S45.1: Vision and nocturnal activities in wading birds and shorebirds
Raymond McNeil1, Luz Marina Rojas1, Thérèse Cabana1 & Pierre Lachapelle2
1Département de sciences biologiques, Université de Montréal, C.P. Succ. Centre-ville, 6128, Montréal, Québec, H3C 3J7, Canada, fax 1 514 343 2293, e-mail mcneilr@ere.umontreal.ca; 2 McGill University, Montréal, Québec, Canada
McNeil, R., Rojas, L.M., Cabana, T. & Lachapelle P. 1999. Vision and nocturnal activities in wading birds and shorebirds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2691-2710. Johannesburg: BirdLife South Africa.Activity during daytime and night-time is a characteristic of most birds that wade in search for food. Some species forage exclusively with the same feeding strategy, both at night and during daytime, e.g. visual pecking or tactile searching (probing and or sweeping). Conversely, other species are visual peckers during daylight and on moonlight conditions but switch to tactile feeding on moonless nights or under low moonlight conditions. It can be expected that species which switch from visual feeding during daytime to tactile foraging at night have a poor night vision capability compared to species which are always sight foragers, irrespective of the time of the day. This issue is examined in this study by comparing the retinal structures and the rod- and cone-mediated ERGs (electroretinograms) of five shorebird, four ardeid, one ibis, one spoonbill, and one skimmer species. ERGs were obtained at different light intensities from anaesthetised birds, and the retinae were subsequently processed for histological observations. Under the tested conditions (i.e. -3.8 to 0 log units), all species are characterised by distinct ERG responses, both under scotopic and photopic conditions. These features are in accordance with photoreceptor ratios and densities, and with night-time and daytime activities and foraging strategies.
INTRODUCTION
In this study, in accordance with the North American use, 'wading birds' is applied to the Ciconiiformes, and 'shorebirds' to the Charadriidae, Scolopacidae and Recurvirostridae families of the Charadriiformes.. Nocturnal foraging is widespread among wading birds and shorebirds (McNeil 1991; McNeil et al. 1992, 1993b; McNeil & Rodríguez 1996a, 1996b). Among the wading birds, the night herons Nycticorax specialise in this strategy (see McNeil et al. 1993b). However, most egrets Egretta are considered as diurnal (see McNeil et al. 1993b). Others like the Great Blue Heron Ardea herodias feed during the day and at night, but are mainly crepuscular feeders (see Horvath & Moholt 1986; McNeil et al. 1993a, 1993b). The spoonbills Ajaia and Platalea feed more actively in the morning, evening and night than during the daytime (see Robert et al. 1989; McNeil et al. 1993b). Nevertheless, there is no evidence that any ibis species feed at night with any regularity (see McNeil et al. 1999). Among the shorebirds, foraging during daytime and nighttime is a characteristic of most species, and some species, especially in the plovers and allied genera, forage mainly at dusk and at night (McNeil 1991; McNeil & Rodríguez 1996a, 1996b; McNeil et al. 1992).
Visual foraging predominates in herons and egrets, the prey being usually seized in the bill, sometimes impaled (Kushlan 1978). Most crepuscular and nocturnal herons are passive foragers (stand and wait at the water's edge or in shallow water for prey to appear, peering, walk slowly in shallow water) but, in contrast, most diurnal egrets use active foraging methods which involve successive and rapid movements (walk quickly, running forward rapidly, hopping and jumping, and open-wing) (Meyerriecks 1962; Kushlan 1976; Willard 1977; Rodgers 1983; Martínez-Vilalta & Motis 1992). Ibises mostly feed by non visual probing of muddy substrata with their curved bill (Hancock et al. 1992; Matheu & del Hoyo 1992). They also use head swinging, groping and pecking when appropriate (Kushlan 1977; Hancock et al. 1992). Spoonbills forage tactilely by wading through shallow water, sweeping the partly open spatulate bill from side to side through water and silt (Kushlan 1978; Matheu & del Hoyo 1992).
Two basic types of foraging techniques are used by shorebirds: visual searching for prey or indication of their presence on or near the substratum surface, and tactile searching (Schneider, 1983; McNeil et al. 1992). Some species adopt the same feeding strategy both during daytime and at night, and even on moonless nights, e.g. visual pecking by Charadrius plovers, and tactile probing by Limnodromus dowitchers (McNeil & Robert 1988; Robert & McNeil 1989; McNeil et al. 1992; Thibault & McNeil 1994, 1995). Other shorebird species may vary their foraging strategies according to the feeding habitat conditions, e.g. the Black-winged Stilt Himantopus himantopus or between day and night, e.g. the Tringa species (McNeil & Robert 1988; Robert & McNeil 1989; McNeil et al. 1992). Both the plovers and Willet Catoptrophorus semipalmatus, while foraging on evasive prey like fiddler crabs, run rapidly forward their prey to catch them (see Piersma et al. 1966a, 1966b).
Many swimming prey of wading birds are more active, closer to the water surface, and thus more easily accessible at dusk and night than during daytime, and most invertebrates, living in the muddy areas of intertidal habitats where many wading birds forage, are more active or closer to the sediment surface at night (McNeil et al. 1992, 1993b, 1995). Consequently, it can be expected that wading birds that detect prey by touch, without having to see them, would be able to feed as fast during darkness as during daytime, whereas birds that detect prey primarily by sight would be disadvantaged at night, unless having specialised adaptations favouring nocturnal vision (McNeil et al. 1992). In such situations, the blindly probing techniques of ibises, spoonbills and dowitchers should allow night foraging (McNeil & Robert 1988; Robert & McNeil 1989; Hancock et al. 1992; Matheu & del Hoyo 1992).
