S44.5: Why does monogamy prevail in the Alpine Water Pipit Anthus spinoletta?
Kurt Bollmann1 & Heinz-Ulrich Reyer2
1Schweizer Vogelschutz SVS – BirdLife Switzerland, PO Box, CH–8036 Zürich, Switzerland, e-mail birdlife.svs@bluewin.ch; 2Zoologisches Institut, Universität Zürich, Winterthurerstr. 190, CH–8057 Zürich, Switzerland, fax 41 1 635 68 17, e-mail ulireyer@zool.unizh.ch
Bollmann, K. & Reyer, H.-U. 1999. Why does monogamy prevail in the Alpine Water Pipit? In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2666-2688. Johannesburg: BirdLife South Africa.A basic assumtion in the study of mating systems is that the amount and disribution of food and sexual partners influence differences in reproductive success. In this study the ecological, demographic and phenotypical determinants of variation in the social and genetic mating patterns and in reproductive success were analized for a population of Water Pipits Anthus spinoletta. The breeding habitat of the species lies in a variable alpine environment. Conditions at the study site varied markedly in time and space. This is due to a patchy vegetation pattern with large differences in food resources, an increasing population size over the study years and differences in nest predation. The operational sex ratio and the age distribution were stable between breeding seasons. Out of 278 social mating patterns studied, most were monogamous (86%), followed by bachelors (9%), polygyny (3%) and polyandry (2%). For paired individuals the fitness differences of the various mating patterns were small. The population showed an age-assorted mating system. Variation in reproductive success was best explained by nest predation and age of males and females. DNA fingerprints of 393 young from 95 nests revealed extrapair parentage in 7.1% of the young from 18.9% of the nests. Since there was no significant relation between territory-specific prey biomass and reproductive success, and the occurrence of nest predation was stochastic and unpredictable, age may be the most reliable criterium for mate choice. Under these circumstances, monogamy can be expected to be the dominant pair bond, especially under conditions where both parents are required to successfully rear the young.
INTRODUCTION
Exactly thirty years ago David Lack (1968) stated that monogamy is the predominant mating pattern in birds (90% of species) because 'each male and female will, on average, leave most descendants if they share in raising a brood'. Today, behavioural ecologists commonly agree that social monogamy only occures where there is limited opportunity for polygamy. Two concepts – the polygyny threshold model PTM (Verner 1964; Verner & Willson 1966; Orians 1969) and the operational sex ratio model (Emlen & Oring 1977) – have particulaly influenced the change of opinion and provided ecological explanations for the observed variation in social mating patterns. The evolution of monogamy has been explained with a reversion of the PTM by Wittenberger (1979) and Wittenberger & Tilson (1980). And in cases where the reproductive output is limited more by male than female care an extension of the model explains the evolution of cooperative polyandry through mate choice (Wittenberger 1979; Gowaty 1981). Today, from an increasing number of studies using new molecular techniques we even know that social pair bonds do not necessarily reflect mating patterns and parentage (Wickler & Seibt 1983; Gowaty 1985; Birkhead & Møller 1996). Because of that social and sexual monogamy have to be distinguished (Gowaty 1985). It is generally agreed that this discrepancy reflects differences in the relative benefit from pursuing extra-pair activities and the costs from neglecting the own mate, nest or dependent young (e.g. Birkhead & Møller 1992). The outcome of this trade-off will depend on species-specific and sex-characteristics as well as on ecological conditions. But explanations about the significance of these factors in describing the variability of social and genetic mating patterns are controversial, mainly because detailed ecological data is usually lacking in such studies (Westneat 1993).
Water Pipits Anthus spinoletta offer good opportunities to study relationships between ecology and social as well as genetic mating systems because the birds breed in a variable environment. The aim of this paper is first to describe the spatial and temporal distribution of food resources – a potential key factor in describing territory quality – and to relate them to the social mating patterns of this species whose ground-nests are exposed to a high level of nest predation. Second, intra- and intersexual differences in reproductive success are related to the relevant ecological and phenotypic determinants. In a third step we examine the amount and the effect of the occurence of extra-pair parentage on the apparent reproductive success of both sexes. In the discussion we address the reasons for the dominance of social monogamy in the system studied.
METHODS
Study area, climate and weather
The study was carried out in the northern continental zone of the central Alps in the valley of Dischma (46°6' N / 9°53' E) in Switzerland. The valley bottom runs from north-west to south-east, rising from 1560 to 2100 m. s. l. over a distance of 15 km. The highest mountain bordering the valley rises up to 3147 m.s.l. The study area encompasses 1.5 km(superscript)2 and lies in the upper, forest free part of the valley at elevations between 1830 and 2300 m. s. l. It is mainly exposed to south-west, with slopes ranging from 0 to 50 ° (mean: 22.6 ± 12.0 S. D. °). From 1990 to 1992, the main study years, mean temperature for January, July and the whole year were –6.7° C, +10.4° C, and +1.1° C, respectively – all of them being higher than the long-time averages. Average annual precipitation amounted to 1213 mm (from 1990 to 1992). In all summer month there is a chance of snowfall, even on the valley bottom, with snow cover persisting up to 2–3 days. Further details about the study area and its climate are given by Bollmann (1996).
Birds, material and methods
The water pipit is a small insectivorous, sexually mono-morphic, migrant passerine with multipurpose summer territories. Males return from their wintering grounds in late April and May when most of the area is still snow covered. They show a territorial behaviour with pronounced aggressive display, song-flight and much time spent in surveying the territory from perches on exposed look-out posts (Böhm 1986; Bollmann 1996). Contests at the boundary are often performed as parallel-walk display with the tail raised and wings drooped and subsequent air chases. Female Water Pipits, on average, arrive a few days later (Bollmann et al 1997). As in the twin species, the Rock Pipit Anthus petrosus (Askenmo et al 1994), in general females are tolerant against intruders of both sexes and do not participate in territorial contests (Verbeek 1970; Pätzold 1984). Nests are built on the ground, usually under bushes, tussocks or in rock crevices. Nest building and incubation is done by the female exclusively. Both parents provision food to the nestlings, usually five, whereas only females brood the nestlings (Rauter & Reyer 1997).
From 1990 to 1992 data were collected during the whole breeding season, in 1993 only during the periods of territory establishment, pairing and egg laying. Observations started between 22 April and 1 May which is about the time when the first individuals arrived. Adult birds were captured with mist nets either during the migration into the valley or at the nests, but there only when the chicks were older than one week and thus able to thermoregulate. Each bird was measured, weighed, sexed and individually colour-banded. In cases were a sex determination by absence or presence of a brood patch or morphological data was not possible, behavioural observations were used to get certainty. Since some birds had been born before the study began, the ages used in the analyses refer to minimum ages.
