S45.3: Photoreceptors and molecular genetics of visual pigments
James K. Bowmaker 1, Susan E. Wilkie 2 & David M. Hunt 2
1Department of Visual Science and 2Department of Molecular Genetics, Institute of Ophthalmology, University College London, Bath Street, London EC1V 9EL, UK, fax 44 171 608 6850, e-mail j.bowmaker@ucl.ac.uk; s.wilkie@ucl.ac.uk; d.hunt@ucl.ac.uk
Bowmaker, J.K., Wilkie, S.E. & Hunt, D.M. 1999. Photoreceptors and molecular genetics of visual pigments. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2729-2742. Johannesburg: BirdLife South Africa.Avian colour vision is subserved by a complex of cone photoreceptors in the retina. In diurnal birds, the cones contain brightly coloured oil droplets, a feature that is restricted to birds and reptiles. We have asked the following questions. How many classes of cone are present? What combinations of visual pigments and oil droplets occur? What is the evolutionary relationship of avian visual pigments to those of other vertebrates? Using microspectrophotometry, we have established that most birds possess four spectrally-distinct single cones containing visual pigments with maximum absorbance at about 570, 505, 440 nm and either close to 420 nm or at about 365 nm in the near ultraviolet. The three longer-wave spectral classes contain oil droplets that act as specific long-pass cut-off filters which narrow the sensitivity functions of the cones and displace their maximum sensitivities to longer wavelengths. The shortest-wave cones contain a transparent droplet. Double cones are also present with a broad sensitivity that is maximal at about 570 nm. Sequence comparisons of the genes that encode the different visual pigments have shown that avian cone opsins belong to the four ancestral vertebrate opsin classes, and that the spectral shift into the ultraviolet probably occurred separately within the major vertebrate groups.
Photoreceptors
Diurnal neognathus birds probably have, at least at the retinal level, one of the most elaborate mechanisms for colour vision within the vertebrates. The retinas of these avian species contain a complex complement of photoreceptors, rods, double cones and at least four classes of single cone. The cones are characterised by brightly coloured oil droplets, a feature restricted to birds and some reptiles (for a review, see Bowmaker 1991). The droplets are located in the distal ellipsoid region of the inner segment and act as selective cut-off (long pass) filters interposed between the incident light and the visual pigment (Bowmaker & Knowles 1977; Goldsmith et al. 1984; Partridge 1989). The four classes of single cone are spectrally distinct with maximum sensitivities extending from the near ultraviolet, close to 360 nm, to the red, close to 600 nm and are thought to subserve tetrachromatic colour vision (Goldsmith 1991; Maier & Bowmaker 1993; Bowmaker et al. 1997).
Although such general statements can be made concerning avian colour vision, in fact few species have been studied in any detail. In the Chicken Gallus gallus four cone visual pigments with wavelengths of maximum absorbance (l max) at about 415, 460, 505 and 562 nm, have been identified by visual pigment extraction techniques (Fager & Fager 1981; Yen & Fager 1984; Okano et al. 1989; Yoshizawa & Fukada 1993) and electroretinography (Govardovskii & Zueva 1977). Recently, the genes encoding these four cone visual pigments and the rod pigment have been isolated, sequenced and expressed (Takao et al. 1988; Kuwata et al. 1990; Okano et al. 1992; Wang et al. 1992), confirming their presence in the retina and suggesting a close evolutionary relationship between the spectrally similar rod pigment and the green-sensitive cone pigment.
Detailed microspectrophotometric analysis of the photoreceptors of the Chicken (Bowmaker & Knowles 1977; Bowmaker et al. 1997) and the closely related Japanese Quail Coturnix coturnix japonica (Bowmaker et al. 1993), both members of the Galliformes, has established the location of the four cone pigments within distinct cone classes. The pattern identified in these two species appears to be common amongst diurnal birds and can be used as a model for other species (Fig 1 and Fig 4). The red-sensitive pigment, with l max 569 nm (P569), dominates the retina and is found in both members of the double cones and in a class of single cone containing a red (R-type) oil droplet. The filtering effect of the R-type droplet, with a cut-off at about 570 nm (Fig. 2), narrows the spectral sensitivity of the single cone class by removing short wavelengths, and displaces the maximum sensitivity of the cell to longer wavelengths above 600 nm. In contrast, the oil droplet (P-type) of the principal member of the double cones has a cut-off at much shorter wavelengths so that the spectral sensitivity of the double cones is broad with a maximum close to 570 nm. The three remaining classes of single cone are identified as green-sensitive with a P505 associated with a yellow (Y-type) droplet with a cut-off at about 510 nm, blue-sensitive with a P455 associated with a clear or colourless (C-type) droplet cutting off at about 450 nm, and violet-sensitive with a P418 associated with a transparent (T-type) droplet that exhibits no significant absorbance throughout the spectrum (Fig 1 and Fig 2).