The retinal receptors of vertebrates are the rods and cones (see Meyer 1977; Martin 1985; Tansley & Erichsen 1985). Nocturnal birds have a greater preponderance of rods in their retinae while, in diurnal birds, most photoreceptors are cones (see Tansley & Erichsen 1985; Waldvogel 1990). Rods are generally associated with night vision; cones, on the other hand, are associated with good visual acuity at high light levels (Tansley & Erichsen 1985).
The purpose of this study was to examine in a selected group of shorebirds and wading birds if diurnal and nocturnal foraging and the use of visual or tactile feeding strategies could be correlated with the structure and/or function of their retina. The following predictions were tested: (1) visual foragers that forage mainly near dawn or dusk or at night have a higher rods:cones ratio and a better night vision capability than visual feeding species which forage only during daytime; (2) visual nocturnal foragers have a better night vision capability not only than tactile diurnal feeders, but also than tactile nocturnal feeders; (3) as the feeding strategies of some shorebird species change as a function of light conditions, species which are always or predominantly sight foragers, irrespective of the time of the day, should have a better night vision capability than species which are always tactile feeding or switch from visual feeding during daytime to tactile foraging at night; and finally (4) diurnal visual foragers should have proportionately more cones, and consequently a better visual acuity, than diurnal tactile foragers.
METHODS
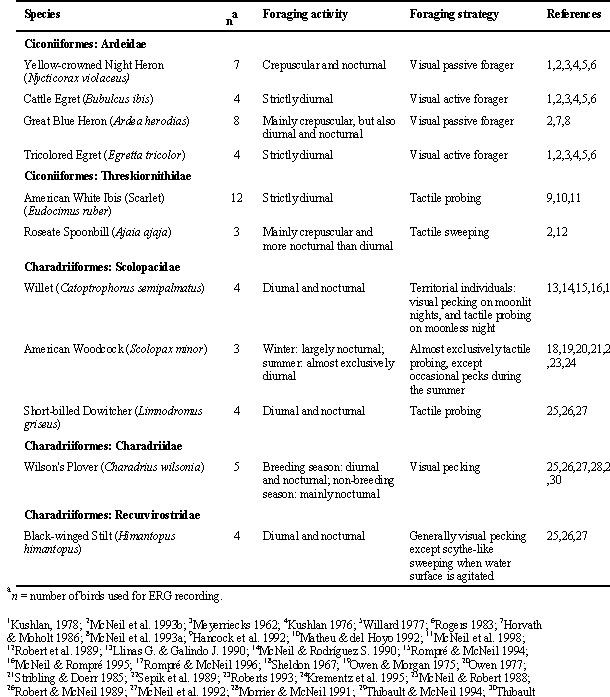
Bird species were selected according to behavioural criteria given in Table 1. The Wilson's Plovers Charadrius wilsonia, Black-winged Stilts, Willets, Yellow-crowned Night Herons Nycticorax violaceus, Cattle Egrets Bubulcus ibis, Tricolored Egrets Egretta tricolor, and American White Ibises Eudocimus ruber (scarlet race) were mist-netted in Laguna de Patos and Chacopata Lagoon in northeastern Venezuela. We were unsuccessful in capturing live spoonbills. As a consequence, the Roseate Spoonbills Ajaia ajaja were shot in Chacopata Lagoon and were unfortunately not available for ERG recording. The Great Blue Herons and the American Woodcocks were obtained near Montréal, Province of Québec (Canada). The Short-billed Dowitchers (Limnodromus griseus) were mist-netted near Rimouski (Province of Québec). Except the spoonbills, the birds were brought alive to the laboratory at the Universidad de Oriente in Cumaná (Venezuela) or the Université de Montréal.
ERG recording
Electroretinograms were recorded with the use of a LKC EPIC-2000 visual electrodiagnostic system (LKC Technologies Inc., Gaithersburg, MD), which includes the LKC Ganzfeld-2503B stimulator, using a method previously reported in more details (Rojas et al. 1997). Briefly, after a period of 4 hours of dark adaptation, the birds were anaesthetised and immobilised with the head kept inside the Ganzfeld, the left eye maintained open upward, and the eyelids and nictitating membrane kept retracted. The maximum pupil diameter (mm) was measured at the beginning and at the end of the experiment. ERG responses (average of 6 at 10.1-sec intervals) were evoked to flashes of increasing luminance of -3.8, -3.0, -2.6, -2.0, -1.4, -1.0, and 0.0 log units of attenuation (maximal intensity: 3.31 cd m -2 sec -1. The birds were then light-adapted for 10 min to a background luminance of 35.7 cd m -2 sec -1, following which the photopic ERGs (average of 10 at 4.1-sec intervals) were evoked to flashes of decreasing luminance (0.0, -0.6, -1.0, and -1.4 log units; maximal intensity: 3.31 cd m -2 sec -1). In order to take into account the fact that the size of the pupil varies with species, the light intensity with which the ERGs were evoked was transformed in Troland unit (Wyszecky & Stile 1967). To facilitate comparison of data between species of birds, luminance-response function curves were generated from the scotopic and photopic ERG responses and the maximal saturated amplitude (Vmax) was calculated.
Morphological observations
Once the ERG recordings completed, four individuals of each species were used for histological analysis whereas the others were given enough time to recover from anaesthesia and were returned to their original habitat. The former were euthanized with sodium pentobarbital. Working with the left eye in the fixative (2.5% glutaraldehyde), the anterior segment was removed and the retina, still attached to the choroid, was cut into 9 sectors, using the pecten as landmark. This division is the same as that used by Rojas de Azuaje et al. (1993) and Rojas et al. (1997), and corresponds to that of Meyer & May (1973) and Begin & Handford (1987), although the sector numbering is different. Thereafter, each sector was processed for histological observations; for detailed methodology, see Rojas et al. (1997, 1999). Rods, cones and ganglion cells were counted in 310-µm wide fields of semi-thin (0.7 µm) sections, for a total of 15 counts for each of the nine sectors. Double cones were counted as two cones. In addition, the length and diameter of the outer and inner segments of rods and cones, and the thickness of each retinal layer were measured in the same observation fields.