Behavioural observations and territories:
From arrival of the first birds throughout the breeding season observations were made on average six days per week. Each part of the study area was checked for individuals every third or fourth day during the breeding season. Daily observations were conducted from dawn to 1300 and from 1700 to dusk (Greenwich –1h). After the first nests had been initiated, evening observation were combined with nest controls. At these occasions egg-laying, brood-size, incubation and nestling period, breeding success and nest predation were registered. In order to determine the territory and the mating status of males and females, the presence/absence and the behaviour of all individuals were recorded during each visit and locations were entered into a map (scale 1:2500). Besides the frequencies of singing and calls, display, agonistic, courtship, foraging and nest-oriented behaviour were registered on a standardized form. Each day after field work these data were analysed and the actual territory pattern map and status of each individual was used for subsequent nest searches. Territory size and shape were determined by the minimum concave polygon method (Clutton-Brock et al. 1982) based on the typical observations point of males. These included starting and landing points in song-flights, look-out posts, sites of male-male displays and fights as well as locations of courtship flights and regular feeding activity, but excluded occasional distant feeding trips to a communal feeding site (meadow, little swamp). The estimate of territory size increased with the observation time and the number of registrations. At least 45 minutes of continuous observation time were needed to reach asymptotic size estimates. In this paper territory shape and size refer to the males' territories during the pre-breeding and egg-laying periods. The territory system is very stable at this time (Askenmo et al. 1994; own observations). The ascertainment of territory location, shape and size was done with CAD-software (Grafsoft 1992).
Nest search:
66% of all nests (n = 162) were found before hatching. Considering some nest failures before hatching – a rare event – about 95% of the nests in the study area were found. Nests were marked by a 50 to 60 cm long vertical bamboo stick placed in a distance of two meters from the nest entrance. It allowed observers to quickly re-find the nest without enhancing predation risk by directly marking it. Nests were not visited when snow covered the vegetation around the nest.
Measure of breeding synchrony:
Some researchers have used standard deviation in clutch initiation dates or the number of deviations from the modal clutch initiation date as a measure of synchrony (e.g. Brown & Bomberger Brown 1987). For this study we follow Westneat (1992) who counted for each nest the number of other nests in the study area initiated within a 5-d window around the date the first egg in the focal nest was laid. This method provides for each nest a number of other nests that are at a similar stage. These numbers we refer to as temporal neighbours.
Mating status: Four categories of mating patterns could be distinguished:
(1) Unpaired male or bachelor: territorial male that was present in the study area for at least two weeks and defended its territory at least until the year-specific median egg laying date (1990 to 1992) or until end of May (1993), but did not succeed in pairing. Males that stayed less than two weeks in the study area were assumed to be still on migration.
(2) Monogamy: male-female pair bond lasting for one or two (serial monogamy) breeding attempts within the same season. Pair bonds were determined on the basis of one or more of the following criteria: A male mate guarding a female during the pre-laying period, a male feeding a female on or close to the nest, a male giving alarm calls close to a female's nest, observation of a male feeding nestlings. Most pair-bonds could be determined by these criteria, but in some cases the necessary observations of male behaviour were missing. Then we considered a female mated with the owner of the territory she nested in. These criteria for a pair-bond also hold for polygyny and polyandry.
(3) Polygyny: simultaneous breeding of two females in the territory of one male; primary females breed earlier, secondary females breed later during the same breeding period. Primary females raise their brood with the males' contribution, secondary females and their broods usually do not receive paternal assistance (one exception). Serial polygyny was not observed.
(4) Polyandry: sequential breeding of a female with two different males after a successful first breeding attempt. First males had only one breeding attempt because they took care of the fledged young. Males of the second breeding attempt were former bachelors or males that were deserted by their first female after brood failure. This means that in polyandry only females had two breeding attempts per season. Simultaneous polyandry was not observed.
Breeding history: According to their breeding history birds were divided into three categories:
(1) Surviving adults: Birds ringed as adults in one year that returned to the study area the next year. This category includes males and females with breeding experience and two former bachelors.
(2) Recruits: One year old birds, that were ringed in the study area as nestlings and returned in the subsequent year.
(3) Immigrants: Since all nestlings that fledged within the study area were ringed, this category consists of yearlings born outside of the study area and of unringed adults from a previous year. When a nest failed due to predation (about 50%), we often did not succeed to ring its adults. On average 58% of the breeding population could be ringed (Bollmann 1996). Since, in general, males are faithful to their territory and females often to their mate some of these birds could be caught in subsequent years and assigned to category (1) with the help of DNA-fingerprints. In 1992 an intensive search was carried out for dispersed birds in an area extending 4.6 km from the study site. Only exceptional records of former breeders could be made (0 out of 54 males, 2 out of 52 females). Therefore, we think it is reasonable to assume that the vast majority of the unringed birds consisted of one year old birds born outside of the study area and thus represented true immigrants. Further evidence comes from the age pyramid of the population (Bollmann 1996).
Measures of reproductive success:
A breeding attempt is defined as the appearance of at least one egg in a nest. To determine laying date of the first egg, final clutch size, number of chicks hatched and number of young fledged, nest inspections were carried out every 2 to 3 days, but usually daily when hatching or fledging could be expected. In this way the causes of nestling mortality could be ascertained in most cases. All nestlings were individually colour-ringed at day 8 or 9 of nestling age. After day 10 nestlings were not handled any more to prevent premature fledging. Offspring survival was estimated by recording the recruitment of locally fledged young to the breeding population in the study area. Clutch size refers to the number of eggs in a nest. For nests that were found after hatching the number of nestlings was assumed to represent the original clutch size. This seems justified because nests found before and after hatching did not differ in the number of eggs or nestlings respectively (Bollmann 1996). Number young fledged refers to the number of young that successfully left the nest. Broods where at least one young fledged are termed successful breeding attempts. Seasonal reproductive success of a pair refers to the total number of young fledged during a breeding season.
Parentage analysis:
To ascertain genetic parentage multilocus DNA fingerprinting was used. Therefore, from all adult Water Pipits mistnetted, and from all nestling that survived to 8–9 days of age blood samples were taken. Details about DNA fingerprinting, scoring fingerprints and parentage analysis are described by Bollmann (1996) and Reyer et al.(1997). Several key terms require definitions: Apparent reproductive success is defined as the number young fledging from a male's or female's nest in the actual season. Realized reproductive success corresponds to the total number of young that a male had fertilized during the season as measured by DNA fingerprints. Reduced parentage means that some young in a brood are not offspring of at least one social parent. Broods with reduced parentage are called broods with extrapair young, in contrast to legimate broods in which all young were offspring of the social male and female, respectively. We defined social parents as the female attending the nest, and the male holding the surrounding territory and guarding the female during the time the nest was built and eggs were laid. A young was assigned extrapair fertilization status (EPF) when it was not a relative of at least one social parent. When a female's brood contained at least one young that was not fertilized by the social male but was related to her, she was assigned extrapair paternity status (EPP). EPP-males were assigned paternity to extrapair young on the basis of DNA-fingerprints. Parents suffered intraspecific brood parasitism (IBP) when their brood contained young that were not offspring of either social parent. Quasi-brood parasitism (QBP) occured when a extrapair female dumped an egg in a nest of another female that was paired with the male that fertilized the dumped egg.
Prey biomass and territory quality:
Prey availability was mesured in a standardised way through sweep netting every second week throughout the breeding season. The sampling along a 50 m x 50 m grid-square represented each vegetation type and altitude in proportion of their occurrence. An exception was made for copes which were not used as breeding sites by Water Pipits. The sum of the nine prey taxa which are most important as nestling food was used as an estimate of the total biomass of available prey of a grid square. Further details about prey sampling are given by Brodmann et al. (1997).