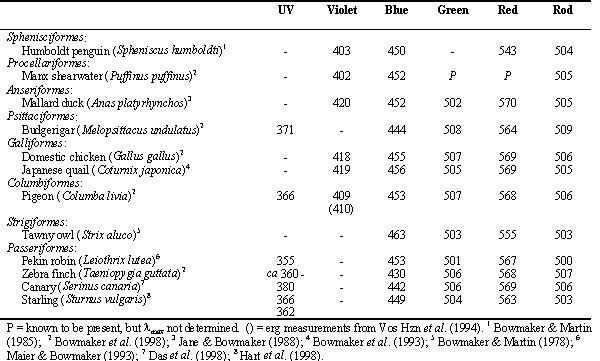
The role of the four classes of single cone in colour vision is supported by the behaviourally- determined increment spectral sensitivity function of the passeriform bird, the Pekin Robin or Red-billed Leiothrix, Leiothrix lutea (Maier 1992). Leiothrix shows high sensitivity in the near ultraviolet with the sensitivity function exhibiting four marked peaks with maxima at about 370, 460, 520 and 620 nm (Fig. 3). These sensitivity maxima closely match spectral sensitivity functions for the four classes of single cone derived from microspectrophotometric analysis of their visual pigments and oil droplets (Maier & Bowmaker 1993). As was inferred from the behavioural data and confirmed by microspectrophotometry, the fourth class of single cone in Leiothrix contains an ultraviolet-sensitive visual pigment with l max at about 365 nm and not at about 420 nm as in the two galliform species. Ultraviolet-sensitive visual pigments with l max close to 365 nm have been recorded by microspectrophotometry from a number of passeriforms, Zebra Finch Taeniopygia guttata (Bowmaker et al. 1997), Canary Serinus canaria (Das et al. 1998), Starling Sturnus vulgaris (Hart et al. 1998) and the psittaciform, Budgerigar or Shell Parakeet Melopsittacus undulatus (Fig. 4) (Bowmaker et al. 1997; Wilkie et al. 1998). In addition to species possessing a fourth cone visual pigment with l max at either 420 or 360 nm, other species such as Humboldt Penguin Spheniscus humboldti (Bowmaker & Martin 1985), Manx Shearwater Puffinus puffinus (Bowmaker et al., 1997 ) and Pigeon Columba livia (Bowmaker et al. 1997; Yokoyama et al. 1998) have a violet/ultraviolet-sensitive pigment with l max close to 400 nm (Table 1).
Ultraviolet sensitivity is well established in avian species and has been most fully demonstrated in Pigeon, both behaviourally (Wright 1972; Romeskie & Yager 1976; Kreithen & Eisner 1978; Emmerton & Delius 1980; Emmerton 1983; Emmerton & Remy 1983; Remy & Emmerton 1989) and electrophysiologically (Blough 1957; Graf & Norren 1974; Norren 1975; Chen et al. 1984; Wortel et al. 1984; Chen & Goldsmith 1986; Vos Hzn et al. 1994), but has also been shown in three species of Humming bird (Goldsmith 1980; Goldsmith et al. 1981), 13 species of passerines (Chen et al. 1984; Chen & Goldsmith 1986), two species of Boobies Pelecaniformes (Reed 1987), Leiothrix lutea (Maier 1992), Kestrel Falco tinnunculus (Viitala et al. 1995), Zebra finch Taeniopygia guttata (Bennett et al. 1996), Bluethroat Luscinia svecica (Andersson & Amundsen 1997), Starling, (Bennett et al. 1997) and Blue Tit Parus caeruleus (Andersson et al. 1998; Hunt et al. 1998).
Although four spectrally distinct classes of single cone have been demonstrated in a number of birds with ultraviolet sensitivity subserved by a single class of cones with l max between about 360 nm and 420 nm, the possibility that Pigeon have five classes of single cone, with two classes maximally sensitive in the violet/ultraviolet range, cannot be ruled out. Microspectrophotometric analysis of Pigeon photoreceptors (Bowmaker, 1977; Bowmaker et al. 1997) has identified four cone pigments with l max at about 405, 460, 515 and 567 nm, but accumulating evidence from behaviourally determined wavelength discrimination functions (Wright 1979; Emmerton & Delius 1980), and from electrophysiological studies (Graf & Norren 1974; Norren 1975; Romeskie & Yager 1976; Wortel et al. 1984; Vos Hzn et al. 1994) indicate that two spectrally distinct violet/ultraviolet cone mechanisms may be present with maxima at about 410 nm and 365 nm. If this is the case, then Pigeon may have the potential for pentachromatic colour vision, perhaps a recent evolutionary feature restricted to the Columbiformes.