Statistical treatment The species were compared by utilising a conventional statistical analysis, the 95% confidence interval on the means.RESULTS
Photoreceptor density
Overall, photoreceptor density varies from a species to another among the species analysed (Fig. 1). In the wading birds, the Yellow-crowned Night Heron is the only species where the rods outnumber the cones, with a resulting overall rods:cones ratio of 2.3:1.0. In contrast, the Great Blue Heron, and particularly the Cattle Egret, the Tricolored Egret and the American White Ibis, have a cone-dominated retina, with overall rods:cones ratios ranging from 0.6:1.0 to 0.3:1.0. In the spoonbill, cones slightly outnumber rods for a rods:cones ratio of 0.9:1.0. Rod density is significantly higher in the Yellow-crowned Night Heron than in the five other wading bird species; it is also higher in the Great Blue Heron and the spoonbill, compared to the ibis and the Tricolored and Cattle egrets. Cone density is higher in the Cattle Egret, followed by the Tricolored Egret, and then by the ibis; it is lower in the Yellow-crowned Night Heron. In the shorebirds, rods outnumber cones in the Wilson's Plover, the American Woodcock and the Black-winged Stilt, with overall rods:cones ratios of 1.3:1.0, 1.2:1.0, and 1.1:1.0, respectively. The Willet, on the other hand, has a clearly cone-dominated retina, with an overall rods:cones ratio of 0.7:1.0, whereas the dowitcher has a mixed retina with similar densities of rods and cones (1.0:1.0). The Willet has proportionately less rods than the plover and the stilt, but its cones outnumber those of the woodcock, the dowitcher and the plover. Overall, in the shorebirds, rod density exceeds that of any of the wading birds, except the Yellow-crowned Night Heron. In general, except for the latter, cone dominance is higher in the wading birds than in the shorebirds (Fig. 1). The two egret species have higher cone density than all shorebird species studied.
While in the Great Blue Heron, the spoonbill and the ibis rod and cone densities do not vary from one sector to another (for comparative values, see Rojas et al. in press), in the Cattle Egret the cones are more numerous in the central retina (sector 5), and in the Tricolored Egret they are more concentrated in the temporal retina (sector 3) where the rods also show the lowest density (Fig. 2). In all shorebird species (for comparative data, see Rojas et al.1999), but particularly in the woodcock and the Willet, cones tend to be more numerous in sector 5 than in other sectors (Fig. 2). The same trend is observed with rods for all shorebird species, although less clearly for the Willet and the dowitcher (Rojas et al.1999).
Rod outer segments are or tend to be longer in all nocturnally active species, i.e. the shorebird species, the two heron species, and the spoonbill (Table 2). They are or tend to be the shortest in the strictly diurnal egrets and ibises. There is no clear between-species trend in the other morphometric parameters of rods and cones.
In the Yellow-crowned Night Herons, Cattle Egrets and Roseate Spoonbills, and in all shorebird species studied, the thickness of the outer nuclear, inner nuclear, and inner plexiform layers, as well as the density of ganglion cells are or tend to be greater in sector 5. However, in the Tricolored Egret, the thickness of these layers and ganglion cell density are or tend to be greater in sector 3. In all wading bird and shorebird species, the optic nerve fiber layer is or tends to be thinner in dorsal and temporal sectors and thicker in the ventral ones (the region of the optic nerve and pecten). For detailed information on the values of these parameters, see Rojas et al. (in press;1999).
Retinal physiology
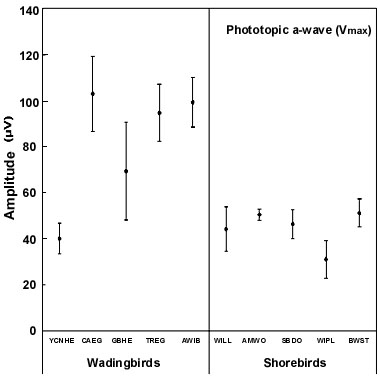
The scotopic and photopic ERGs obtained for each species differ in shape and amplitude between species (see Rojas et al. 1997;1999; in press). Representative examples are given for three shorebird species in Figure 3. The luminance-response functions generated from amplitude measurements in scotopic conditions are represented in Figure 4. Overall, the scotopic and photopic maximal b- and a-wave amplitudes (Vmax) vary from a species to another among the species analysed (Fig 5, Fig 6, Fig 7 and Fig 8). The maximal scotopic b-wave amplitudes measured in the Cattle Egret and the Black-winged Stilt are higher than in all other species studied (Fig. 5). The responses of the Short-billed Dowitchers, American Woodcocks and Willets tend to be the lowest in many cases when compared to that of other species. The scotopic a-wave amplitude of the Yellow-crowned Night Heron and the Wilson's Plover are lower than that of most other species (Fig. 6). For photopic responses, the maximal b- and a-wave amplitudes measured in the strictly diurnally foraging species (the two egret species and the American White Ibis) exceed or tend to exceed those recorded for the shorebird species and the two heron species, which all forage at night (Figs 7 and 8). The Wilson's Plover has the lowest photopic responses of all species studied.