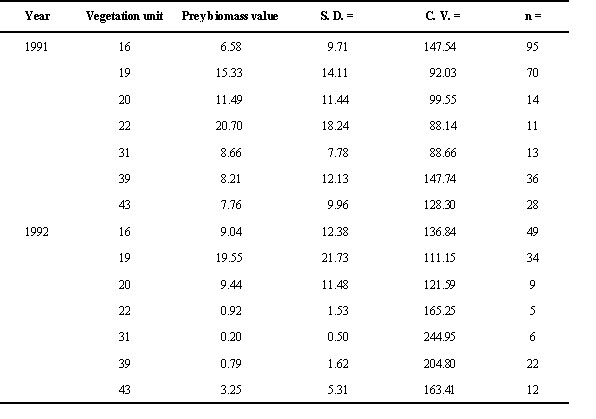
In 1991 and 1992, we used territory size and territory-specific prey biomass as measures for territory quality. Both measures are independent from each other since prey biomass is related to the grid-system. The average prey biomass density (mg dryweight per sample) – hereafter referred to as prey biomass – was calculated separately for each of the vegetation units and the first breeding period (FBP) and the late breeding period (LBP) as described by Bollmann et al. (1997). The FBP comprised all first breeding attempts, the LBP included replacement clutches and second breeding attempts. With the help of the vegetation-map we determined the proportions of various vegetation types for each territory with CAD-software (Grafsoft 1992). These proportions and the concerning vegetation-specific prey biomass of the specific year were than used to calculate territory-specific prey biomass for each breeding period separately. Vegetation-specific prey values are given in Appendix 1.
Statistics
For territories in hilly terrain a two-dimensional projection of territory size (s) onto a map produces a measure that differs from the real territory size (s*). The same is true for vegetation cover and other area-related data taken from topographical maps. Therefore we corrected such data with the following equation:
real area s* = s / cos
aHere,
a is the slope of a territory calculated from the projection of the fall-line through the centre of each territory (x) and the difference in elevation between its two cross-points with the territory border (y). The quotient y/x defines the tangent of the slope.Statistics were calculated on JMP 3.0.2 (SAS Institute 1994). Non-parametric statistics were used where requirements for parametric tests were not fulfilled. Estimates of errors are standard errors unless stated otherwise. Pairwise comparisons are two-tailed.
RESULTS
Vegetation and prey biomass
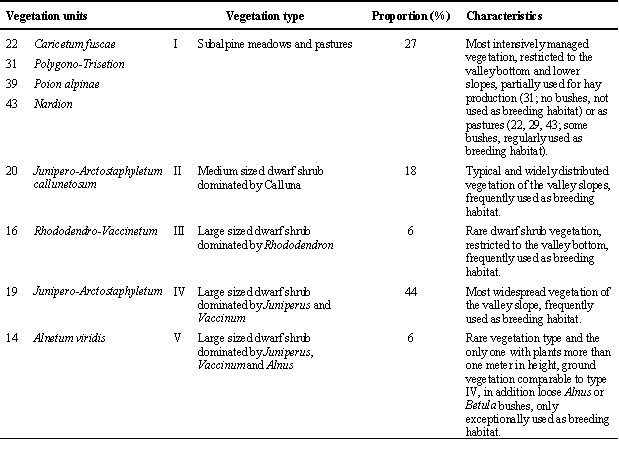
In the study area there is a mosaic of different environmental conditions. As a ground breeding passerine, the water pipit settles over a distinct altitudinal gradient. With increasing altidude, average temperatures decrease of which causes a heterogeneous snow melt pattern and a shorter breeding season in higher areas. Due to this climatic gradient, eight different vegetation units are found in the study area (Table 1). These were grouped into five vegetation types according to the abundance of various dwarf shrubs and the main management practise. Dwarf shrubs and pastures were stocked by cattle and cows in summer, meadows were used for hay production.
The different vegetation types cause differences in the abundance and biomass of insects – the main nutritiounal resource of the Water Pipit (see Appendix 1). There was a strong relationship between the vegetation-specific prey biomasses of the first with the late breeding period (Spearman's rank correlation: rs = 0.86, P < 0.001, n = 135). Thus, independent of its size, a vegetation type or a territory with high biomass resources during the first breeding period offers also good conditions later on in the breeding season or vice versa.
Territory system
During the study, population density increased from 3.44 territories per 10 ha in 1990 to 6.78 in 1993. Territory distribution was irregular because some vegetation types (meadows and copes) were not used for breeding (Fig. 1). In meadows there was a lack of suitable nesting sites, but this vegetation type was used for foraging early and late in the season. Copes were avoided as soon as they came into leaf and became dense subsequently. Out of the 279 recorded territories 43.9% consisted of two, 34.8% of one, 20.5% of three and 0.8% of four vegetation types. Mean territory size ranged from 0.98 to 1.47 ha and differed between years (Kruskal-Wallis 1-way ANOVA: H2 = 37.78, P < 0.001, n = 232). This variation was caused by on average smaller territories in 1993 – the year with the highest population density (U-test: 1991 vs. 1992: Z = 0.11, P = 0.92, n = 132; 1991 vs. 1993: Z = 5.23, P < 0.001, n = 160; 1992 vs. 1993: Z = 5.04, P < 0.001, n = 172).
Territories are mainly exclusive but 31% (n = 232) overlap with one or several neighbouring territories by 5 to 65% of their area (estimates of territory map). For 1992 male territories have been projected onto female home ranges to calculate the overlap. In 52% out of 64 territories home ranges of females lay completely within their males' territory boundaries. About every second female used additional areas outside of her male's territory. These areas amounted on average to 25.4 ± 24.5 S. D. % (range 2.0 to 99.0, n = 31) of the male's territory size and were negatively related to territory sizes (Spearman rank correlation: rs = –0.38, P < 0.03, n = 31). Thus, the smaller her male's territory the more a female tended to leave it.
Mating system: mating status and age of the sexes
In all study years the sex ratio was slightly male biased but did not differ from equality. Table 2 summarizes the frequencies of mating patterns for the total study period and broken down by years. There was no significant variation in the frequencies of the systems between the years (1990 to 1992, Chi-square test: Chi-square4 = 1.99, P = 0.74). In all years the most common pattern was one male with one female (monogamy). On average, 86% of the males and 94% of the females were monogamous. Out of the 148 cases of monogamy from 1990 to 1992, in only two cases (1.3%) the same pair performed a second breeding attempt after successfully raising a first brood (serial monogamy with same mate). Next in frequency (9%) were unpaired territorial males (bachelors). Cases where one male was simultaneously paired to two females (polygyny) or one female sequentially mated with two males (polyandry) were rare and amounted only to 3% and 2%, respectively.
As in most migrant passerines female Water Pipits return later than males. Whether females choose mate or territory qualities is hard to separate as they are likely to covary. Our data show an age-assortative mating pattern with 71% of one year old females mated to yearling males (n = 41) and 82% of older females paired with older males (n = 39; Chi-square1 = 22.50, P < 0.001).
Table 3 shows the ages of males and females in different mating patterns. Considering only individuals whose exact age is known, there were no age-related differences among females, but among males there were. The proportion of males remaining bachelors throughout the season or of those mating only late with females that had a first brood with another male (polyandry) decreases with age. After including unbanded immigrants and assuming that they are yearlings, this relationship between male age and mating pattern is strengthened and becomes obvious from a positive correlation between the percentage of at least two year olds and the ratio of females per male (rs = 1.0, P = 0.05).
Resources: territory size and food supply
Fig. 2 is a graphic representation of the relationship between male mating status and territory size. Compared with monogamous birds bachelor territories were 1.7 times smaller, territories of polygynous males 2.8 times larger. Males of polyandric females held territories that equalled in size those of monogamous males. This is to be expected since the pair bond during one single breeding attempt was monogamous for both sexes.