Molecular genetics
Avian vision extends over a wide spectral range from the near ultraviolet around 300 nm to the red, about 750 nm, but the rod visual pigments and the four spectrally distinct families of cone visual pigments that subserve vision throughout the spectral range all share a common structure and evolutionary origin. All vertebrate visual pigments are composed of an opsin protein bound to a chromophore that, in most vertebrates and exclusively in birds, is 11-cis-retinal, the aldehyde of Vitamin A. Since the chromophore is identical in all avian visual pigments, the spectral locations of the five different pigment families commonly found in birds must be determined by different amino acid sequences of the opsin protein and their varying interactions with retinal. Opsin consists of a single polypeptide chain composed of about 350 amino acids which form seven membrane-spanning hydrophobic regions linked by extra-membrane hydrophilic loops (Fig. 5). The hydrophobic transmembrane segments are formed of a -helices each composed of about 26 amino acids of which only the central 18 are embedded within the membrane, whereas the remaining hydrophilic regions are presumed to be straight chains (Hargrave et al. 1984; Baldwin 1993; Schertler et al. 1993; Unger et al. 1997). The seven membrane helices form a bundle or palisade within the membrane that diverges towards the extracellular surface creating a cavity that acts as a ligand-binding pocket. Although the opsin polypeptides vary in length, the transmembrane helices and the cytoplasmic loops are highly conserved, with the major differences in length restricted primarily to the extracellular loops and tail. In all visual pigments, retinal is covalently bound via a Schiff’s base linkage to a lysine residue in the centre of helix VII (Bownds 1967; Wang et al. 1980) and in the majority of pigments the Schiff’s base is protonated, with the positive charge stabilized by a glutamate residue acting as a counterion located in helix III, close to the extracellular membrane surface (Fig. 5) (Sakmar et al. 1989; Zhukovsky & Oprian 1989; Nathans 1990).
The different opsins share a number of features along with other heptahelical G protein-coupled receptors (Fig. 5). There are two glycosylation sites near the amino terminus on the extracellular side and a series of phosphorylation sites near the carboxyl terminus on the cytoplasmic side (Hargrave 1982). Also, regions of the cytoplasmic loops linking the helices are involved in interacting with the membrane-bound G protein. In addition, there are two highly conserved cysteine residues in the first and second extracellular loops that form a disulphide bridge thought to be essential for the correct folding and formation of the molecule (Karnik et al. 1988) and further cysteines in the carboxyl tail that are palmitoylated, anchoring the tail and forming a fourth cytoplasmic loop (Fig. 5) (Ovchinnikov et al. 1988).
Within the vertebrates, sequence homology and phylogenetic identity of the opsins assign the pigments to a maximum of four cone classes, with the rod opsins forming a fifth class showing similarities with the green-sensitive cone pigments of fish, reptiles and birds (Fig 6). The so-called red and green cone pigments of humans fall into a single cone class along with the red-sensitive cone pigments of all other vertebrate groups, whereas the human (and mammalian) so-called blue-sensitive cone pigment falls into a further class along with the violet- and ultraviolet-sensitive cone pigments of fish, amphibians, reptiles and birds. The fourth class of cone opsins comprise the blue-sensitive pigments of fish, amphibians, reptiles and birds. Mammals appear to have lost two classes of cone opsin, the middle-wave, green-sensitive and the short-wave, blue-sensitive, that are retained by all other vertebrate groups.
Only a few opsin sequences are available for avian visual pigments. Those from Chicken (Takao et al. 1988; Kuwata et al. 1990; Okano et al. 1992; Wang et al. 1992) fall into the five vertebrate classes and the sequence of the opsin of the ultraviolet-sensitive P370 cone pigment from Budgerigar (Wilkie et al. 1998) and the violet-sensitive P400 from Pigeon (Yokoyama et al. 1998) are grouped with the violet-sensitive P418 of Chicken. Additional sequences for the green-sensitive cone and rod pigments from the Mallard Duck Anas platyrhynchos and Budgerigar (Heath et al. 1997) confirm the similarity of these two opsin groups, a feature underlined by the spectral similarity of the two classes of pigment which have almost identical l max in any given avian species (Table 1). The opsin sequences from the rod and four cone visual pigments from the Canary have also recently been published (Das et al. 1999).