DISCUSSION
Overall, the rods:cones ratio exceeds 1.0 in all species that are always or predominantly sight foragers at night (Yellow-crowned Night Heron, Wilson's Plover, Black-winged Stilt) or need vision for nocturnal flights that form parts of the nocturnal feeding activities (American Woodcock). Rod density of all nocturnally active species (the two herons, the spoonbill, and all shorebird species) exceeds rod density of the strictly diurnal species (Cattle and Tricolored egrets, American White Ibis). In the shorebirds, rod density exceeds rod density of all wading birds, except the Yellow-crowned Night Heron. As a consequence, the combined data confirm our first initial prediction that visual foragers that forage mainly near dawn or dusk or at night (night herons, plovers and stilts) have a higher rods:cones ratio than visual feeders which forage only during daytime (the two egrets).
Overall, our second prediction is also confirmed. Indeed, the night herons, plovers and stilts, all visual nocturnal foragers, have a higher rods:cones ratio and, consequently, a better night vision capability not only than tactile diurnal feeders (ibises), but also than tactile nocturnal feeders (spoonbills, woodcocks, dowitchers). Rod outer segments are or tend to be longer in all nocturnally active species. Surprisingly, in comparison with all other nocturnally active species studied, the rods:cones ratio and the rod density of the Great Blue Heron are low. Rod density in this species is in some ways counterbalanced by the length of their outer segments, the longest of all studied species, which almost double in length those of the diurnal species, and significantly exceed those of the spoonbill and the night heron.
In accordance with our third initial prediction, the Wilson's Plover and the Black-winged Stilt, which are always or predominantly sight feeders irrespective of light condition, have a higher rod density than the three other shorebird species which either are always tactile foragers, or switch from daytime visual feeding to tactile foraging on moonless nights.
Based on maximum scotopic b-wave amplitudes, all the species studied, including the strictly diurnal egrets and ibises which have the lowest rods:cones ratios and rod densities, appear to have roughly comparable night vision capability. As a consequence, based on scotopic responses, the fact that the two egrets and the ibis do not forage at night does not appear to result from poor night vision capability. For the three species, and particularly for the ibis (see Rojas et al. 1997), we would have expected the contrary, based on rod densities and rods:cones ratios. It would seem from our study that the factor which best discriminates between the crepuscular and nocturnal foraging species and the strictly diurnal ones resides in the daytime visual capabilities. Indeed, contrary to the fourth initial prediction, the strictly diurnal tactile ibises and visual egrets have or tend to have similar cone densities, cones:rods ratios, and photopic b- and a-wave amplitudes, generally higher than the nocturnally active species, excepting the Willet for cone density.
There is a trend in the Cattle Egret, but also in the Wilson's Plover and the Willet, to have higher photoreceptor density, particularly of the cones, and greater thickness of the nuclear, inner nuclear, and inner plexiform layers, as well as ganglion cell density in the central retina (sector 5). Such thickened areas with higher cone and ganglion cell densities were not found in the ibis, the spoonbill, or the two herons. In the Tricolored Egret, the values of these parameters are higher in the temporal sector 3. These two egret species, the plover and the Willet use active foraging methods which involve successive and rapid movements (see introduction) for feeding on evasive small prey such as insects, locusts, grasshoppers, frogs, tadpoles, fish, fiddler crabs, etc. (Morrier & McNeil 1991; Martínez-Vilalta & Motis 1992; McNeil & Rompré 1995). Areas of circumscribed thickening of the retina brought about by an increase in the number of visual cells (predominantly cones), combined with a concomitant increase in the number of bipolar and ganglion cells, correspond to the so-called 'central' and 'temporal' areas of acute vision, or fovea, in higher vertebrates (see Walls 1942; Meyer 1977; Martin 1985). It has been reported that increased ganglion cell density is usually found in the retinal regions subserving higher visual acuity (see Binggeli & Paule 1969; Ikushima et al. 1986; Hayes & Brooke 1990; Inzunza et al. 1991; Suburo et al. 1991), and could provide for a better capacity to discriminate details. Ganglion cells are movement-, direction- and contrast-sensitive, and receive most of their input from bipolar and amacrine cells of the inner nuclear layer (see Dowling 1987). All these features of the thickened retina in the central sector 5 could provide the species with a better monocular perception of contrasts and prey movement on either side of their body (see Smythe 1975). On the other hand, the same features in the temporal sector 3 could provide the Tricolored Egret with a good binocular visual acuity (see Smythe 1975), for striking at its evasive fish prey while foraging in shallow water (Meyerriecks 1962; Martínez-Vilalta & Motis 1992). The other wading bird species studied are either tactile foragers or passive visual foragers (see introduction).
Together, these ERG responses, both under scotopic and photopic conditions, photoreceptors ratios and densities, and other morphological features of the retina of the species studied can be correlated with their nighttime and daytime foraging activities. For example, the main prey of the two herons species (crabs, frogs, worms, insects, etc.) are more active, and more available at dusk and during darkness than during daytime (see Hancock & Kushlan1984). The swimming prey of the Black-winged Stilt are more active, closer to the water surface, and thus more easily accessible at dusk and night than during daytime, and most invertebrates, living in the muddy areas of intertidal habitats where the dowitchers and ibises forage, are more active or closer to the sediment surface at night (McNeil et al. 1992, 1993, 1995). The same is true for the terrestrial annelids (Lumbricus) on which woodcocks mostly feed (Dugan 1981). Consequently, it is believed that shorebirds that detect prey by touch, without having to see them, would be able to feed as fast during darkness as during daytime, whereas birds that detect prey primarily by sight would be disadvantaged at night, unless having specialised adaptations favouring nocturnal vision (McNeil et al. 1992). The Uca fiddler crabs, the preferred prey of the Wilson's Plover and territorial Willets, contrary to above-mentioned invertebrates, although swarming by thousands on intertidal mudflats both in the day and at night, are more active during daytime (McNeil & Rompré 1995; Thibault & McNeil 1995). On moonless nights, the territorial Willets have impaired vision, and abandon their feeding territory to move to habitats where they feed tactilely with non-territorial individuals (McNeil & Rompré 1995; Rompré & McNeil 1996). Present results show that the Wilson's Plover has the lowest photopic responses of all species studied. Contrary to most other Charadrius plovers, the Wilson's Plovers are solitary foragers (Morrier & McNeil 1991) and thus, while feeding during daylight, suffer higher individual risks of being captured by aerial predators (Thibault & McNeil 1994). During the non-breeding season, they congregate in small groups and remain motionless on roosts where substrata offer better concealing than the wide mudflats (Thibault & McNeil 1994). Between 20 to 30 min after dusk, the plovers leave diurnal roosting sites and distribute themselves all over the foraging mudflats. The main reason why Wilson's Plovers are largely nocturnal appears to be the avoidance of diurnal predators (Thibault & McNeil 1994). Thus, the plover can advantageously trade-off photopic vision for the scotopic one.