An index of territory quality independent of territory size, is prey biomass, measured in mg dryweight per sample (see Methods). Prey biomass ranged from 0.0 to 19.6 with an overall mean of 11.1 ± 0.4 S. D. (n = 132). No difference between the two years was found (Z = –0.18, P = 0.86). Prey biomass increased from territories of bachelors through those of monogamous males (including males mated to polyandrous females) to territories of polygynous males (Fig. 2). Territory size and territory specific prey biomass showed a significant positive correlation (rs = 0.36, P < 0.001; n = 132). Thus, territories of polygynous males provided not only more food because they were larger, they were also more profitable per unit area. To test which of the two correlated variables influences the mating pattern most, we performed a logistic regression with prey biomass and territory size as independent variables. Size significantly influenced male mating status (P < 0.002) whereas the influence of prey biomass was neglectable (P = 0.50). Both factors together explained 18% of the models variance (Chi-square6 = 29.72, P < 0.001, n = 130).
Reproductive output
Nest predation occurred in 44 percent of the breeding attempts (n = 131) and explained by far the most variance in reproductive success. The main predator was the Adder Vipera berus. The spatial occurrence of nest predation (two catragories: yes/no) seemed to have a stochastic and unpredictable character and did neither depend on the vegetation composition of a territory (logistic regression: Chi-square5 = 2.50, P = 0.78, n = 131) nor on the study plot a nest lay in (Chi-square1 = 1.15, P = 0.29, n = 131).
Per nest:
Now, we consider how the number of young fledged per nest varied among the different mating combinations. The analysis is based on a total of 137 breeding attempts during 1990 to 1992. Reproductive output per nest did not vary significantly across the different mating patterns (Table 4), neither when only successful attempts were considered (H3 = 1.97, P = 0.58, n = 75), nor when all attempts were included (H3 = 4.66, P = 0.20, n = 137), nor when the percentage of nest failure was tested (Chi-square3 = 3.40, P = 0.36, n = 137). This is probably due to small sample sizes for mating patterns other than monogamy. It is interesting, however, that the secondary females in polygynous matings which received no male help (one exception) performed worst in all three measures of reproductive success. Only two out of seven successfully raised a brood. In the other five cases unsuccessful breeding attempts originated twice from nest desertion, once from nestling starvation and twice from nest predation.
For the breeding attempts in 1991 and 1992 the average number of young fledged per nest did not differ with age of males (Z = 1.07, P = 0.30, n = 106) nor with age of females (Z = 2.60, P = 0.11, n = 106). Also, territory size and territory specific prey biomass did not explain variation in reproductive success (linear regression: R2 = 0.01, df = 2, P = 0.49, n = 104).
Per season:
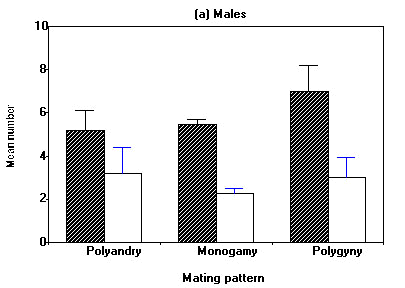
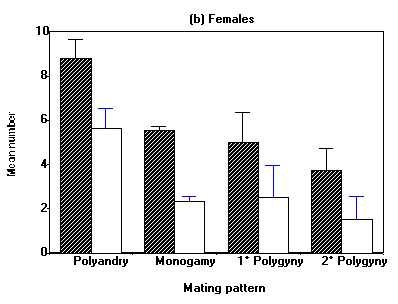
Differences in reproductive output of different mating patterns become more obvious when seasonal reproductive performance is considered (Fig. 3). While males of the various mating patterns do not differ in the total number of eggs and the total number of young fledged within a season (Fig. 3a), polyandric females laid significantly more eggs and fledged more young than all other three mating combinations (Fig. 3b). In contrast to some polyandric females, females that stayed with her mate for a second brood did not succeed to raise it successfully. For the two cases of serial monogamy with the same mate we registered 9.5 ± 0.7 eggs and 4.0 ± 2.2 young fledged per season.
The mean number of young fledged per season, however, did differ for various age classes in both sexes (Table 5). Three year old males and females fledged 1.5 to 2.1 times more young within a season than one or two year old conspecifics. Birds that bred for their first time did not differ from two year old parents. These results are probably the consequence of differences in the nest predation rate. The percentage of predated nests was 38.2, 51.7 and 0.0 for one, two and three or more year old males (n = 55, 29, 7), respectively, and 37.5, 47.6 and 14.3 for females of the same age classes (n = 64, 21, 7). So, for both sexes predation was lowest at nests of three year old birds, but only for males was the age-dependence significant (Chi-square2 = 6.42, P < 0.04).
Genetic parentage
We performed paternity analyses on 95 families with a total of 393 nestlings (Table 6), involving 77 different males and 77 females. These figures include 21 unringed males and 14 females. Except for one male and female, each, where we could show by DNA-fingerprinting that they were parents in a former year, unringed birds were treated as one individual each. For other unringed individuals such an analysis was negative or we had no evidence (e.g. territory fidelity, behaviour, plumage traits) that they had been breeding in a former year. A two-way ANOVA with percent extrapair fertilized-(EPF-)young as the dependent variable (arcsine square-root transformed; F16, 78 = 0.54, P = 0.96) showed no effect of male identity (P = 0.96), nor of year (P = 0.81). Similarly, there was no effect of female identity (P = 0.85) and year (P = 0.77) on the model (F16, 78 = 0.69, P = 0.86). Results did not differ when we included only data of ringed individuals. Therefore, we are confident that our use of broods as database does not create a problem of pseudoreplication.
Patterns of extrapair fertilizations:
In 67 (70.5%) out of the 95 broods, blood samples were available from both adults and the young, in 12 (12.6%) from the female and the young, in 4 (4.2%) from the male and the young, and in 12 (12.6%) from the young only. In most families all nestling fingerprints matched the fingerprints of both putative parents or the offspring were fullsibs. Each year the mean frequency of broods with extrapair offspring was moderate to low with no significant variation between years (Chi-square2 = 1.18, P = 0.55, n = 95; Table 6). Averaged over 1990 to 1992 the mean proportion of extrapair young per year was 7.0% (S. D. = 2.0) and the mean proportion of broods with extrapair young 20.4% (S. D. = 6.5). Pooled over all three years the proportion of broods with extrapair young was 18.9% (18 of 95 broods) and the total proportion of EPF-young 7.1% (28 out of 393 nestlings). Within the 18 broods with extrapair offspring we found 15 cases (83.3%) of extrapair paternity and three cases (16.7%) of extrapair maternity.
(a) Extrapair paternity (EPP): The proportion of EPP-young per brood varied from 20% (1 nestling out of 5) to 80% (4/5) with a mode of one EPP-young or a total of 42% in the EPP-broods sired by an extrapair-male. Within each brood, all EPP-young were always sired by the same male. In two out of the 15 EPP-broods we know the extrapair-male. One was a next neighbour bachelor at the time of its successful fertilization, the other performed the EPF during the nestling period of his own brood (assuming a fertile period from –6 to +3, with date of first egg = 0: Lake 1975; Birkhead et al. 1989). In this case, seven males were established at shorter distances and two territories lay between the EPP-brood and his territory. This male suffered from having EPP-young in his own brood in the same year. Extrapair paternity occurred in all observed mating patterns, except in broods of primary females of polygynous males. In its frequency none of the four other mating patterns differed from expectation (Fisher's Exact Test: 0.12 _ P _ 0.52). Three (20%) out of the 15 cuckolded males did not feed their nestlings.
