S45.2: Visual fields, foraging and binocularity in birds
Graham R. Martin1 & Gadi Katzir2
1 Schools of Biological Sciences & of Continuing Studies, The University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK, fax +44 121 414 5619, e-mail g.r.martin@bham.ac.uk; 2Department of Biology, University of Haifa, Oranim, 36006, Israel, e-mail gkatzir@research.haifa.ac.il
Martin, G.R. & Katzir, G. 1999. Visual fields, foraging and binocularity in birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2711-2728. Johannesburg: BirdLife South Africa.Interspecific differences in the size and optical structure of bird eyes have often been noted. Optical structure influences the eye’s visual field (that portion of space imaged upon the retina at any one moment) and the combination of two eyes result in marked interspecific differences in the topography of the total field about a bird’s head and in the size and position of the binocular field. Comparative data on visual fields in a range of birds which differ in their phylogeny and ecology, and in the size and optical structure of their eyes, are summarised. We propose that the visual fields of birds can be classified into three main types and hypothesise that the principal factor with which visual field topography is associated is the extent to which feeding involves visual guidance towards food items which may be taken directly in the bill or with the feet. We propose that binocularity in birds, perhaps with the exception of owls (Strigiformes), is not associated with stereopsis. We hypothesise that binocularity is best understood in terms of neural processes involved in the extraction of information from the visual flow field. Specifically, we suggest that the functional importance of binocular overlap lies not in the fact that each eye can image the same portion of the frontal field simultaneously, but in the fact that binocularity is the result of the monocular field of each eye projecting contralaterally. Such contralateral projections mean that each monocular field encompasses a point from which a linear optic flow field expands in a radially symmetrical fashion as an object is approached in forward motion. It is from this symettrically expanding flow field pattern that time to contact and the exact position of an object or surface can be determined.
INTRODUCTION
Interspecific differences in the size and optical structure of bird eyes have often been noted (Walls 1942; Polyak 1957; Martin 1985; Evans & Martin 1993). It is known that these differences in optical structure influence the quality, size, and brightness of the image produced upon the retina (Land 1981) and general functional explanations for these differences in terms of both ecology and behaviour have been advanced (Martin 1993; Martin 1994a).
The visual field an eye (that portion of space imaged upon the retina at any one moment) is also influenced by its optical structure and the position of the two eyes within the skull influences the total (cyclopean) visual field about an animal’s head. Thus, eye structure and position result in marked interspecific differences in the total visual field (Martin 1994a). Furthermore, the ways in which the visual fields of each eye overlap and produce a binocular portion within the total visual field also shows marked interspecific variation (Martin & Katzir 1995). Particular attention has be paid to the extent and position of binocular overlap and its possible function in both birds and mammals (Hughes 1977; McFadden 1993). These functional explanations have often been influenced by the assumption that binocular vision (two eyes viewing the same position in space) results in stereopsis (the perception of three dimensional space achieved through binocular vision and the detection by neural processes of the disparity in the images of the two eyes). However, while binocular vision is essential for stereopsis, the assumption that binocularity inevitably results in stereopsis has recently been questioned in birds (McFadden 1993; 1994; Davies & Green 1994).
Much of the discussion on the function of interspecific differences in visual field topography has, however, been limited by the paucity of data collected under comparable conditions in a range of species. We summarise here comparative data on visual fields and eye structure in a range of birds which differ in their phylogeny and ecology, and in the size and optical structure of their eyes. We elaborate the proposal that the visual fields of birds can be classified into three main types and hypothesise that the principal factor with which visual field topography is associated is the extent to which feeding involves visual guidance towards individual food items which may be taken directly in the bill or with the feet. There appears to be good evidence that binocular field size is not maximised in any bird, even in so-called frontal eyed species such as owls. Across a wide range of species there appears to be convergence upon a binocular field width of approximately 20° - 30° . This convergence may result from similarities in foraging behaviour and its sensory guidance.
We propose that the presence of binocularity in most birds is not associated with stereopsis and hypothesise that binocularity is best understood in terms of neural processes involved in the extraction of information from the visual flow field.
[Note: The taxonomic classification employed in this paper follows that of (Sibley & Monroe 1990) and common names of birds follow the recommendations of (British Ornithologists' Union 1992). Common name, scientific name, Family and Order for each species discussed are given in Table 1.]
METHODS
Published estimates of the extent of visual fields have been determined in two principal ways: 1. The visual projection of the pupil margins (e.g. Martinoya et al. 1981; Bischof 1988). 2. The visual projection of the retinal margins (e.g. Hughes 1977; Martin & Young 1983).The first method has the advantage that it is simple and can use direct observation or photographic techniques, while the second requires more elaborate, but still relatively simple, ophthalmoscopic techniques. Both techniques require the bird to be alert and with its head stabilised at the centre of a co-ordinate system.
The first technique is based upon the assumption that if it is possible to see the pupil ('seeing the pupil' is in fact looking through the lens into the posterior chamber) from a particular location about the head, the bird will be able to see the viewer, i.e. the viewer is within the bird’s visual field. However, more detailed analysis using the ophthalmoscopic technique shows that this assumption can lead to significant errors especially as regards over estimates of the size of the frontal binocular field. Two separate definitions of visual fields are required:
(i) Optical Field; the angular extent of space which is imaged by the eye’s optical system. It is defined operationally by the angular limit from which the pupil can be seen.
(ii) Retinal Field; that portion of the optical field which is served by the retina. This is the visually functional field and defines the segment of space in which stimuli must lie if they are to be seen by the bird and are thus capable of eliciting a behavioural response.