The vision capability of the above-mentioned species was compared by utilising a conventional statistical analysis: the 95% confidence interval on the means. However, according to Felsenstein (1985) and Garland et al. (1993), different species cannot be considered as points drawn independently from the same distribution if they are descended from common ancestors. For instance, two bird species could have a similar rod density mainly because they are closely related. These hierarchical relationships may lead to an overestimation of the degrees of freedom used in conventional statistical tests and hence to untrustworthy significance levels (Garland et al. 1993 and references therein). We re-analysed the data using a method allowing to correct for bias due to phylogeny (Garland et al. 1993). Overall, the analysis which takes into account the phylogenetic lineage maintains the significance, although generally at a lower level, of the between-species differences in the rod and cone densities previously found using conventional 95% confidential interval of the means (Rojas 1998). However, the significance of the between-species differences in the Vmax amplitude is not maintained (Rojas 1998). This can be due to the small size of the samples as well as to important inter-individual differences in the physiological responses while recording ERGs. This may also be due to possible aberrations in the retained phylogenetic hypothesis which is based on information taken from Sheldon (1987) and Sibley & Ahlquist (1990).
ACKNOWLEDGEMENTS
This study was supported by research grants from the Natural Sciences and Engineering Research Council of Canada, the Université de Montréal, and Universidad de Oriente through a collaboration agreement between the two universities. We thank Jacques Charrette, Yves de Repentigny, Martine Émond, Guy Fitzgerald, Claude Laplante, Louise Pelletier, Michel Thibault, Jean-Luc Verville of the Université de Montréal, and colleagues of the Universidad de Oriente, in particular Marcos Tulio Díaz, José Ramón Rodríguez Silva, Gedio Marín, Alpidio Boada, José Guilarte, and Dwight Arrieche, for assistance during the field and laboratory work.REFERENCES
Begin, M.R. & Handford, P. 1987. Comparative study of retinal oil droplets in grebes and coots. Canadian Journal of Zoology 65: 2105-2110.
Binggeli, R.L. & Paule, W.J. 1969. The pigeon retina: quantitative aspects of the optic nerve and ganglion cell layer. Journal of Comparative Neurology 137: 1-18.
Dowling, J.E. 1987. The retina. Cambridge; Belknap Press, Harvard University Press.
Dugan, P.J. 1981. The importance of nocturnal foraging in shorebirds: a consequence of increased invertebrate prey activity. In: Jones, N.V. & Wolff, W.J. (eds) Feeding and survival strategies of estuarine organisms. New York; Plenum Press: 251-260.
Felsenstein, J. 1985. Phylogenies and the comparative method. American Naturalist 124: 1-15.
Garland, T., Jr., Dickermann, A.W., Janis, C.M. & Jones, J.A. 1993. Phylogenetic analysis of corariance by computer simulation. Systematic Biology 42: 265-292.
Hancock, J.A. & Kushlan, J.A. 1984. The herons handbook. London; Croom Helm.
Hancock, J.A., Kushlan, J.A. & Kahl, M.P. 1992. Storks, ibises, and spoonbills of the world. London; Academic Press.
Hayes, B.P. & Brooke, M, de L. 1990. Retinal ganglion cell distribution and behaviour in procellariiform seabirds. Vision Research 30: 1277-1289.
Horvath, E.G. & Moholt, R.K. 1986. Diurnal feeding cycle at an inland Great Blue Heron colony. Murrelet 67: 27-28.
Ikushima, M., Watanabe, M. & Ito, H. 1986. Distribution and morphology of retinal ganglion cells in the Japanese Quail. Brain Research 376: 320-334.
Inzunza, O., Bravo, H., Smith, R.L.& Angel, M. 1991. Topography and morphology of retinal ganglion cells in falconiforms: a study on predatory and carrion-eating birds. Anatomical Record 229: 271-277.
Krementz, D.G., Seginak, J.T. & Pendleton, G.W. 1995. Habitat use at night by wintering American Woodcock in coastal Georgia and Virginia. Wilson Bulletin 107: 686-697.
Kushlan, J.A. 1976. Feeding behavior of North American herons. Auk 93: 86-94.
Kushlan, J.A. 1977. Foraging behavior of the White Ibis. Wilson Bulletin 89: 342-345.
Kushlan, J.A. 1978. Feeding ecology of wading birds. In: Sprunt IV, A., Ogden, J.C. & Winckler, S. (eds) Wading birds. New York; National Audubon Society Research Report 7: 249-297
Llinas, G., J. & Galindo J., J.M. 1990. Algunos aspectos del comportamiento alimenticio del Zarapito, Catoptrophorus semipalmatus (Scolopacidae), en la Ensenada de la Paz, Baja California Sur, México. Southwestern Naturalist 35: 237-240.
Martin, G.R. 1985. Eye. In: King, A.S. & McLelland, J. (eds) Form and function in birds. New York; Academic Press: 311-373.