(b) Extrapair maternity (EPM): Out of the three cases of extrapair maternity, twice intraspecific brood-parasitism (IBP) and once quasi-brood parasitism (QBP) occurred. In one IBP-brood one egg was dumped, in the other one dumped egg was accompanied by a young sired by EPF or QBP (no blood of both parents). None of the females suffering from extrapair maternity had own young sired by EPF in her nest. In both IBP-broods hatching took place within one day and the morphological measures of the extrapair young were intermediate between those of the legitimate ones. Thus, it seems likely that the eggs were dumped during the egg laying period of the parasitized female. In the QBP-nest hatching was spread over two days. This nest was found on 6 June when it contained four eggs. Between the nest controls of 8 and 9 June, one egg was added. Since all females laid one egg per day (Bollmann 1996) and the EPM-young was the smallest and lightest of the brood, we conclude that the last egg was the dumped one and that with high probability the social male engaged in EPC after the laying period of its pair-female.
Correlates of extra-pair parentage and effects on reproductive success
We investigated potential ecological (time, territory size, prey biomass) and phenotypic (age, tarsus lenght) correlates of EPP. The analysis revealed breeding synchrony to be the only factor significantly related to the occurence of EPP. So the proportion of EPP-clutches was higher in territories with fewer temporal neighbours (logistic regression: Chi-square = 4.29, P < 0.04, n = 92). Within EPP-broods, the percentage of EPP-young increased with territory size (rs=0.613, P < 0.045, n = 11; only data of 1991 and 1992) and also with the distance from territories to communal feeding sites (rs = 0.592, P < 0.020, n = 15; 1990–1992). A measure of individual quality is fertility. If females engage more often in EPCs when their social mates are of low fertility, we would expect more clutches with infertile eggs and a higher proportion of unhatched eggs per brood among EPP- than in legitimate broods. Both expectations, however, were not fulfilled. Neither did clutches with infertile eggs occur more frequently in EPP-broods (13.3%, 2 out of 15) than in legitimate broods (9.1%, 7 out of 77; Fisher's Exact Test, one-tailed: P = 0.451), nor did the proportion of unhatched eggs in EPP-broods (2.4 ± 2.2 S. E., n = 15) differ from those in legitimate broods (2.5 ± 1.0 S. E., n = 77; ANOVA: F1, 90 = 0.04, P = 0.85, squared-root arcsine transformed values).
Compared with their apparent reproductive success, the realized reproductive success (rRS) of males that suffered from EPFs decreased by 40.7%. However, this figure may be too pessimistic since we know from at least one cuckolded male, raising one EPF-young, that he improved his rRS by two EPFs. Thus, a figure of approximately 35% may be more realistic. The parasitized females' (n = 3) realized reproductive success was 21.4% lower than their apparent reproductive success.
Considering the level of the whole population, the frequency of EPF-young did not strongly bias the measure of males' or females' RS. Even when we restricted the analysis to successful broods only, we found a strong positive correlation between apparent and realized reproductive success for both sexes (males: r = 0.81, P < 0.001, n = 85; females: r = 0.98, P < 0.001, n = 85). Since we knew only a few of the extrapair males and females, the estimates for the sex-specific average rRS of the population are minimum figures and the correlations may be even stronger.
DISCUSSION
Phenotypic parameters
Selection pressure on female Water Pipits to choose by reliable territory and/or male characteristics must have been strong because (a) individuals suffer a high degree of nest predation and often complete loss of all breeding attempts, and (b) individuals are likely to survive only one breeding season due to the bottom-heavy age pyramid (Bollmann 1996). In a breeding habitat where environmental parameters cannot be assessed reliably at the time of pairing, female preference for males that survived at least one breeding season could be the solution. This may have resulted in the observed age-assortative mating pattern. In short-lived species, age and its correlates can be good and reliable indicators of male quality and were shown to be used as mate choice criteria in other studies (Millington & Grant 1983; Grant & Grant 1989). Older males have survived more threats from food shortage, predators, parasites, and pathogens, and therefore they could be a selected group with higher than average genetic quality (Hamilton & Zuk 1982; Searcy 1982; Manning 1985).
Social mating system
In noncolonial passerines polygyny probably evolved at least partially in response to differences in food availability between territories (Wittenberger 1976, 1979). Numerous studies have shown high proportions of polygynous matings in birds breeding in habitats of two-dimensional vegetation structure – like marshes, steppes, and savannahs (Verner & Willson 1966; Orians 1969, 1980; Holm 1973; Wittenberger 1976; Catchpole et al.1985; Ezaki 1990; Bensch & Hasselquist 1992). Verner & Willson (1966) mentioned some evidence that the potential range of difference in productivity between two sites is greater in a two-dimensional system than in a three dimensional one. Therefore these habitats should have a high environmental potential for polygyny. My Water Pipit population fits several assumptions of the polygyny threshold model (PTM) for the occurrence of polygyny fairly well:
(1) Ecological variation:
The birds breed in an open alpine steppe with high variation in ecological factors, including prey abundance.
(2) Costs to females of sharing a male:
Secondary females had costs in terms of reproductive success. However the same difference could have resulted from poorer quality females associating as secondary females with polygynous males.
(3) Females can choose:
On average females spent 18 days between arriving in the breeding area and the initiation of the clutch (Bollmann 1996). This time could be used to visit various territories or males.
(4) Females settle on male territories:
Male territory boundaries were rather stable during a breeding season. About fifty percent of the females remained within their males' territory boundaries (Bollmann et al. 1997). We have no indication at all that females built up own territories and that males adapted their territory boundaries to monopolize females as they do in the Dunnock Prunella modularis (Davies 1992).
(5) Females are free to settle where they choose to:
Aggression between females which theoretically could influence female settlement patterns is lacking. Over four years we only have one observation of a female-female aggression occurring during the second breeding period, well after territory establishment.
(6) Breeding synchrony:
In addition to the spatial distribution of resources, the temporal distribution of mates influences the potential for polygamy. Among species in which both sexes contribute to parental care, a moderate degree of breeding asynchrony is essential for the expression of polygamy (Emlen & Oring 1977). Often the operational sex ratio (OSR) becomes skewed with the lenght of the breeding season. In the Water Pipit, this condition was fulfilled. During the breeding season nest losses occurred frequently due to predation and snow and subsequently there was a more or less constant pool of renesting and thus fertile females in the population. The period of egg-laying was continuous and amounted to 52 to 53 days in each year (Bollmann, unpublished manuscript).
Although the assumptions of the PTM and the OSR-model apply more or less well to the Water Pipit, monogamy was the predominant mating pattern and polygyny occured only exceptionally (1990 to 1993: 0.0–4.3%). These figures lie below the generally accepted level of 5% for non-accidental occurrence of polygyny (Verner & Willson 1966). Since both, male and female, can be expected to optimize their number of offspring and thus maximize their individual fitness (Trivers 1972; Alatalo et al. 1981; Davies & Houston 1986; Davies 1989, 1992) we have to consider costs and benefits of the different mating patterns for both sexes separately to explain why monogamy prevails:
(a) Males:
For males seasonal reproductive success does not show any difference between polyandry, monogamy and polygyny. Only one out of seven polygynous males fledged more offspring from the two nests combined (9) than the maximum from a single nest (6). In general the fitness gain in mating polygynously seems to be slight, similar to other species where polygyny is rare (Dhondt 1987; Grant & Grant 1989). The reason may lie in the low reproductive success of secondary unassisted females which performed poorly in our study (Table 5). In addition, even a slight fitness gain is probably outweighed by the costs, since polygyny requires a large territory. Monopolization of such a territory might be costly due to intrasexual competition. Evidences for competition comes from the following results: (i) territories became smaller with increasing population density (Bollmann 1996), (ii) meadows and copes were unsuitable as breeding territories, and thus intrasexual competition of successful males focussed on pasture or dwarf shrub vegetation, (iii) the sex ratio was slightly male biased (Bollmann 1996), (iv) inexperienced yearlings were more often bachelors and defended smaller territories with lower prey biomass, (v) among cuckolded males the proportion of extrapair young was higher in large than in small territories. During the pre-laying period Water Pipits often visited undefended communal foraging sites that lay mainly in meadows (Bollmann et al. 1997), and males faced the trade-off between mate guarding and territory defense.