In all bird species investigated to date the difference between the boundaries of the optical and retinal fields is found to be quite large especially in the frontal portion of the monocular visual field where it may exceed 20°. However, in other portions of the monocular fields, especially to the rear of the head, the boundaries of the two fields typically coincide. This means that estimating visual fields, especially the frontal binocular field by reference to the view of the pupil in the region of the bill, can be quite erroneous and can lead to over estimates of more than two-fold in the extent of the binocular field. For example, in Tawny Owls Strix aluco the functional (retinal) field = 47° while the apparent (optical) field = 110° ; in Short-toed Eagles Circaetus gallicus retinal field = 20° while the optical field = 42° ( Fig. 1). Put simply, appearances can be deceptive. Just because a bird appears to have a large binocular field (by virtue of the observer being able to see the pupils of both eyes simultaneously) this may not be the case. In fact, it has been observed, for example in herons Ardeidae, owl Strigidae, and eagle Accipitridae, (Martin 1984; Martin & Katzir 1994b; Martin & Katzir in press) that at certain elevations the pupils can be seen simultaneously in both eyes, and therefore the bird appears to have binocular vision, but there is no functional visual field in that region.
Details of the procedures employing the ophthalmoscopic reflex technique to measure visual fields (both optical and retinal) and how they may be altered by eye movements, have been published a number of times (e.g. Martin 1994b; Martin & Katzir 1994a; Katzir & Martin 1998). It is necessary to distinguish between the following subdivisions or components of the total visual field. Monocular Field, the visual field of a single eye; Binocular Field, the area where monocular fields overlap; Cyclopean Field, the total visual field produced by the combination of both monocular fields; Projection of the Optic Axes, the directions of the approximate axis of symmetry of the optical components of each eye; Divergence of the Optic Axes, the angular separation of the optic axes. The size of an eye is indicated by its axial length (cornea - retina) and the separation of the eyes in the skull by the separation between the nodal points of the two eyes.
In some species it has been possible to model the optical structure of the eye and calculate the position of the limits of the optical field relative to the optic axis of the eye. There is generally good agreement between the measured and calculated widths of the optical fields e.g. in Tawny Owl (Martin 1984), European Starling Sturnus vulgaris (Martin 1986a).
RESULTS
Table 1 summarises visual field parameters in bird species investigated using the same ophthalmoscopic reflex technique. This use of common procedures should give greater validity to interspecific comparisons and to general principles which emerge from them. The species in which measurements have been made vary considerably in their general ecology and feeding behaviour as well as in their phylogeny. They also differ markedly in overall body size and eye size. All data refer to binocular and cyclopean field widths in an approximately horizontal plane when the head and eyes have adopted what appears to be their characteristic, or resting, position. These were determined from photographs of unrestrained birds in a posture considered typical for the species and which defined bill position relative to the horizontal. The accompanying diagrams of visual fields (Fig. 2, Fig 3, Fig 4 and Fig 5) depict the birds’ heads in these typical positions.
In some species eye movement amplitude has been determined by observing the change in position of the limits of the retinal field at each elevation when eye movements are induced by simple non-invasive means (see Martin & Katzir (1994a) for methods) . However, eye movements are not simply translational in any particular plane but involve complex rotations of the eye with the result that eye movement amplitude varies as a function of elevation. In some species eye movements are apparently absent, or are at least not readily elicited by the techniques employed (e.g. Stone-curlew Burhinus oedicnemus (Martin & Katzir 1994b), Tawny Owl (Martin 1984), Short-toed Eagle Circaetus gallicus (Martin & Katzir in press)) while in other species eye movements of relatively large amplitude occur spontaneously (e.g. European Starling (Martin 1986a), herons Ardeidae (Martin & Katzir 1994a, Katzir & Martin 1998). Where eye movements are present, the largest translations in the position of the field margins occur in the region of the planes containing the optic axes and the bill, the smallest movements are to the rear of the head. The result is that the position of the margins of the visual field behind the head are little, if at all, altered by eye movements. However, large changes to the degree of binocular overlap in front of the head can occur. In some species eye movements can result in the spontaneous abolition of binocularity e.g. European Starlings (Martin 1986a) and herons (Martin & Katzir 1994a; Katzir & Martin 1998).
DISCUSSION
Aspects of the particular relationships between eye structure and visual fields have been discussed in the individual papers from which data has been extracted to construct Table 1. We concentrate here on more general findings which this comparative approach can reveal.
Visual Field Topography
The data summarised in Table 1 supports the hypothesis that the visual field topographies of birds fall into three distinctive types (Martin 1994b). Examples of these are shown in Figs 2 - 5. Table 1 shows that these visual field types appear to be independent of eye size, the separation of the eyes within the skull, body size, and phylogeny. We hypothesise that these visual field types are convergent features associated with feeding behaviour, in particular the extent to which visual cues are used to detect and procure individual food items. We characterise these field types by reference to the principal cue used to guide the detection of food items and the method by which items are procured.
Type 1. Visual guidance to food items taken in the bill or feet.
This topography is typified by an extensive cyclopean field giving near comprehensive coverage of the frontal and dorsal regions about the head and a vertically long but relatively narrow binocular field. The visual projection of the bill-tip falls either at the centre, or a little below the centre, of the binocular region. This type of topography is found in species which forage in water, in air and through the air-water interface. It occurs irrespective of body size, eye size or the separation of the eyes within the skull and is found across a range of avian families of quite different phylogeny. Examples described to date are drawn principally from species which take prey directly in the bill although there is one example of a predator whose initial prey strike is usually with the feet.