Martínez-Vilalta, A. & Motis, A. 1992. Family Ardeidae (herons). In: del Hoyo, J., Elliott, A. & Sargatal, J. (eds) Handbook of the birds of the world, vol 1. Barcelona; Lynx Edicions: 376-429.
Matheu, E. & del Hoyo, J. 1992. Family Threskiornithidae (ibises and spoonbills). In: del Hoyo, J., Elliott, A. & Sargatal, J. (eds) Handbook of the birds of the world, vol 1. Barcelona; Lynx Edicions: 472-506.
McNeil, R. 1991. Nocturnality in shorebirds. Acta Congressus Internationalis Ornithologici 20: 1098-1104.
McNeil, R. & Robert, M. 1988. Nocturnal feeding strategies of some shorebird species in a tropical environment. Acta Congressus Internationalis Ornithologici 19: 2328-2336.
McNeil, R. & Rodríguez S., J.R. 1990. When does the Willet ‘plough’ the water to catch fish? Wader Study Group Bulletin 58: 50-51.
McNeil, R. & Rodríguez S., J.R. 1996a. Ecological significance and sensorial aspects of nocturnal foraging in shorebirds. In: Cabana, T. (ed.) Animals in their environment. Frelighsburg, Québec; Orbis: 23-58.
McNeil, R. & Rodríguez S., J.R. 1996b. Nocturnal foraging in shorebirds. International Wader Studies 8: 114-121.
McNeil, R. & Rompré, G. 1995. Day and night feeding territoriality in Willets Catoptrophorus semipalmatus and Whimbrel Numenius phaeopus during the non-breeding season in the tropics. Ibis 137: 169-176.
McNeil, R., Drapeau, P. & Goss-Custard, J.D. 1992. The occurrence and adaptive significance of nocturnal habits in waterfowl. Biological Review 67: 381-419.
McNeil, R., Benoît, R. & DesGranges, J.L. 1993a. Daytime and nighttime activity at a breeding colony of Great Blue Herons in a non tidal environment. Canadian Journal of Zoology 71: 1075-1078.
McNeil, R., Drapeau, P. & Pierotti, R. 1993b. Nocturnality in colonial waterbirds: occurrence, special adaptations, and suspected benefits. In: Power, D.M. (ed.) Current ornithology, vol 10. New York; Plenum Press: 187-246.
McNeil, R., Díaz, D.O., Liñero A., I. & Rodríguez S., J.R. 1995. Day- and night-time prey availability for waterbirds in a tropical lagoon. Canadian Journal of Zoology 73: 869-878.
McNeil, R., Genevois, F., Kushlan, J.A. & Martin, M.R. 1999. Foraging and nocturnality in colonial waterbirds. In: Cézilly, F., Hafner, H. & Nettleship, D.N. (eds) Colonial breeding in waterbirds: evolutionary causes and functional consequences. Oxford; Ithaca; Cornell University Press: - .
Meyer, D.B. 1977. The avian eye and its adaptations. In: Crescitelli, F. (ed) The visual system in vertebrates, vol VII/5. Berlin; Springer Verlag: 549-611.
Meyer, D.B. & May, H.C. 1973. The topographical distribution of rods and cones in the adult chicken retina. Experimental Eye Research 17: 347-355.
Meyerriecks, A.J. 1962. Diversity typifies heron feeding. Natural History 71(6): 48-59.
Morrier, A. & McNeil, R. 1991. Time-activity budget of Wilson's and Semipalmated plovers in a tropical environment. Wilson Bulletin 103: 598-620.
Owen, R.B., Jr. 1977. American Woodcock (Philohela minor = Scolopax minor of Edwards 1974). In: Sanderson, G.C. (ed.) Management of migratory shore and upland game birds in North America. Washington; International Association of Wildlife Agencies: 146-186.
Owen, R.B., Jr. & Morgan, J.W. 1975. Summer behavior of adult radio-equipped woodcock in central Maine. Journal of Wildlife Management 39: 179-182.
Piersma, T. & Wiersma, P. 1966a. Family Charadriidae (plovers). In: del Hoyo, J., Elliott, A. & Sargatal, J. (eds) Handbook of the birds of the world, vol 3. Barcelona; Lynx Edicions: 384-442.
Piersma, T., van Gills, J. & Wiersma, P. 1966b. Family Scolopacidae (sandpipers, snipes and phalaropes). In: del Hoyo, J., Elliott, A. & Sargatal, J. (eds) Handbook of the birds of the world, vol 3. Barcelona; Lynx Edicions: 444-533.
Robert, M. & McNeil, R. 1989. Comparative day and night feeding strategies of shorebird species in a tropical environment. Ibis 131: 69-79.
Robert, M., McNeil, R. & Leduc, A. 1989. Conditions and significance of night feeding in shorebirds and other water birds in a tropical lagoon. Auk 106: 94-101.
Roberts, R.H. 1993. The ecology and management of wintering woodcocks. In: Longcore, J.R. & Sepik, G.F. (eds.) Proceedings of the eighth American Woodcock symposium. Washington; Biological Report 16, U.S. Department of the Interior, Fish and Wildlife Service: 87-97.
Rodgers, J.A., Jr. 1983. Foraging behavior of seven species of herons in Tampa Bay, Florida. Colonial Waterbirds 6: 11-23.
Rojas, L.M. 1998. Étude comparative et morpho-fonctionnelle de la vision nocturne chez deux groupes d'oiseaux aquatiques. Thèse de doctorat, Université de Montréal, Montréal.
Rojas, L.M., McNeil, R., Cabana, T. & Lachapelle, P. 1997. Diurnal and nocturnal visual function in two tactile foraging waterbirds: the American White Ibis and the Black Skimmer. Condor 99: 191-200.
Rojas, L.M., McNeil, R., Cabana, T. & Lachapelle P. 1999. Diurnal and nocturnal visual capabilities in shorebirds as a function of their feeding strategies. Brain Behavior and Evolution 53: 29-43.