Although Davies et al. (1995) expect 'dunnock-like' variability in mating systems in species feeding on small prey items and breeding in dense vegetation, Water Pipits better fit the widespread assumption of females settling on a male's territory. The pronounced aggressive behaviour of males resulted in a stable territory pattern also existing in Rock Pipits, even after food supplementation during the pre-breeding period (Askenmo et al. 1994). Such a stable pattern in turn might reduce the costs of extrapair fertilizations by evicting neighbours and preventing settlement by floaters. An aggressive territorial behaviour is typical for primary monogamous species (e.g. Arcese 1987, 1989; Freed 1987).
(b) Females:
For females seasonal reproductive success was higher in polyandry compared to monogamy and polygyny which did not differ. This difference was an effect of polyandric females having two broods and for two reasons did not seem to depend on differences in food supply: (i) prey biomass differed between territories of bachelors and paired males but not between paired males of different mating status, (ii) territory-specific prey biomass was not related to reproductive success and pairs succeeded to fledge broods of the most common size (5) within a wide range of territory-specific prey biomass (3.1 to 19.6 mg dryweight per sample or 14.9 to 200.6 mg dryweight per territory). Further, reproductive output did not differ between the years although population density increased (Bollmann 1996). Therefore we conclude that the study area had a low environmental potential for polygyny, at least in terms of food supply. This conclusion is supported by observations on the close related Rock Pipit (Askenmo & Neergaard 1990). The authors assume that females trade safety of a nest site against male assistance and that there is a negative relationship between the frequency of polygyny and nest predation pressure.
Although we have only a small sample size, polyandric females seem to have an advantage over serially monogamous females. The former could shorten their breeding season by re-mating with a new mate and breeding immediately after fledging the first brood because the first male continued to provision the fledged young; the latter may have problems initiating a second clutch in a territory where the young are not yet independent and are constantly begging. For the five polyandric females the average time period between fledging the first brood and laying the first egg of the second clutch on average amounted to 6.8 ± 0.4 days (range: 6–8 d), for one of the two serially monogamous females it was 13 days. Such a time gain of about one week might be an important precondition for a successful second breeding attempt since adults start to moult during the time of the last broods in the population.
Genetic mating system
During the three years of the water pipit study we found moderate to low frequencies of extrapair parentage: 4.9% to 8.9% of the 393 young and 15.6% to 27.8% of the 95 broods. These figures include one case of extrapair maternity in each year. The frequent occurrence of nest predation (44%) restricted the number of broods that could be analysed through DNA-fingerprinting to 95 out of 162 and of breeders caught since they were normally mistnetted at the nest around day 10 of nestling age. Thus we were not able to identify all the EPP-males and EPM-females. Nevertheless we know from band-sharing coefficients that all EPF-young within a brood were sired by the same male. Despite the lack of identifying all extrapair adults, we can conclude that in our population the low level of extrapair parentage did not bias the measure of reproductive success (RS) and thus, the apparent RS of male and female Water Pipits adequately reveals the realized RS on the level of the population.
CONCLUSIONS
In conclusion, monogamy is the dominant social mating pattern in our study because ecological differences among breeding territories (i) are not large enough to compensate for the loss of paternal care, (ii) are reduced because about every second female leaves the territory for foraging and (iii) cannot be assessed reliably at the time of settlement (Bollmann et al. 1997). Consequently the environmental potential for polygyny threshold is not reached. The few cases in which we do find deviations from the prevailing monogamous mating pattern can be better explained through differences in the age of males.
Next to the dominance of social and genetic monogamy, both extrapair paternity and maternity occurred in our population of Water Pipits and resulted in multiple parentage. For a larger sample, Reyer et al. (1997) could show that this pattern seems to be an 'ecological epiphenomenon' with low potential for selection. For females extra-pair activities can be very costly if males are able to detect it and reduce paternal care (e.g. participating in the provisioning of nestlings; Møller 1988; Burke et al. 1989). Presence or absence of male participation can not only increase or decrease the number of young fledged, but also determine whether or not a breeding attempt will fledge young at all. Also males suffer from extra-pair activities of their mates. The partial spatial separation between breeding territories and feeding sites poses a dilemma between mate guarding and territory defence.
In summary, under the harsh alpine breeding conditions of Water Pipits the potential for a sexual conflict seems to be small. Age is the best phenotypic parameter that explaines differences in reproductive success. We suggest that this trait is important for territorial species under conditions where the significant habitat charactersitics can not be assessed reliably at the time of pairing.
ACKNOWLEDGMENTS
We are grateful to Paul Brodmann for providing the basic prey data and to Angela Schymainda and Gaby Klecack for developing an performing the fingerprinting techniques. A special thank goes to all other colleagues participating in the Water Pipit project and to our hosts in the valley of Dischma. This research was financed by the Swiss National Science Foundation (SNF) through a grant to H.-U. Reyer (No. 31–26308.89).
References
Alatalo, R. V., Carlson, A., Lundberg, A. & Ulfstrand, S. 1981. The conflict between male polygamy and female monogamy: the case of the pied flycatcher Fidecula hypoleuca. American Naturalist 117: 738-753.
Arcese, P. 1987. Age, intrusion pressure and defence against floaters by territorial male song sparrows. Animal Behaviour 35: 773-784.
Arcese, P. 1989. Territory acquisition and loss in male song sparrows. Animal Behaviour 37: 45-55.
Askenmo, C. & Neergaard, R. 1990. Polygyny and nest predation in the rock pipit: Do females trade male assistance against safety? In: Blondel, J. Gosler, A. Lebreton, I.-O. & McCleery, R. (Eds), Population Biology of Passserines Birds. Berlin; Springer Verlag: 331-343.
Askenmo, C. Neergaard, R. & Arvidsson, B. 1994. Food supplementation does not affect territory size in rock pipits. Animal Behaviour 47: 1235-1237.
Bensch, S. & Hasselquist, D. 1992. Evidence for active female choice in a polygynous warbler. Animal Behaviour 44: 301-311.
Birkhead, T. R. & Møller, A. P. 1992. Sperm Competition in Birds: Evolutionary Causes and Consequences. London; Academic Press: 282pp.
Birkhead, T. R. & Møller, A. P. 1996. Monogamy and sperm competition in birds. In: Black, J.M. (ed.) Partnerships in Birds: The Study of Monogamy. London; Oxford University Press: 323-343.
Birkhead, T. R., Hunter, F. M. & Pellatt, J. E. 1989. Sperm competition in the zebra finch, Taeniopygia guttata. Animal Behaviour 38: 935-950.