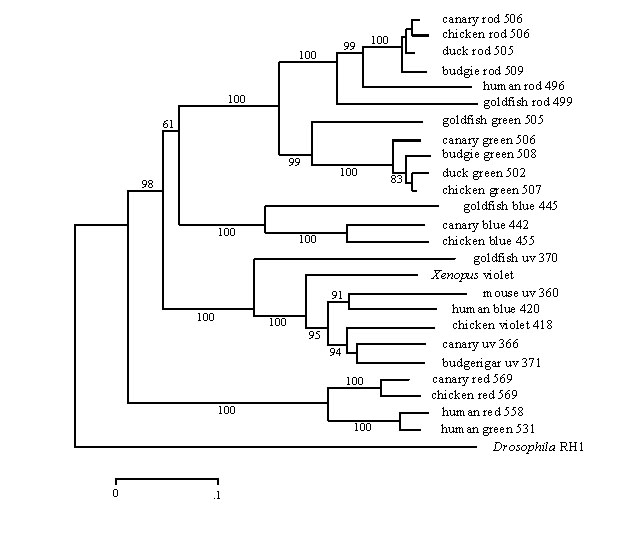
From these sequences it is possible to construct a phylogenetic tree that clearly illustrates the division of vertebrate visual pigments into five opsin families (Fig. 6). These families are ancient, having evolved rapidly in the early stages of vertebrate evolution, probably originating about 350 million years ago (MYA) so that they are represented in the major vertebrate classes. A notable feature of the tree is the indication that the spectral divisions within the violet/ultraviolet group have evolved separately within birds and mammals. The ancestral gene, expressed as a visual pigment with l max somewhere between about 350 and 430 nm, has presumably mutated differently within the birds and mammals to tune cone pigments with l max apparently clustering at three locations close to 365, 400 and 420 nm (Table 1) in birds and at about 365 nm and 420 to 440 nm in mammals (Jacobs 1993).
It is possible by sequence comparisons to identify specific amino acids within opsins that are potential tuning sites, a task perhaps more straightforward within an opsin family than between families (Bowmaker & Hunt 1998). Amino acid substitutions located within the membrane helices and in close proximity to the chromophore are most likely to have major effects on the spectral tuning of the pigment. Further, replacements of charged for non-charged or polar for non-polar amino acids are likely to have more significant effects on the chromophore. Comparisons between the 420-nm Chicken sequence and the 365-nm sequences from Budgerigar and Canary (Wilkie et al. 1998) suggest three possible amino acid sites (81, 88 and 113) which contain alanine, threonine (polar) and alanine respectively in the ultraviolet-sensitive pigment (Fig. 5), but are replaced by serine (polar), valine and threonine in the violet-sensitive pigment. All three sites are in the vicinity of the glutamate residue (Glu-108) which occurs in the same position in helix III as the glutamate counterion to the protonated Schiff’s base in longer-wave pigments. From the position of the l max and the narrower band width of the beta-band of the absorbance spectrum, it has been suggested that the Schiff’s base may be unprotonated in ultraviolet-sensitive pigments (Hárosi & Sandorfy 1995). If this is the case, then a possible effect of the potential tuning sites in the budgerigar ultraviolet-sensitive pigment would be to neutralize the effect of Glu-108.
CONCLUSION
It is becoming clear, as the photoreceptors of more vertebrate species are studied, that many diurnal vertebrates, notably birds, are potentially tetrachromatic, extending their visual range outside of the human visual spectrum into the near ultraviolet (Bowmaker 1998). Indeed, it is only the mammals that are almost universally restricted to a ‘colour deficient’ dichromacy, with just some primates having re-evolved a trichromatic system. With our increasing understanding of ultraviolet sensitivity and tetrachromacy in some fish, reptiles and birds, it is not surprising that recent behavioural studies demonstrate that such animals make use of the wealth of visual information available in the ultraviolet (Derim-Oglu & Maximov 1994; Viitala et al. 1995; Andersson 1996; Bennett et al. 1996; Burkhardt 1996; Andersson & Amundsen 1997; Bennett et al. 1997; Andersson et al. 1998; Hun et al. 1998). Perhaps the more interesting question is why many mammals, including primates and humans, have lost sensitivity to these short wavelengths.
REFERENCES
Andersson, S. 1996. Bright ultraviolet coloration in the Asian whistling thrushes (Myiophonus spp.). Proceedings of the Royal Society of London B 263: 843-848.
Andersson, S. & Amundsen, T. 1997. Ultraviolet colour vision and ornamentation in bluethroats. Proceedings of the Royal Society of London B 264: 1587-1591.
Andersson, S., Örnborg, J. & Andersson, M. 1998. Ultraviolet sexual dimorphism and assortative mating in blue tits. Proceedings of the Royal Society of London B 265: 445-450.
Baldwin, J.M. 1993. The probable arrangement of the helices in G protein-coupled receptors. European Molecular Biology Organisation Journal 12: 1693-1703.