The key behavioural-sensory feature of these birds’ foraging appears to be the need to place the bill or feet precisely with respect to food items whose location is determined and then rapidly approached using exclusively visual cues. Table 1 indicates that this topography occurs in birds which: (1) peck at individual, mainly immobile, items such as seeds, fruit and vegetation (e.g. Rock Pigeon Columba livia); (2) peck at a mixture of immobile objects and smaller terrestrial invertebrates (e.g. European Starling); (3) peck at a mixture of immobile food items and more evasive, mainly terrestrial, invertebrates (e.g. Stone-curlew, Ostrich Struthio camelus); (iv) take evasive and sometimes highly mobile prey directly in the bill (e.g. Cattle Egret, Reef Heron, Squacco Heron, Night Heron, Manx Shearwater Puffinus puffinus, Humboldt Penguin Spheniscus humboldti, King Penguin Aptenodytes patagonicus, Black-browed and Grey-headed Albatrosses Diomedea chrysostoma , D. melanophris);or in the feet, typically after a short approach flight (e.g. Short-toed Eagle). While the diets and feeding techniques of all of these species are known to varying degree, experimental evidence that their foraging is guided by vision is available only for Rock Pigeons (Zeigler et al. 1993) and Herons (Katzir 1993; Katzir & Intrator, 1987).
Within this visual field type there is variation, particularly in the extent of the blind area above and to the rear of the head and in the vertical extent of the frontal binocular field. However, there does appear to be convergence in the maximum width of the binocular field which occurs approximately in the plane of the bill and equals between 20° - 30° . Vertically, the longest binocular field is that of the herons which extends through 180° , while in a range of species including Ostrich, Stone-curlew, Short-toed Eagle, and Albatrosses the maximum binocular field extends vertically through approximately 80° . In many species the binocular field is made smaller, and may even be abolished, by eye movements. However, this does not result in a significant decrease in the size of the blind area above and to the rear of the head (e.g. herons, albatrosses, Rock Pigeon, European Starling).
The extreme (180° ) vertical extent of the binocular field in herons has been interpreted as a feature which permits these birds to monitor the position of potential prey in a wide area below them while holding the head immobile (Martin & Katzir 1994a). In these birds a head or body movement could trigger an escape response from their highly evasive prey. These birds generally wait motionless until prey enters a zone in which capture by a single bill-strike is possible (Hancock & Kushlan 1984; Voisin 1991; Katzir 1993; Katzir & Martin 1994).
In amphibious species entry into water results in the reduction of the visual fields due to the loss of the refractive power of the cornea. In the amphibiously foraging penguins and albatrosses the binocular field is close to 30° rather than the 20° found in terrestrially foraging species, and his may be associated with the reduction of the binocular field upon immersion. In these species it has been shown that immersion of the eye will result in the near abolition of the frontal binocular field. However, the bill remains centrally placed within this reduced field (Martin & Young 1984; Martin 1998).
Type 2. Non-visual guidance to food items taken in the bill
This visual field topography is typified by comprehensive visual coverage of the hemisphere above the head while the bill lies outside, or on the very periphery, of the visual field. The binocular field is narrow (< 10° ) and extends from just above the bill and close to the horizontal, through approximately 180° to the horizontal behind the head. Such fields have been described in detail in only two species, Eurasian Woodcock Scolopax rusticola and Mallard Anas platyrhynchos but has been observed (personal observation) in one other species of duck, Northern Shoveler A. clypeata. In these species, foraging appears primarily to be mediated by tactile and chemical cues from the bill and tongue and can take place naturally under conditions of low light levels and in the laboratory in the absence of visual cues (Berkhoudt 1985; Gerritsen & Sevenster 1985; Gottschaldt 1985; Martin 1990). The food items of these birds are generally not evasive and are not approached rapidly. These birds may probe into soft substrates for buried invertebrates or search in turbid waters for vegetable matter and invertebrates. Food items are ingested without visual inspection. The bill at the periphery of the visual field would appear to be sufficient to place it accurately with respect to surfaces but this topography is perhaps not sufficient for accurate control of bill position in rapid pecking. Apparently freed from the constraint of placing the bill within the binocular field, the eyes of these birds are placed high within the skull and thus provide comprehensive visual coverage of the space about the bird’s head.
Type 3. Non-visual guidance to food items taken in the feet.
This visual field topography is typified by a relatively large blind area to the rear of the head and a relatively broad (maximum width 48° ) but vertically small (80° ) binocular field. The projection of the bill falls outside of the visual field at the lower edge of the binocular field. A visual field with this overall topography has been described in detail in only one species, Tawny Owl. However, such a field topography may be typical of other species of owl from both the Strigidae and the Tytonidae. It has been argued that this particular visual field topography may be associated with three main factors (Martin 1990): (i) The acoustic location of prey. This is a characteristic feature of many species of Strigiformes, it being demonstrated that in a number of owls acoustic cues alone are sufficient for successful foraging under both natural and laboratory conditions (see (Martin 1990) for a review of evidence). Also, the region of highest accuracy in auditory localisation lies in front of the head in the region of the binocular field (see Volman (1994) for a review). (ii) The taking of prey items in the feet. Just prior to prey capture the feet are swung up into the region of binocular vision. (iii) Although these birds take evasive prey they rely upon stealth (slow and silent flight) rather than rapid approach when taking items. Thus, these birds may not be subject to the same requirements, as seen in species with Type 1 fields, for precise control of feet or bill position during rapid manoeuvring or pecking.
It seems that neither nocturnal foraging nor the taking of prey in the feet are of themselves associated with the broader binocular field of owls. Thus, the mainly nocturnally foraging Black-crowned Night Heron Nycticorax nycticorax has a Type 1 visual field topography typical of that found in species which use visual guidance to food items taken in the bill (Katzir & Martin 1998) and Eurasian Woodcocks, which regularly feed throughout the night, have the Type 2 fields associated with non-visual guidance to food items taken in the bill (Martin 1994b). Also, Short-toed Eagles, which take prey in their feet after a rapid approach (Cramp & Simmons 1980), have the Type 1 binocular field.