Böhm, Ch. 1986. Revierverhalten und Revierkriterien beim Wasserpieper (Anthus spinoletta). Ökologie der Vögel 8: 145-156.
Bollmann, K. 1996. The mating system of the alpine water pipit Anthus spinoletta in a variable environment: ecological, demographic and fitness aspects. PhD Thesis, University of Zürich, Zürich, Switzerland.
Bollmann, K. Reyer, H.-U. & Brodmann, P. A. 1997. Territory quality and reproductive success: can Water Pipits Anthus spinoletta assess the relationship reliably? Ardea 85: 83-98.
Brodmann, P. A. Reyer, H.-U. Bollmann, K. Schläpfer, A.R. & Rauter, C. 1997. The importance of food quantity and quality for reproductive performance in alpine water pipits (Anthus spinoletta). Oecologia 109: 200-208.
Brown, C. R. & Bomberger Brown, M. 1987. Group-living in cliff swallows as an advantage in avoiding predators. Behavioral Ecology and Sociobiology 21: 97-107.
Burke, T., Davies, N. B., Bruford, M. W. & Hatchwell, B. J. 1989. Parental care and mating behaviour of polyandrous dunnocks Prunella modularis related to paternity by DNA fingerprinting. Nature 338: 249-251.
Catchpole, C. K., Leisler, B. & Winkler, H. 1985. The evolution of polygyny in the great reed warbler Acrocephalus arundinaceus: a possible case of deception. Behavioral Ecology and Sociobiology 16: 285-291.
Clutton-Brock, T. H., Guiness, F. E. & Albon, S. D. 1982. Red Deer: Behavior and Ecology of two Sexes. Edinburgh; Edinburgh University Press. 378pp.
Davies, N. B. 1989. Sexual conflict and the polygamy threshold. Animal Behaviour 38: 226-234.
Davies, N. B. 1992. Dunnock Behaviour and Social Evolution. Oxford; Oxford University Press. 272pp.
Davies, N. B. & Houston, A. I. 1986. Reproductive success of dunnocks Prunella modularis in a variable mating system. II. Conflicts of interest among breeding adults. Journal of Animal Ecology 55: 139-154.
Davies, N. B., Hartley, I. R., Hatchwell, B. J., Desrocher, A., Skeer, J. & Nebel, D. 1995. The polygynandrous mating system of the alpine accentor, Prunella collaris. I. Ecological causes and reproductive conflicts. Animal Behaviour 49: 769-788.
Dhondt, A. A. 1987. Reproduction and survival of polygynous and monogamous blue tit Parus caeruleus. Ibis 129: 327-334.
Emlen, S. T. & Oring, L. W. 1977. Ecology, sexual selection and the evolution of mating sytems. Science 197: 215-223.
Ezaki, Y. 1990. Female choice and the causes and adaptiveness of polygyny in great reed warblers. Journal of Animal Ecology 59: 103-120.
Freed, L. A. 1987. Territory takeover and sexually-selected infanticide in tropical house wrens. Behavioral Ecology and Sociobiology 19: 197-206.
Gowaty, P. A. 1981. An extension of the Orians-Verner-Willson model to account for mating systems besides polygyny. American Naturalist 118: 851-859.
Gowaty, P. A. 1985. Multiple parentage and apparent monogamy in birds. In: Gowaty, P.A. & Mock, D.W. (eds) Avian Monogamy. Washington, DC; American Ornithology Union: 11-21.
Grafsoft. 1992. MiniCad+ User Manual. Version 4.0. Elicott City, MD, USA; Diehl Grafsoft Inc.
Grant, B. R. & Grant, P. R. 1989. Evolutionary Dynamics of a Natural Population: The Large Cactus Finch of the Galápagos. Chicago; Univ. of Chicago Press. 350pp.
Hamilton, W. D. & Zuk, M. 1982. Heritable true fitness and bright plumage in birds: A role for parasites? Science 218: 384-387.
Holm, C. H. 1973. Breeding sex ratios, territoriality, and reproductive success in the red-winged blackbird (Agelaius phoeniceus). Ecology 54: 356-365.
Lack, D. 1968. Ecological Adaptations for Breeding in Birds. London; Methuen. 409pp.
Lake, P. E. 1975. Gamete production and the fertile period with particular reference to domesticated birds. Symposium of the Zoological Society London 35: 225-244.
Manning, J. T. 1985. Choosy females and correlates of male age. Journal of Theoretical Biology 116: 349-354.
Millington, S. J. & Grant, P. R. 1983. Feeding ecology and territoriality of the cactus finch Geospiza scandens on Isla Daphne Major, Galápagos. Oecologia 58: 76-83.
Møller, A. P. 1988. Paternity and paternal care in the swallow, Hirundo rustica. Animal Behaviour 36: 996-1005.
Orians, G.H. 1969. On the evolution of mating systems in birds and mammals. American Naturalist 103: 589-603.
Orians, G.H. 1980. Some Adapatations of Marsh-nesting Blackbirds. Princeton; Princeton University Press. 295pp.
Pätzold, R. 1984. Der Wasserpieper Anthus spinoletta. Wittenberg; A. Ziemsen: 108pp.
Rauter, C. & Reyer, H.-U. 1997. Incubation pattern and foraging effort in the female Water Pipit Anthus spinoletta. Ibis 139: 441-446.
Reyer, H.-U. Bollmann, K. Schläpfer, A.R., Schymainda, A. & Klecack, G. 1997. Ecological determinants of extrapair fertilizations and egg dumping in Alpine water pipits (Anthus spinoletta). Behavioral Ecology 8: 534-543.
SAS Institute. 1994. JMP: Statistics and Graphics Guide. Version 3. Cary, NC, USA; SAS Institute Inc.
Searcy, W. A. 1982. The evolutionary effects of mate selection. Annual Review of Ecology and Systematics 13: 57-85.
Trivers, R. L. 1972. Parental investment and sexual selection. In: Campbell, B. (ed.) Sexual Selection and the Descent of Man. Chicago; Adline Press: 136-179.
Verbeek, N. A. M. 1970. Breeding ecology of the water pipit. Auk 87: 425-451.
Verner, J. 1964. Evolution of polygamy in the long-billed marsh wren. Evolution 18: 252-261.
Verner, J. & Willson, M.F. 1966. The influence on habitats on mating systems of North American passerine birds. Ecology 47: 143-147.
Westneat, D. F. 1992. Nesting synchrony by female red-winged blackbirds: effects on predation and breeding success. Ecology 73: 2284-2294.
Westneat, D.F. 1993. Polygyny and extrapair fertilizations in eastern red-winged blackbirds (Agelaius phoeniceus). Behavioral Ecology 4: 49-60.
Wickler, W. & Seibt, U. 1983. Monogamy: an ambiguous concept. In: Bateson, P. (ed.) Mate choice. Cambridge; Cambridge Univ. Press: 33-55.
Wittenberger, J.F. 1976. The ecological factors selecting for polygyny in altricial birds. American Naturalist 110: 779-799.
Wittenberger, J.F. 1979. The evolution in mating sytems in birds and mammals. In: Marler, P. & Vandenbergh, J. (eds) Handbook of Behavioral Neurobiology: Social Behavior and Communication. New York; Plenum: 271-349.
Wittenberger, J. F. & Tilson, R. L. 1980. The evolution of monogamy: hypotheses and evidence. Annual Review of Ecology and Systematics 11: 197-232.