Bennett, A.T.D., Cuthill, I.C., Partridge, J.C. & Lunau, K. 1997. Ultraviolet plumage colors predict mate preferences in starlings. Proceedings of the National Academy of Sciences 94: 8618-8621.
Bennett, A.T.D., Cuthill, I.C., Partridge, J.C. & Maier, E.J. 1996. Ultraviolet vision and mate choice in zebra finches. Nature 380: 433-435.
Blough, D.S. 1957. Spectral sensitivity in the pigeon. Journal of the Optical Society of America 47: 827-833.
Bowmaker, J.K. 1991. Photoreceptors, photopigments and oil droplets. In: Gouras, P. (ed) The Perception of Colour; London; Macmillan: 108-127.
Bowmaker, J.K. 1998. Evolution of colour vision in vertebrates. Eye 12: in press.
Bowmaker, J.K., Heath, L.A., Wilkie, S.E. & Hunt, D.M. 1997. Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vision Research 37: 2183-2194.
Bowmaker, J.K. & Hunt, D.M. 1998. Molecular genetics of spectral sensitivity variation in photoreceptors. In: Archer, S. N., Djamgoz, M. B. A., Loew, E. R., Partridge, J. C. & Valerga, S. (eds) Adaptive Mechanisms in the Ecology of Vision; London; Chapman and Hall: in press.
Bowmaker, J.K. & Knowles, A. 1977. The visual pigments and oil droplets of the chicken, Gallus gallus. Vision Research 17: 755-764.
Bowmaker, J.K., Kovach, J.K., Whitmore, A.V. & Loew, E.R. 1993. Visual pigments and oil droplets in genetically manipulated and carotenoid deprived quail: a microspectrophotometric study. Vision Research 33: 571-578.
Bowmaker, J.K. & Martin, G.R. 1985. Visual pigments and oil droplets in the penguin, Spheniscus humboldti. Journal of Comparative Physiology A 156: 71-77.
Bownds, D. 1967. Site of attachment of retinal in rhodopsin. Nature 216: 1178-1181.
Burkhardt, D. 1996. Ultraviolet perception by bird eyes and some implications. Naturwissenschaften 83: 492-497.
Chen, D.M., Collins, J.S. & Goldsmith, T.H. 1984. The ultraviolet receptor of bird retinas. Science 225: 337-340.
Chen, D.M. & Goldsmith, T.H. 1986. Four spectral classes of cones in the retinas of birds. Journal of Comparative Physiology A 159: 473-479.
Derim-Oglu, E.N. & Maximov, V.V. 1994. Small passerines can discriminate ultraviolet surface colors. Vision Research 34: 1535-1539.
Emmerton, J. 1983. Pattern discrimination in the near ultraviolet by pigeons. Perception & Psychophysics 34: 555-559.
Emmerton, J. & Delius, J.D. 1980. Wavelength discrimination in the visible and ultraviolet spectrum by pigeons. Journal of Comparative Physiology 141: 47-52.
Emmerton, J. & Remy, M. 1983. The pigeon's sensitivity to ultraviolet and 'visible' light. Experientia 39: 1161-1163.
Fager, L.Y. & Fager, R.S. 1981. Chicken blue and chicken violet, short wavelength sensitive visual pigments. Vision Research 21: 581-586.
Goldsmith, T.H. 1980. Humming birds see near ultraviolet light. Science 207: 786-788.
Goldsmith, T.H. 1991. The evolution of visual pigments and colour vision. In: Gouras, P. (ed) The Perception of Colour; London; Macmillan: 62-89.
Goldsmith, T.H., Collins, J.S. & Licht, S. 1984. The cone oil droplets of avian retinas. Vision Research 24: 1661-1671.
Goldsmith, T.H., Collins, J.S. & Perlman, D.L. 1981. A wavelength discrimination function for the hummingbird Archilochus alexandri. Journal of Comparative Physiology 143: 103-110.
Govardovskii, V I. & Zueva, L.V. 1977. Visual pigments of chicken and pigeon. Vision Research 17: 537-543.
Graf, V. & Norren, D.V. 1974. A blue sensitive mechanism in the pigeon retina: l max 400 nm. Vision Research 14: 1203-1209.
Hargrave, P.A. 1982. Rhodopsin chemistry, structure and topology. Progress in Retinal Research 1: 1-51.
Hargrave, P.A., McDowell, J.H., Feldmann, R.J., Atkinson, P.H., Rao, J.K.M. & Argos, P. 1984. Rhodopsin's protein and carbohydrate structure: selected aspects. Vision Research 24: 1487-1499.
Hárosi, F.I. & Sandorfy, C. 1995. Retinylidene-opsin Schiff base chromophores and their accessibility to water. Photochemistry and Photobiology 61: 510-517.
