiological properties of magnetic cells in the pigeon's visual system. Journal of Comparative Physiology 9: 619-625.
Semm, P., Nohr, D., Demaine, C. & Wiltschko, W. 1984. Neural basis of the magnetic compass: Interactions of visual, magnetic and vestibular inputs in the pigeon's brain. Journal of Comparative Physiology 155: 283-288.
Semm, P. & Schneider, T. 1991. Magnetic responses in the central nervous system of birds. In: Lieth, H. (ed.) Effects of Atmospheric and Geophysical Variables in Biology and Medicine, Progress in Biometeorology 8: 3-13.
Visalberhi, E. & Alleva, E. 1979. Magnetic influences on pigeon homing. Biological Bulletin 125: 246-256.
Walcott, C. & Green, R.P. 1974. Orientation of homing pigeons altered by a change in the direction of the applied magnetic field. Science 184: 180-182.
Wiltschko, R. & Wiltschko, W. 1978. Evidence for the use of magnetic outward-journey information in homing pigeons. Naturwissenschaften 65: 112.
Wiltschko, R. & Wiltschko, W. 1995a. Magnetic Orientation in Animals. New York; Springer Verlag.
Wiltschko, W. & Wiltschko R. 1995b. Migratory orientation of European Robins is affected by the wavelength of light as well as by a magnetic pulse. Journal of Comparative Physiology 177: 363-369.
Wiltschko, R. & Wiltschko, W. 1998. Pigeon homing: effect of various wavelengths of light during displacement. Naturwissenschaften 85: 165-167.
Wiltschko, W. 1968. Uber den Einfluss statischer Magnetfelder auf die Orientierung von Rotkehlchen (Erithacus rubecula). Zeitschrift fur Tierpsychologie 25: 537-558
Wiltschko, W. 1978. Further analysis of the magnetic compass of migratory birds. In: Schmidt-Koenig, K. & Keeton, W.T. (eds.), Animal Migration, Navigation and Homing. Berlin: Springer Verlag: 302-310.
Wiltschko, W., Munro, U., Beason, R.C., Ford, H. & Wiltschko, R. 1994. A magnetic pulse leads to a temporary deflection in the orientation of migratory birds. Experientia 50:697-700.
Wiltschko, W., Munro, U., Ford, H. & Wiltschko, R. 1993a. Red light disrupts magnetic orientation of migratory birds. Nature 364: 525-527.
Wiltschko, W., Munro, U., Ford, H. & Wiltschko, R. 1993b. Magnetic inclination compass: a basis for the migratory orientation of birds in the northern and southern hemisphere. Experientia 49: 167-170.
Wiltschko, W. & Wiltschko. R. 1981. Disorientation of inexperienced young pigeons after transportation in total darkness. Nature 291: 433-434.
Table 1. Functional Properties of Magnetic Compass Mechanisms in Vertebrates as indicated by Behavioural Studies.

Fig. 1. . Schematic diagram illustrating the avian inclination compass (after Wiltschko 1972). The alignments of the horizontal (Hh) and vertical (Hv) components, as well as the total magnetic field vector (He,) are shown in relationship to the gravity vector (g). (A) In the northern hemisphere, the magnetic field slopes down towards the pole (>>p<<) and away from the equator (>>e<<). For an animal that is unable to detect the polarity of the magnetic field, reversal of either the horizontal component (B) or the vertical component (C) causes the downward slope of the magnetic field lines and, therefore, the poleward and equatorward directions, to be reversed.