Rojas, L.M., McNeil, R., Cabana, T. & Lachapelle P. In press. Behavioral, morphological and physiological correlates of diurnal and nocturnal vision in selected wading bird species. Brain Behavior and Evolution.
Rojas de Azuaje, L.M., Tai, S. & McNeil, R. 1993. Comparison of rod/cone ratio in three species of shorebirds having different nocturnal foraging strategies. Auk 110: 141-145.
Rompré, G. & McNeil, R. 1994. Seasonal changes in day and night foraging of Willets in northeastern Venezuela. Condor 96: 734-738.
Rompré, G. & McNeil, R. 1996. Variability in day and night feeding habitat use in the Willet Catoptrophorus semipalmatus during the non-breeding season in northeastern Venezuela. Wader Study Group Bulletin 81: 82-87.
Schneider, D. 1983. The food and feeding of migratory shorebirds. Oceanus 26: 38-43.
Sepik, G.F., McAuley, D.B., Longcore, J.R. & Derleth. E.L. 1989. Habitat requirements and management of woodcock in the Northeast: Assessment of knowledge and needs. In: Finley, J.C. & Brittingham, M.C. (eds.) Timber management and its effects on wildlife. University Park, PA; Proceedings of the 1989 Pennsylvania State forest resources issue conference: 97-109.
Sheldon, F.H. 1987. Phylogeny of herons estimated by DNA-DNA hybridization data. Auk 104: 97-108.
Sheldon, W.G. 1967. The book of the American Woodcock. Amherst; University of Massachusetts Press.
Sibley, C.G. & Ahlquist, J.E. 1990. Phylogeny and classification in birds: a study in molecular evolution. New Haven, CT; Yale University Press.
Smythe, R.H. 1975. Vision in the animal world. London; MacMillan Press.
Stribling, H.L. & Doerr, P.D. 1985. Nocturnal use of fields by American Woodcock. Journal of Wildlife Management 49: 485-491.
Suburo, A.M., Herrero, M.V. & Scolaro, J.A. 1991. Regionalization of the ganglion cell layer in the retina of the Magellanic Penguin (Spheniscus magellanicus). Colonial Waterbirds 14: 17-24.
Tansley, K. & Erichsen, J.R. 1985. Vision. In: Campbell, B. & Lack, E. (eds) A dictionary of birds. Calton; Poyser: 623-629.
Thibault, M. & McNeil, R. 1994. Day/night variation in habitat use by Wilson’s Plovers in northeastern Venezuela. Wilson Bulletin 106: 299-310.
Thibault, M. & McNeil, R. 1995. Predator-prey relationship between Wilson’s Plovers and fiddler crabs in northeastern Venezuela. Wilson Bulletin 107: 73-80.
Waldvogel, J.A. 1990. The bird’s eye view. American Scientist 78: 342-353.
Walls, G.L. 1942. The vertebrate eye and its adaptative radiation. Bloomfield Hill; Cranbrook Institute of Science.
Willard, D.E. 1977. The feeding ecology and behavior of five species of herons in southeastern New Jersey. Condor 79: 462-470.
Wyszecki, G. & Stile, W.S. 1967. Color science: concepts and methods, quantitative data and formulas. New York; John Wiley & Sons.
Table 1. Behavioural characteristics of the selected species.

Table 2. Measurements of the rod outer segment length in the species studied.
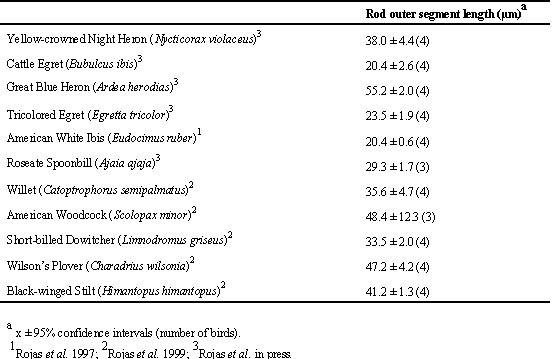
Fig. 1. Mean rod and cone numbers (mean/310 µm ± 95% confidence intervals) and rods:cones ratios of the species. The figures above the columns indicate the rods:cones ratio. AMWO = American Woodcock, AWIB = American White Ibis (Scarlet), BWST = Black-winged Stilt, CAEG = Cattle Egret, GNHE = Great Blue Heron, ROSP = Roseate Spoonbill, SBDO = Short-billed Dowitcher, TREG = Tricolored Egret, WILL = Willet, WIPL = Wilson's Plover, and YNHE = Yellow-crowned Night Heron. Adapted from Rojas et al. (1997, 1999, in press).
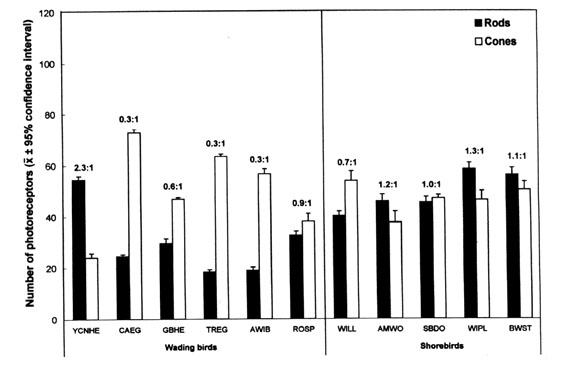
Fig. 2. Mean rod and cone numbers (mean/310 µm ± 95% confidence intervals) of the Wilson's Plover, Willet, Cattle Egret and Tricolored Egret in each of the nine retinal sectors as well as in all sectors averaged. The figures above the columns indicate the overall rods:cones ratio. Adapted from Rojas et al. (1999, in press).