Hart, N.S., Partridge, J.C. & Cuthill, I.C. 1998. Visual pigments, oil droplets and cone photoreceptor distribution in the European starling (Sturnus vulgaris). Journal of Experimental Biology 201: 1433-1446.
Heath, L.A., Wilkie, S.E., Bowmaker, J.K. & Hunt, D.M. 1997. The rod and green cone opsins of two avian species, the budgerigar, Melopsittacus undulatus, and the mallard duck, Anas platyrhynchus. Gene 204: 121-126.
Hunt, S., Bennett, A.T.D., Cuthill, I.C. & Griffiths, R. 1998. Blue tits are ultraviolet tits. Proceedings of the Royal Society of London Series B-Biological Sciences 265: 451-455.
Jacobs, G.H. 1993. The distribution and nature of colour vision among the mammals. Biological Reviews 68: 413-471.
Karnik, S.S., Sakmar, T.P., Chen, H.B. & Khorana, H.G. 1988. Cysteine residue-110 and residue-187 are essential for the formation of correct structure in bovine rhodopsin. Proceedings of the National Academy of Sciences 85: 8459-8463.
Kreithen, M.L. & Eisner, T. 1978. Ultraviolet light detection by the homing pigeon. Nature 272: 347-348.
Kuwata, O., Imamoto, Y., Okano, T., Kokame, K., Kojima, D., Matsumoto, H., Morodome, A., Fukada, Y., Shichida, Y., Yasuda, K., Shimura, Y. & Yoshizawa, T. 1990. The primary structure of iodopsin, a chicken red-sensitive cone pigment. Febs Letters 272: 128-132.
Maier, E.J. 1992. Spectral sensitivities including the ultraviolet of the passeriform bird Leiothrix lutea. Journal of Comparative Physiology A 170: 709-714.
Maier, E.J. & Bowmaker, J.K. 1993. Colour vision in a passeriform bird, Leiothrix lutea: correlation of visual pigment absorbance and oil droplet transmission with spectral sensitivity. Journal of Comparative Physiology A 172: 295-301.
Nathans, J. 1990. Determinations of visual pigment absorbance: identification of the retinylidene Schiff's base counterion in bovine rhodopsin. Biochemistry 29: 9746-9752.
Norren, D.V. 1975. Two short wavelength sensitive cone systems in pigeon, chicken and daw. Vision Research 15: 1164-1166.
Okano, T., Fukada, Y., Artamonov, I.D. & Yoshizawa, T. 1989. Purification of cone visual pigments from chicken retina. Biochemistry 28: 8848-8856.
Okano, T., Kojima, D., Fukada, Y., Shichida, Y. & Yoshizawa, T. 1992. Primary structures of chicken cone visual pigments: vertebrate rhodopsins have evolved out of cone visual pigments. Proceedings of the National Academy of Sciences 89: 5932-5936.
Ovchinnikov, Y.A., Abdulaev, N.G. & Bogachuk, A.S. 1988. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. Febs Letters 230: 1-5.
Partridge, J.C. 1989. The visual ecology of avian cone oil droplets. Journal of Comparative Physiology A 165: 415-426.
Reed, J.R. 1987. Scotopic and photopic spectral sensitivities of boobies. Ethology 76: 33-55.
Remy, M. & Emmerton, J. 1989. Behavioral spectral sensitivities of different retinal areas in pigeons. Behavioral Neuroscience 103: 170-177.
Romeskie, M. & Yager, D. 1976. Psychophysical studies of pigeon color vision - I. Photopic spectral sensitivity. Vision Research 16: 501-505.
Saitou, N. & Nei, M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4: 406-425.
Sakmar, T.P., Franke, R.R. & Khorana, G.H. 1989. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proceedings of the National Academy of Science 86: 8309-8313.
Schertler, G.X., Villa, C. & Henderson, R. 1993. Projection structure of rhodopsin. Nature 362: 770-772.
Takao, M., Yasui, A. & Tokunaga, F. 1988. Isolation and sequence determination of the chicken rhodopsin gene. Vision Research 28: 471-480.
Unger, V.M., Hargrave, P.A., Baldwin, J.M. & Schertler, G.F.X. 1997. Arrangement of rhodopsin transmembrane a -helices. Nature 389: 203-206.
Viitala, J., Korpimäki, E., Palokangas, P. & Koivula, M. 1995. Attraction of kestrels to vole scent marks visible in ultraviolet light. Nature 373: 425-427.
Vos Hzn, J.J., Coemans, M.A.J.M. & Nuboer, J.F.W. 1994. The photopic sensitivity of the yellow field of the pigeon's retina to ultraviolet light. Vision Research 34: 1419-1425.