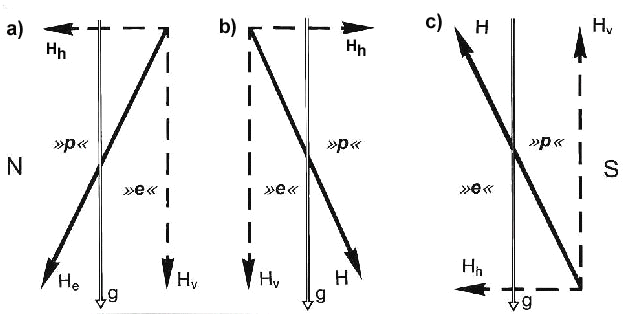
Fig. 2. The liquid crystal model of Edmonds (1996). The strong magnetic fields that are present in the vicinity of single domain particles of magnetite (rectangles) can align light-absorbing molecules (heavy zig-zag lines) in the surrounding medium. If the magnetite particles are free to rotate so that they can track the alignment of the geomagnetic field, the resulting changes in alignment of the light-absorbing molecules can affect the spectral transmission properties of the medium. A,B) When the magnetic field is parallel to the propagation direction of light (thin wavy lines), the molecules will be aligned end on to the incoming light, allowing most of the light to pass through without being absorbed. C,D) In contrast, when the magnetic field is aligned at right angles to the direction of propagation, absorption of light by the medium will be maximal. In a photoreceptor in which this type of spectral filter is located in front of the photopigment-containing outer segments (e.g., in oil droplets present in cone receptors in the retinas of many vertebrates), modulation of the response to light by the magnetic field will show a dependence on the alignment of the axis, but not polarity, of the magnetic field.
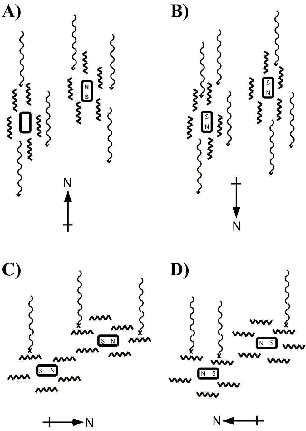
Fig. 3. Disorientation of young pigeons after transportation in total darkness (Wiltschko & Wiltschko 1981). Young inexperienced pigeons that rely on magnetic compass information obtained during displacement to an unfamiliar release site are homeward oriented when displaced in diffuse white light (A), but disoriented if displaced in total darkness (B)
.
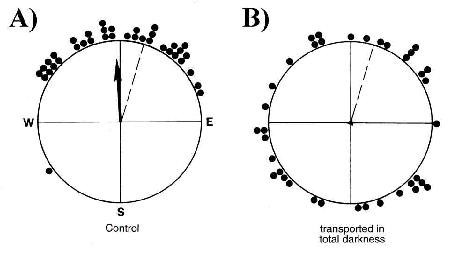
Fig. 4. Wavelength-dependent effects of light on migratory orientation (Wiltschko & Wiltschko 1995b, submitted). European robins tested indoors with the geomagnetic field as the only source of compass information were well oriented (p < 0.05, Rayleigh test) under diffuse full spectrum white (A, controls), 443 nm blue (B) and 565 nm green (C) light. However, under 590 nm yellow (D) and 633 nm red (E) light, they were disoriented. Triangles are the mean vector bearings of individual birds calculated from their responses over several nights of testing. Arrows at the centers of the distributions indicate the mean vectors calculated for the entire group of birds, the length of the arrows is proportional to the mean vector length ('r') with the radius of the circle corresponding to an r value of 1.
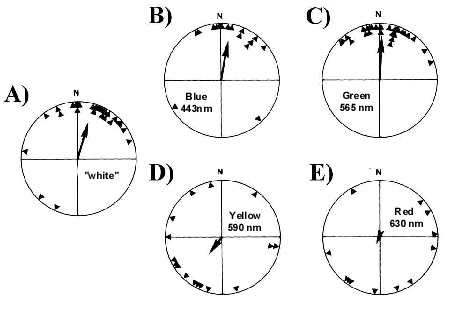
Fig. 5. Electrophysiological recordings from the visual system of pigeons (Semm et al. 1984). (A) Changes in spike frequency of direction-selective cells in the nucleus of the basal optic root in response to a gradual inversion of the inclination of the magnetic field (lower bar; field strength = 42000 nT). (B) Recordings from different direction-selective cells exposed to the gradual inversion of the magnetic field showing responses (i.e., augmentation of spike frequency indicated by horizontal bars) to different inclinations of the magnetic field vector.
.