The Function of Binocular Vision in Birds
Because of its assumed importance for the perception of the relative distances of objects through the mechanism of stereopsis, the extent and position of the area of binocular overlap has often been stressed in discussions of comparative visual anatomy and physiology (e.g. Walls 1942; Polyak 1957; Tansley 1965; Hughes 1977; Martinoya et al. 1981; Bischof 1988; Casini et al. 1993). As well as being based upon assumptions about stereopsis these discussions have often been based upon assumed, rather than measured, binocular field sizes.
In birds, although binocularly driven neurons (assumed to be the substrate for stereopsis) have been described in the telencephalon of owls (Pettigrew 1979), evidence for stereoscopic vision using behavioural tests, such as random dot stereograms, is lacking. Recent reviewers have suggested that stereopsis, if present at all, may not be a general property of avian visual systems (McFadden 1993) (Davies & Green 1994). Even though binocularly driven neurons have been identified in the accessory optic system of Rock Pigeons (Wylie & Frost 1990; Frost et al. 1994; Wylie et al. 1998)), the two receptive fields of each binocular neuron are quite separate, lying up to 180 ° apart in monocular fields on opposite sides of the head, and are clearly not associated with stereopsis. These neurons respond to different patterns of optic flow field information which result from particular types of translational and self-motion.
While the presence of stereopsis has yet to be demonstrated behaviourally in birds, even the utility of binocular vision is not fully established. For example, in Rock Pigeons, pecking behaviour is not diminished in accuracy by monocular compared with binocular viewing (Jäger & Zeigler 1991). Furthermore, in many bird species, eye movements are non-conjugate and can result in the spontaneous abolition of binocularity (Wallman & Pettigrew 1985; Martin & Katzir 1994a). Thus, both binocular fusion, regarded as an essential prerequisite for stereopsis, and the presence of a fixed horopter, which can provide depth cues without binocular fusion [as in amphibia (Collett 1977; Rossel 1983)], would seem to be unavailable to many birds. Furthermore, stereopsis as a means of estimating depth is relatively slow. (Davies & Green 1994) concluded that too much time is required to reach a sufficient level of accuracy in estimating depth through stereopsis for it to guide rapid gross bodily movements in birds, especially during critical rapid flight manoeuvres such as landing. Thus it has been proposed that multiple cues to depth and distance from monocular vision, rather than cues specifically from binocular vision, may be of particular importance in the control of motor movements in birds (Davies & Green 1994; McFadden 1994)
.The above discussion would seem to confirm that a general functional hypothesis for binocularity in birds cannot refer to stereopsis. As noted above there is a marked topographical similarity, and relatively small extent, in the frontal binocular fields of a range of bird species which differ in their phylogeny and ecology but who apparently employ visual guidance to take food items in the bill or feet. This comparative base permits the examination of some general questions concerning the function of binocular vision in birds and the proposal of an alternative functional hypothesis.
Binocularity and optic flow fields
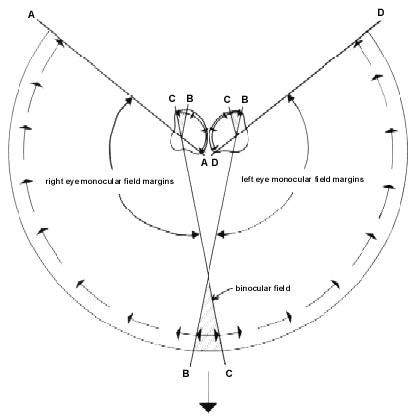
The functional importance of binocular overlap may lie not in the fact that each eye can image the same portion of the frontal field simultaneously, but in the fact that binocularity is the result of the monocular field of each eye projecting contralaterally. Such contralateral projections mean that each monocular field encompasses a point from which a linear optic flow field expands in a radially symmetrical fashion as an object is approached in forward motion.
Information extracted from symmetrical optic flow fields has been considered particularly important for the control of locomotion in birds, mammals and invertebrates. This information includes accurate determination of the point towards which an animal, or its head, is travelling, and the time to contact that point (Lee 1980; Davies & Green 1994; Lee 1994). Both of these need to be determined rapidly and with high precision in birds since they typically move their heads (as in pecking), bodies (as in approaching objects in flight), or both (as in or striking at prey with the feet) at high speed towards hard surfaces and objects which may themselves be moving. The symmetrical pattern of the optic flow field surrounding the point towards which an animal, or its head, is moving, produces a high degree of redundant information which can serve to increase the accuracy of the derived parameter tau (t ) which specifies time to contact and from which spatial information can also be derived (Lee 1994). Such flow-field variables have been shown to control landing behaviour in some, but not all, bird species (Davies & Green 1994). Also, neurons which respond specifically to symmetrically expanding images have been detected in the nucleus rotundus of the Rock Pigeon brain (Frost et al. 1994) and it has been suggested that these equate functionally with similar neurons thought to control self-motion in flying invertebrates (Krapp & Hengstenberg 1996; Srinivasan 1996).
The optic flow field pattern in the eyes of a bird with laterally placed eyes and a small frontal binocular field, and whose head is moving in the direction of its bill, is shown diagrammatically in Figure 6. It is clear that it is only in the temporal periphery portion of each retina, the section which subserves binocular vision, that a symmetrically expanding linear optic flow field can occur. Across the rest of the retina, which provides only monocular vision, the flow pattern, although expanding from the direction of the point of contact, does not possess radial symmetry.