Wang, J.K., McDowell, J.H. & Hargrave, P.A. 1980. Site of attachment of 11-cis retinal in bovine rhodopsin. Biochemistry 19: 5111-5117.
Wang, S.Z., Adler, R. & Nathans, J. 1992. A visual pigment from chicken that resembles rhodopsin: amino acid sequence, gene structure, and functional expression. Biochemistry 31: 3309-3315.
Wilkie, S.E., Vissers, P.M.A.M., Das, D., DeGrip, W.J., Bowmaker, J.K. & Hunt, D.M. 1998. The molecular basis for UV vision in birds: spectral characteristics, cDNA sequence and retinal localization of the UV-sensitive visual pigment of the budgerigar (Melopsittacus undulatus). Biochemical Journal 330: 541-547.
Wortel, J.F., Wubbels, R.J. & Nuboer, J.F.W. 1984. Photopic spectral sensitivities of the red and the yellow field of the pigeon retina. Vision Research 24: 1107-1113.
Wright, A.A. 1972. The influence of ultraviolet radiation on the pigeon's color discrimination. Journal of the Experimental Analysis of Behavior 17: 325-327.
Wright, A.A. 1979. Color-vision psychophysics: a comparison of pigeon and human. In: Granda, A.M. & Maxwell, J.H. (eds) Neural Mechanisms of Behavior in the Pigeon; New York; Plenum: 89-127.
Yen, L. & Fager, R.S. 1984. Chromatographic resolution of the rod pigment from the four cone pigments of the chicken retina. Vision Research 24: 1555-1562.
Yokoyama, S., Radlwimmer, F.B. & Kawamura, S. 1998. Regeneration of ultraviolet pigments of vertebrates. Febs Letters 423: 155-158.
Yoshizawa, T. & Fukada, Y. 1993. Preparation and characterization of chicken rod and cone pigments. In: Hargrave, P.A. (ed.) Photoreceptor cells; San Diego; Academic Press: 161-179.Zhukovsky, E.A. & Oprian, D.D. 1989. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science 246: 928-930.
Table 1. Summary of avian visual pigments,
l max (nm) as determined from microspectrophotometry.
Fig. 1. Mean absorbance spectra of visual pigments from Chicken. Open symbols, before bleaching; filled symbols, after exposure to white light. (A) red-sensitive cones (both single and double cones), (B) green-sensitive single cones, (C) rods, (D) blue-sensitive single cones, (E) violet-sensitive single cones, (F) distribution histograms of the individual l max from all cones. The solid lines in (A) to (E) are visual pigment template curves with l max at 569,507,506, 453 and 418 nm respectively. (From Bowmaker
et al. 1997).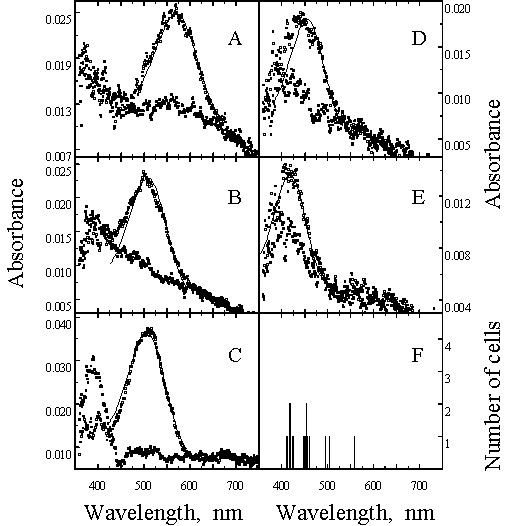
Fig. 2. Mean absorbance spectra of cone oil droplets from Chicken (Gg) and Budgerigar (Mu). Letters indicate droplet type. Red, Yellow, Clear and Transparent are all found in single cones, whereas Pale droplets are located in the Principal member of double cones. (From Bowmaker et al. 1997).
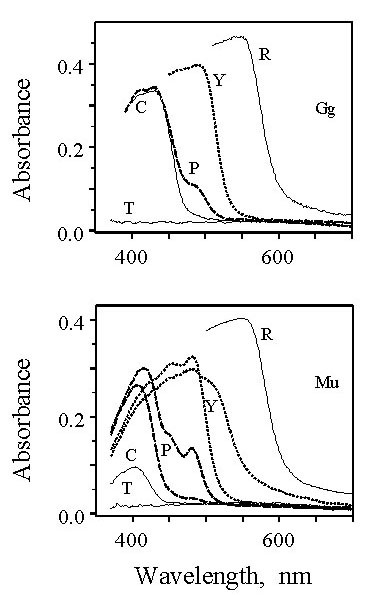
Fig. 3. Comparison of the behaviourally determined spectral sensitivity of Pekin Robin (A) and the effective spectral sensitivities of the four single cone types (B). The functions have been arbitrarily displaced on the log sensitivity axis and the curves in (B) are normalized to log 2. Visual pigment absorbance spectra were corrected for the filtering effects of the relevant oil droplets and for the absorbance of the ocular media (From Maier & Bowmaker 1993).