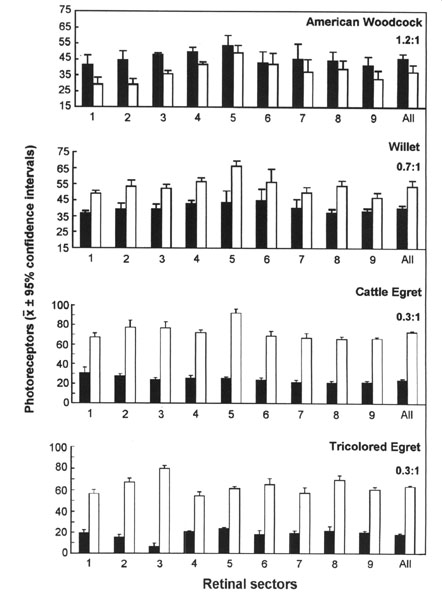
Fig. 3. Representative ERG responses of three shorebird species, the Wilson's Plover, Black-winged Stilt and Willet, obtained under scotopic and photopic conditions. Nomenclature: a, peak of the a-wave; b, peak of the b-wave. The figures on the left represent light intensity values (Log units). Adapted from Rojas et al. (1999).
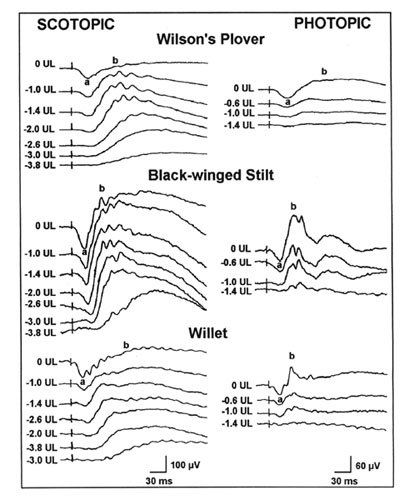
Fig. 4. Luminance-response function (± 95% confidence intervals) of the b-wave of the shorebird (A) and wading bird species (B) under scotopic conditions. The ordinate represents the b-wave amplitude (µV) and the abscissa the stimulus luminance intensity (Log troland-sec). Adapted from Rojas et al. (1999, in press).
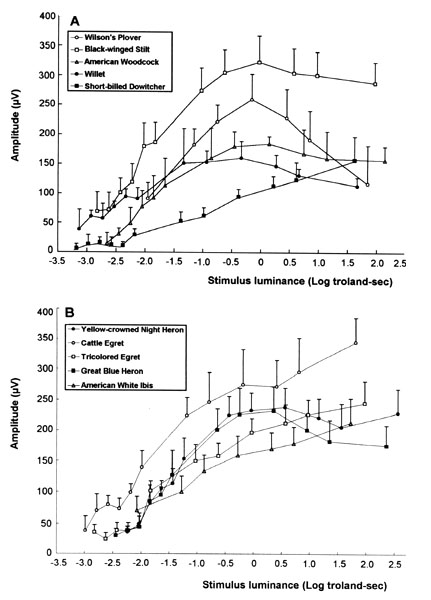
Fig. 5. Maximal scotopic b-wave amplitude (Vmax) of the species studied. For species abbreviations, see Fig. 1. Adapted from Rojas (1998).
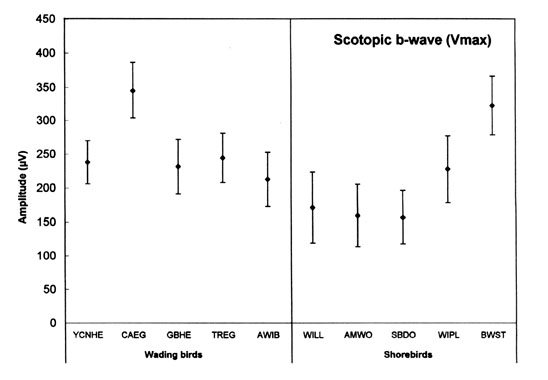
Fig. 6. Maximal scotopic a-wave amplitude (Vmax) of the species studied. For species abbreviations, see Fig. 1. Adapted from Rojas (1998).
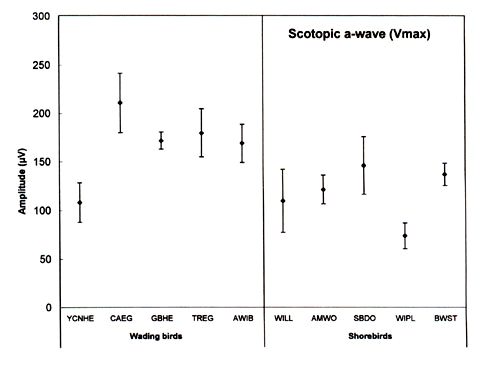
Fig. 7. Maximal photopic b-wave amplitude (Vmax) of the species studied. For species abbreviations, see Fig. 1. Adapted from Rojas (1998).
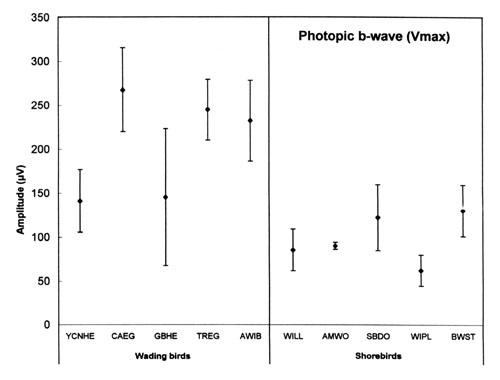
Fig. 8. Maximal photopic a-wave amplitude (Vmax) of the species studied. For species abbreviations, see Fig. 1. Adapted from Rojas (1998).