Thus in normal forward motion of the head the visual field of an eye must extend contrallaterally in order to contain a centre of expansion of the optic flow field which encompasses the object or surface towards which the animal’s head is moving. For movement towards a relatively distant target, the disparity of viewpoint of two eyes with overlapping contralateral fields will be negligible. This will result in the two eyes receiving identical optical flow field information and this may of itself enhance accuracy through redundancy (as, for example, in humans where two eyes are superior to one eye even when binocular viewing is devoid of stereoscopic cues (Jones & Lee 1981). Furthermore, it has been argued that for close objects, movement with respect to a target (as in pecking or bill striking) is precisely specified by the fact that the optic image of the target can be symettrically positioned with respect to the centres of expansion of each flow field (Lee et al. 1991).
Thus, we propose that the essential function of binocularity in birds may lie simply in the provision in each eye of an optic flow field which expands symmetrically about a target point in front of the head. Two eyes with identical or similar flow fields may increase, through redundancy, the accuracy of information with respect to that target, but they are unlikely to provide higher order information which can be extracted exclusively from two eyes imaging the same portion of a scene. Why the maximum width of the binocular field in a range of bird species equals only approximately 20° , or perhaps a more pertinent description is that the radius of the radially symmetrical proportion of the optic flow field equals approximately 10° , is unclear. We would suggest that it represents an optimal width which provides sufficient optic flow field information to ensure accurately controlled rapid approaches towards objects during foraging, while at the same time maximising the width of the peripheral, and hence cyclopean, visual field within constrains imposed by each eye’s optical design.
Binocularity in owls
The function of binocularity in owls is, however, unclear. These birds apparently do posses a neural substrate for stereopsis (Pettigrew 1979) although stereopsis has not been demonstrated behaviourally. Their binocular field width is approximately twice that of other birds. Many owl species hunt habitually at night when they may rely exclusively on auditory cues, coupled with detailed knowledge of their habitat, for the detection and capture of prey (see Martin (1986b) for review). They are capable of slow, silent flight and rely upon stealth, rather than rapid approach, to secure prey (Mikkola 1983). The increased binocular field width in these birds may reflect the decreased density of the optic elements which are potentially available in the flow field at low light levels (Martin 1984). Such a decrease in optical elements is a direct consequence of the physical limitations on the sampling any image as light levels fall (Snyder et al. 1977). Detecting fewer elements over a wider field of view may serve to provide equally accurate extraction of flow field information as is available from a smaller portion of field at higher densities of optic elements (which would be available at daytime light levels).
Binocularity and flight
That the 20° wide binocular field is primarily concerned with flow field information required for the accurate and rapid positioning of the bill or feet with respect to objects when foraging, rather than with the control of body position during flight or when landing, is suggested by the visual field topography of Mallards and Woodcocks. In these birds the frontal portion of the binocular field (facing the direction of movement during flight) is less than 10° (Table 1) but must be sufficient to control flight and landing. However, to develop this analysis further would require analysis of flight and landing speeds in relation to the type of obstacles which each species encounters, especially during landing.
Binocularity and eye movements
It has been shown that in some species, eye movements, which are usually non-conjugate (Wallman & Pettigrew 1985; Martin & Katzir 1994a), can lead to the spontaneous abolition of the binocular field. This abolition of binocularity is not associated with an increase in the extent of the peripheral visual field (and hence total cyclopean field). It seems more likely therefore to be associated with altering the direction of gaze associated with a particular retinal feature such as area, fovea or region of specialised colour vision (Nalbach et al. 1993). Retinal topography in birds is complex, often including more than one fovea (Meyer 1977; Martin 1985), and some birds apparently switch between these during foraging (Pettigrew & Moroney, 19xx). Eye movements which result in the abolition of binocularity may simply result from such switching and have no particular function as regards the alteration of visual fields.
CONCLUSION
The above discussions have clearly highlighted a number of factors which need to be considered in providing functional interpretations of visual field topography in birds. Three principal types of visual field topography have been identified and a number of hypotheses have been framed concerning their relation to feeding behaviour and the sensory cues which are employed. A general hypothesis concerning binocular vision and its role in the extraction of optical flow field information to control the accurate position of the bill, and possibly the feet, during foraging behaviour, has been proposed. Clearly, to test these hypotheses requires both a broader comparative base and more detailed analyses of the feeding and flight behaviour of the same species in both natural and laboratory situations. Of particular importance would be comparative data on visual fields in species which employ other distinctive types of foraging behaviour. These include the trawling of prey from the airspace (e.g. Hirundinidae and Caprimulgidae) or from a water surface (e.g. Rynchopidae, and some species of Procellariidae), carrion feeding which does not rely upon rapid approach to prey (e.g. Cathartidae), and feeding using precise positioning of the bill in the absence of pecking (e.g. Trochilidae).
REFERENCES
Berkhoudt, H. 1985. Structure and function of avian taste receptors. In: King, A.S. & McLelland, J. (eds) Form and function in birds. London; Academic Press: 463-496.
Bischof, H.J. 1988. The visual field and visually guided behavior in the zebra finch (Taeniopygia guttata). Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology 163:329-337.
British Ornithologists' Union 1992. Checklist of birds of Britain and Ireland. Tring, Herts., U.K.; British Ornithologists' Union.
Casini, G., Fontanesi, G. & Bagnoli, P. 1993. Binocular processing in frontal-eyed birds. In: Zeigler, H.P. & Bischof, H.-J. (eds) Vision, brain, and behavior in birds. Cambridge, Massachusetts; MIT Press: 159-171.
Collett, T. 1977. Stereopsis in toads. Nature 267:349-351.