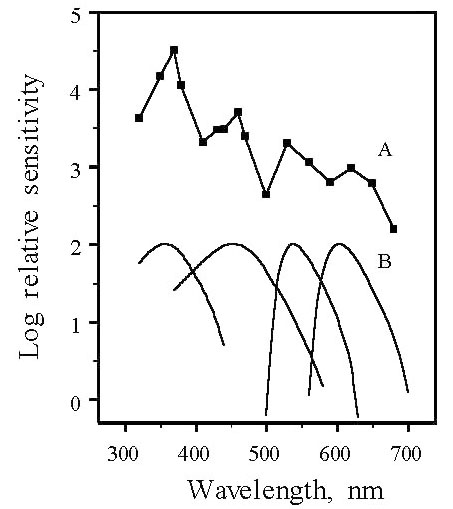
Fig. 4. Mean absorbance spectra of
visual pigments from Budgerigar. Open symbols, before bleaching; filled symbols, after
exposure to white light. (A) red-sensitive cones (both single and double cones), (B)
green-sensitive single cones, (C) rods, (D) blue-sensitive single cones, (E)
violet-sensitive single cones, (F) distribution histograms of the individual l max from all cones. The solid lines in (A) to (E) are
visual pigment template curves with l max at
564,509,509, 444 and 371 nm respectively. (From Bowmaker et al. 1997).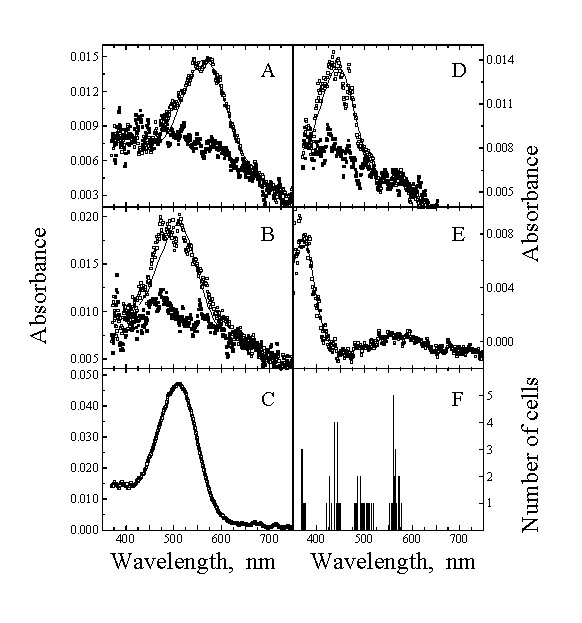
Fig. 5. Two-dimensional model of Budgerigar UV-sensitive opsin. Each a -helical region is shown as 26 residues in length, although only the central 18 are thought to be embedded in the membrane. The sites of the retinal Schiff’s base linkage (Lys-291) and the potential counterion (Glu-108) are highlighted in black. The disulphide linkage between Cys-105 and Cys-182 is shown, and the positions of the potential glycosolation, palmitoylation and phosphorylation sites are indicated. Potential UV spectral tuning sites are shaded. (From Wilkie et al. 1998).
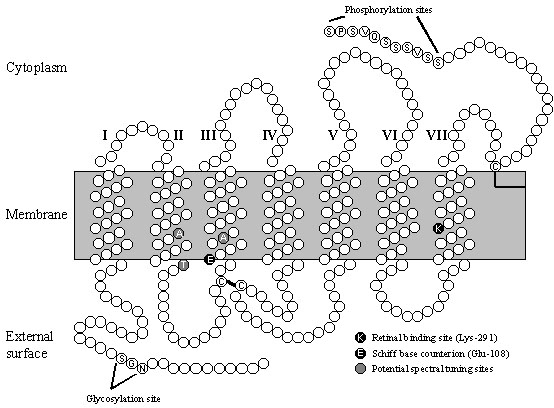
Fig. 6. Phylogenetic tree of vertebrate opsins. The tree was generated by the neighbour-joining method (Saitou and Nei 1987) from the frequency of substitutions between amino acid sequences of the opsins, using the Drosophila Rh1 opsin as an outgroup. The bootstrap confidence values based on 500 replicates are shown for each branch. The scale bar is calibrated in substitutions per site.