Cramp, S. & Simmons, K.E.L. 1980. Handbook of the birds of Europe, the Middle East and North Africa. The birds of the western Palearctic. Vol. II; Oxford, London and New York; Oxford University Press.
Davies, M.N.O. & Green, P.R. 1994. Multiple sources of depth information: An ecological approach. In: Davies, M.N.O. & Green, P.R. (eds) Perception and motor control in birds: an ecological approach. Berlin; Springer-Verlag: 339-356.
Evans, H.E. & Martin, G.R. 1993. Organa sensuum. Baumel, J.J. (ed) Handbook of avian anatomy: nomina anatomica avium. Cambridge, Massachusetts; Nuttall Ornithological Club: 585-611.
Frost, B.J., Wylie, D.R. & Wang, Y.C. 1994. The analysis of motion in the visual systems of birds. In: Davies, M.N.O. & Green, P.R. (eds) Perception and motor control in birds: an ecological approach. Berlin; Springer-Verlag: 248-269.
Gerritsen, A.F.C. & Sevenster, J.G. 1985. Foraging behaviour and bill anatomy in sandpipers. Fortschritte der Zoologie 30:237-240.
Gottschaldt, K.M. 1985. Structure and function of avian somatosensory receptors. In: King, A.S. & McLelland, J. (eds) Form and function in birds. Vol. 3; London; Academic Press: 375-461.
Hancock, J. & Kushlan, J. 1984. The herons handbook. London; Croom Helm.
Hughes, A. 1977. The topography of vision in mammals of contrasting life style: comparative optics and retinal organization. In: Crescitelli, F. (ed) Handbook of sensory physiology. Vol. VII/5; Berlin; Springer-Verlag: 613-756.
Jäger, R. & Zeigler, H.P. 1991. Visual field organization and peck localization in the pigeon (Columba livia). Behavioural Brain Research 45:65-70.
Jones, R.K. & Lee, D.N. 1981. Why two eyes are better than one: The two views of binocular vision. Journal of Experimental Psychology: Human Perception and Performance 7:30-40.
Katzir, G. 1993. Visual mechanisms of prey capture in water birds. In: Zeigler, H.P. & Bischof, H.-J. (eds) Vision, brain and behavior in birds. Cambridge, Mass; MIT Press: 301-315.
Katzir, G. & Intrator, N. 1987. Striking of underwater prey by reef herons, Egretta gularis schistacea. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology 160:517-523.
Katzir, G. & Martin, G.R. 1994. Visual fields in herons (Ardeidae) - panoramic vision beneath the bill. Naturwissenschaften 81:182-184.
Katzir, G. & Martin, G.R. 1998. Visual fields in the Black-crowned Night Heron Nycticorax nycticorax: nocturnality does not result in owl-like features. Ibis 140:157-162.
Krapp, H.G. & Hengstenberg, R. 1996. Estimation of self-motion by optic flow processing in single visual interneurons. Nature 384: 463-466.
Land, M.F. 1981. Optics and vision in invertebrates. in: Autrum, H. (ed) Handbook of sensory physiology. Vol. VII/6B; Berlin; Springer: 471-592.
Lee, D.N. 1980. The optic flow field: the foundation of vision. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 290:169-179.
Lee, D.N. 1994. An eye or ear for flying. In: Davies, M.N.O. & Green, P.R. (eds) Perception and motor control in birds: an ecological approach. Berlin; Springer-Verlag: 270-291.
Lee, D.N., Reddish, P.E. & Rand, D.T. 1991. Aerial docking by Hummingbirds. Naturwissenschaften 78:526-527.
Martin, G.R. 1984. The visual fields of the tawny owl, Strix aluco L. Vision Research 24:1739-1751.
Martin, G.R. 1985. Eye. In: King, A.S. & McLelland, J. (eds) Form and function in birds. Vol. 3; London; Academic Press: 311-373.
Martin, G.R. 1986a. The eye of a passeriform bird, the European starling (Sturnus vulgaris): eye movement amplitude, visual fields and schematic optics. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology 159:545-557.
Martin, G.R. 1986b. Sensory capacities and the nocturnal habit of owls (Strigiformes). Ibis 128:266-277.
Martin, G.R. 1986c. Total panoramic vision in the mallard duck, Anas platyrhynchos. Vision Research 26:1303-1306.
Martin, G.R. 1990. Birds by night. London; T & A D Poyser.
Martin, G.R. 1993. Producing the image. In: Zeigler, H.P. & Bischof, H.-J. (eds) Vision, brain, and behavior in birds. Cambridge, Massachusetts; MIT Press: 5-23.
Martin, G.R. 1994a. Form and function in the optical structure of bird eyes. in: Davies, M.N.O. & Green, P.R. (eds) Perception and motor control in birds: an ecological approach. Berlin; Springer: 5-34.
Martin, G.R. 1994b. Visual fields in woodcocks Scolopax rusticola (Scolopacidae; Charadriiformes). Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology 174:787-793.
Martin, G.R. 1998. Eye structure and amphibious foraging in albatrosses . Proceedings of the Royal Society of London. Series B: Biological Sciences 265:1-7.
Martin, G. R. in press. Eye structure and foraging in King Penguins Aptenodytes patagonicus. Ibis
Martin, G.R. & Brooke, M.D.L. 1991. The eye of a procellariiform seabird, the Manx shearwater, Puffinus puffinus: visual fields and optical structure. Brain, Behavior and Evolution 37:65-78.
Martin, G.R. & Katzir, G. 1994a. Visual fields and eye movements in herons (Ardeidae). Brain, Behavior And Evolution 44:74-85.
Martin, G.R. & Katzir, G. 1994b. Visual fields in the stone curlew Burhinus oedicnemus. Ibis 136:448-453.
Martin, G.R. & Katzir, G. 1995. Visual fields in ostriches. Nature 374:19-20.
Martin, G.R. & Katzir, G. in press. Visual field in Short-toed eagles Circaetus gallicus and the function of binocularity in birds. Brain, Behavior and Evolution
Martin, G.R. & Young, S.R. 1983. The retinal binocular field of the pigeon (Columba livia): English racing homer. Vision Research 23:911-915.
Martin, G.R. & Young, S.R. 1984. The eye of the Humboldt Penguin, Spheniscus humboldti: visual fields and schematic optics. Proceedings of the Royal Society of London. Series B: Biological Sciences 223:197-222.
Martinoya, C., Rey, J. & Bloch, S. 1981. Limits of the pigeon's binocular field and the direction for best binocular viewing. Vision Research 21:1197-1200.
McFadden, S.A. 1993. Constructing the three-dimensional image. In: Zeigler, H.P. & Bischof, H.-J. (eds) Vision, brain and behavior in birds. Cambridge, Massachusetts; MIT Press: 47-61.
McFadden, S.A. 1994. Binocular depth perception. In: Davies, M.N.O. & Green, P.R. (eds) Perception and motor control in birds: an ecological approach. Berlin; Springer-Verlag: 54-73.
Meyer, D.B. 1977. The avian eye and its adaptations. In: Crescitelli, F. (ed) Handbook of sensory physiology. Vol. VII/5; Berlin; Springer:
Mikkola, H. 1983. Owls of Europe. Calton; T & A D Poyser.
Moroney, M.K. & Pettigrew, J.D. 1987. Some observations on the visual optics of kingfishers (Aves, Coraciformes, Alcedinidae). Journal of Comparative Physiology. A Sensory, Neural, and Behavioral Physiology 160:137-149.
Nalbach, H.O., Wolf-Oberhollenzer, F. & Remy, M. 1993. Exploring the image. In: Zeigler, H.P. & Bischof, H.-J. (ed) Vision, brain and behavior in birds. Cambridge, Mass; MIT Press: 25-46.
Pettigrew, J.D. 1979. Binocular visual processing in the owl's telencephalon. Proceedings of the Royal Society of London. Series B: Biological Sciences 204:435-454.
Polyak, S. 1957. The vertebrate visual system. Chicago; University of Chicago.
Rossel, S. 1983. Binocular stereopsis in an insect. Nature 302:821-822.
Sibley, C.G. & Monroe, B.L. 1990. Distribution and taxonomy of birds of the world. New Haven and London; Yale University Press.
Snyder, A.W., Laughlin, S.B. & Stavenga, D.G. 1977. Information capacity of eyes. Vision Research 17:1163-1175.
Srinivasan, M.V. 1996. Flies go with the flow. Nature 384:411.
Tansley, K. 1965. Vision in vertebrates. London; Chapman Hall.
Voisin, C. 1991. The herons of Europe. London; T & A D Poyser.
Volman, S.F. 1994. Directional hearing in owls :neurobiology, behaviour and evolution. In: Davies, M.N.O. & Green, P.R. (eds) Perception and motor control in birds: an ecological approach. Berlin; Springer-Verlag: 292-314.
Wallman, J. & Pettigrew, J.D. 1985. Conjugate and disjunctive saccades in two avian species with contrasting oculomotor strategies. Journal of Neuroscience 5:1418-1428.
Walls, G.L. 1942. The vertebrate eye and its adaptive radiation. Michigan; Cranbrook Institute of Science.
Wylie, D.R.W., Bischof, W.F. & Frost, B.J. 1998. Common reference frame for neural coding of translational and rotational optic flow. Nature 392:278-282.
Wylie, D.R. & Frost, B.J. 1990. Binocular neurons in the nucleus of the basal optic root (nBOR) of the pigeon are selective for either translational or rotational visual flow. Visual Neuroscience 5:489-495.
Zeigler, H.P., Jager, R. & Palacios, A.G. 1993. Sensorimotor mechanisms and pecking in the pigeon. In: Zeigler, H.P. & Bischof, H.-J. (eds) Vision, brain and behavior in birds. Cambridge, Massachusetts; MIT Press: 265-283.
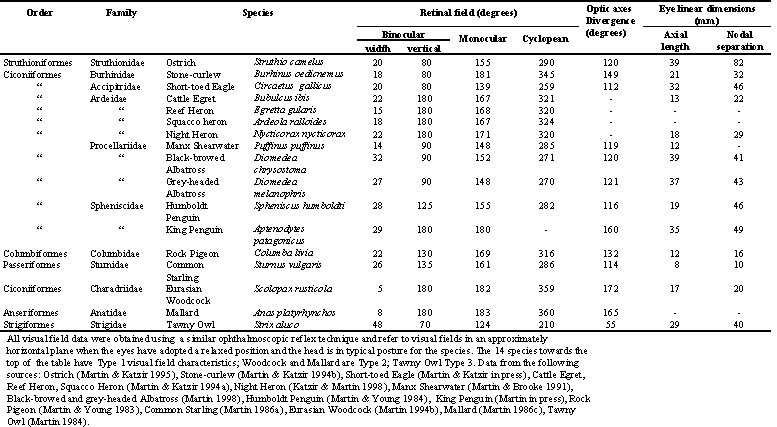
Table 1. Retinal visual fields and eye size in 17 bird species

Fig. 1. Schematic horizontal section through the eyes, skull and visual fields of a bird (based upon data for Short-toed Eagle). The diagram depicts the relationship between the retinal and optical field margins of each eye and how they combine to give the functional binocular (retinal) and apparent binocular (optical) fields and the blind area to the rear of the head. Note that to the rear of the head the optical and retinal field margins coincide.
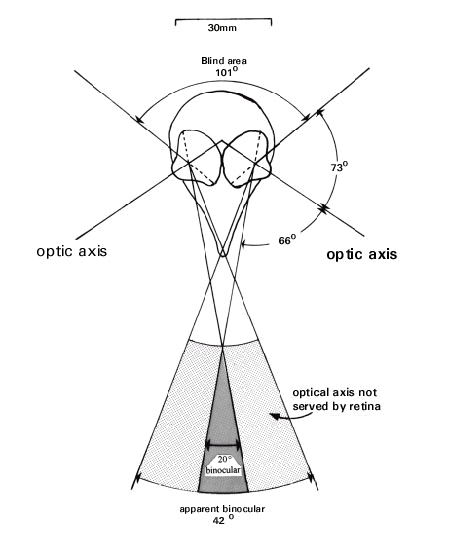
Fig. 2. Type 1 visual fields in Cattle Egret and Black-crowned Night Heron. Diagrams shows the region of maximum binocular overlap and the extent of the optical fields not served by the retinae (blind optical field) as a function of elevation when the eyes have adopted their maximally forward position (maximum binocular overlap). The width of the 'apparent binocular field' is defined by the outer limits of the optical fields not served by the retina. The visual projections of the pectens and of the line of the bill are also indicated. The diagram uses an orthographic projection based upon a conventional latitude and longitude co-ordinate system with the equator aligned vertically in the median sagittal plane of the bird. The birds’ heads are shown in the correct orientation for the co-ordinate system. It should be imagined that each bird’s head is positioned at the centre of the sphere with the features of the visual field projected onto its surface. Grid at 20° intervals.
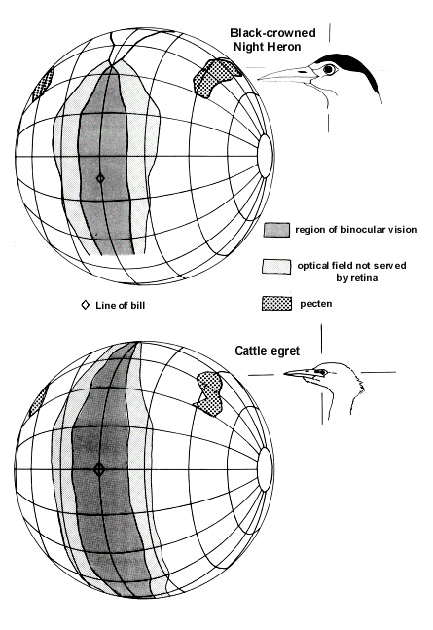
Fig. 3. Type 1 visual field in Short-toed Eagle (conventions as for Fig. 2).
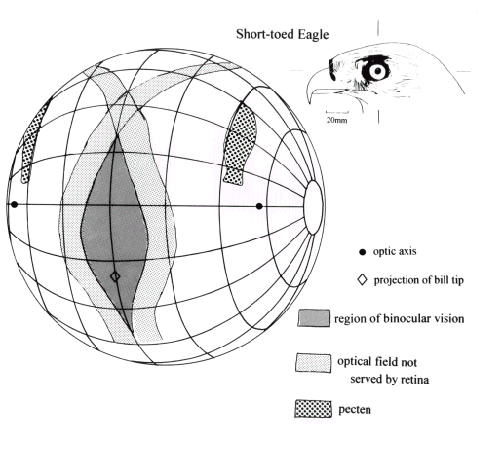
Fig. 4. Type 2 visual field in Eurasian Woodcock (conventions as for Fig. 2).
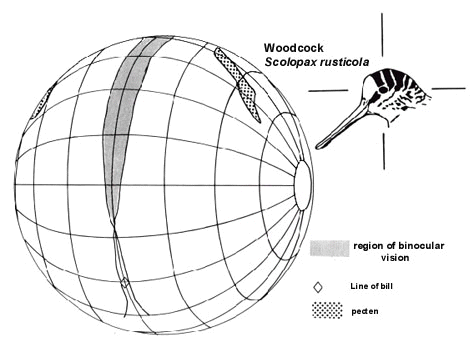
Fig. 5. Type 3 visual field in Tawny Owl (conventions as for Fig. 2).
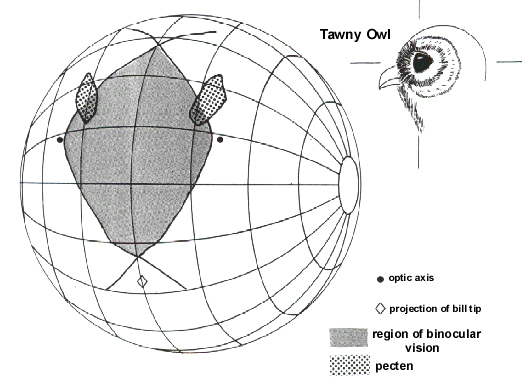
Fig. 6. Schematic horizontal section through the retinal visual fields of a bird. A, B, C, D are points within the cyclopean field and their projections onto the retina of each eye are indicated by similar letters. The large arrow at the bottom of the diagram indicates the direction of forward movement of the bird’s head. The arrows around the edge of the cyclopean field indicate schematically the directions in which elements in the optic flow field appear to travel as the head moves forward. The directions of movement across the retina of the images of these element’s are indicated by arrows within each eye. Indicated by hatching is the section in each retina which corresponds to the binocular field. In these sections the images of elements in the optic flow field travel in both directions away from the image of the point towards which the head is moving.