Zumbühl, G. & Burnand, J. 1986. Vegetationskarte Davos-Parsenn-Dischma. 1:25'000. In: Wildi, O. & Ewald, K. (eds) Der Naturraum und dessen Nutzung im alpinen Tourismusgebiet von Davos: Ergebnisse des MAB-Projektes Davos. Beilage. Birmensdorf; Eidg. Anstalt für forstliches Versuchswesen.
Table 1. Classification and characterization of the vegetation in the study area. Eight vegetation units (after Zumbühl & Burnand 1986) were grouped into five vegetation types according to the abundance of various dwarf shrubs and the management practise.

Table 2. Frequencies of the various social mating patterns for the years 1990 to 1993.
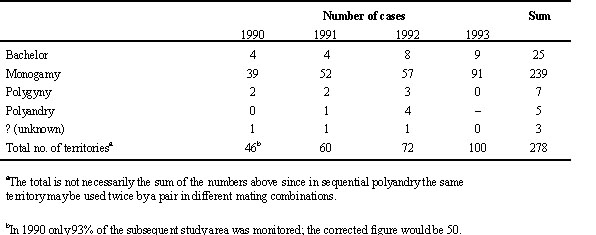
Table 3. Ages of males and females involved in the various mating patterns from 1991 to 1993. The total column includes unbanded immigrants – assuming that they all belong to the age class one year – for the years 1992 and 1993. The numbers for 1991 were excluded from this column since in this year unbanded birds could have also included some surviving adults (see Methods). The proportion of males in the different mating systems varies significantly with exact age (pooled for ff / m < 1.0 vs. ff / m ³ 1.0) and with age classes (1, 2, ³ 3 yr: Chi-square = 15.10, df = 2, P < 0.001). The proportion of females does not differ significantly with exact age (pooled for mm / f < 1.0 vs. mm / f ³ 1) and with age classes (1, 2, ³ 3 yr: Chi-square = 0.47, df = 2, P = 0.79).
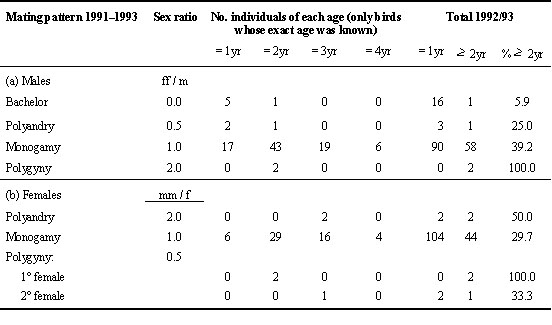
Table 4. The number of young fledged per nest as well as percentages of nest failures (0 young fledged) in the different mating patterns (1990–1992).
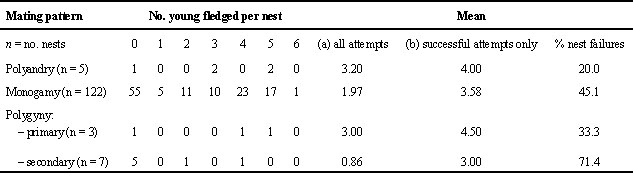
Table 5. Seasonal reproductive success of male and female Water Pipits of different age classes. Only birds whose exact minimal age was known are considered. Data given are means ± 1 S. E., sample sizes in brackets. Significance levels refer to 2-tailed t-tests between age class
³ 3 and the others: (*) P < 0.1; * P < 0.05; ** P < 0.01.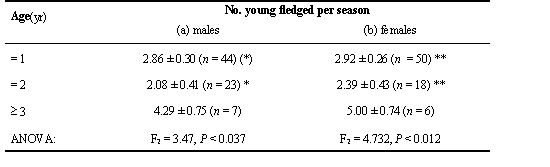
Table 6. Population size, territory density (territories per 10 ha), total number of broods with young reaching banding age (8–9 d) and number of broods and young in the Water Pipit population that were analyzed by DNA fingerprinting from 1990 to 1992. Percentages (in brackets) refer to the sample size in the line above.
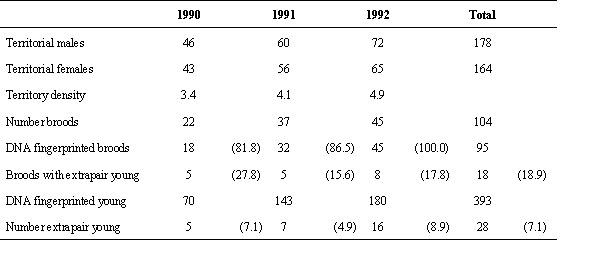
Fig. 1. Vegetation map of the study area (a) and distribution of Water Pipit territories in the breeding season 1992 (b). The border of the study area is marked with the thick line. Note that the territories are distributed irregularly. Vegetation map after Zumbühl & Burnand (1985): 14 = Alnetum viridis, 16 = Rhododendro-Vaccinetum, 19 = Junipero-Arctostaphyletum, 20 = Junipero-Arctostaphyletum callunetosum, 22 = Caricetum fuscae, 31 = Polygono-Trisetium, 39 = Poion alpinae, 43 = Nardion.
Fig. 2. Differences in territory size (hatched bars) measured in ha and territory-specific food biomass (open bars) measured in mg dryweight per sample for males in different mating patterns based on data from May of 1991 and 1992. Shown are means with standard errors and sample sizes. Pairwise comparisons for territory size: territories of polygynous males are larger than all others (Polyandry P < 0.01, others P < 0.001), and monogamous males have larger territories than bachelors (P < 0.001); for prey biomass: territories of bachelors show prey biomass values that are significantly lower (on average 33–45%) than those of monogamous (P < 0.01) and from polygynous males P < 0.03).
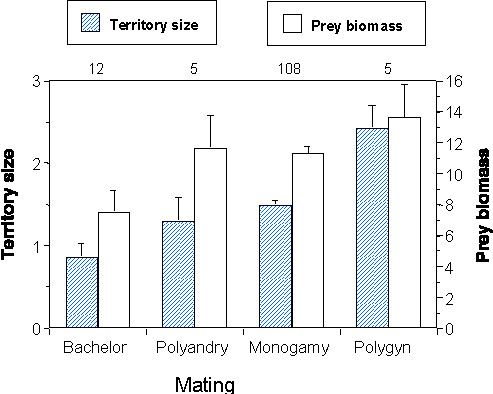
Fig. 3. Seasonal reproductive performance of male (a) and female (b) Water Pipits for various social mating patterns. Hatched and open columns represent mean number of eggs and fledged young, respectively. Vertical lines denote standard errors. (a) One-way ANOVA: eggs: F2, 98 = 0.86, P < 0.43; fledglings: F2,98=0.56, P<0.57. No pairwaise comaprisons significant. (b) One-way ANOVA: eggs: F3, 96 = 5.92, P < 0.001; fledglings: F3, 96 = 4.14, P < 0.01. Pairwaise comparisons (t-test): eggs: Clutches of polyandric matings are bigger than all others (1° Polygyny P < 0.05, others P < 0.001); young fledged: polyandric birds fledge more young than monogamous and 2° polygynous ones (P < 0.001 resp. 0.01).
Appendix 1. Estimates of available prey biomass (mg dryweight per sample) for first breeding attempts in 1991 and 1992 for 7 different vegetation units according to a vegetation map of the study area (Zumbühl & Burnand 1986). S. D. = standard deviation, C. V. = coefficient of variation, n = sample size. Vegetation: 16 = Rhododendro-Vaccinetum, 19 = Junipero-Arctostaphyletum, 20 = Junipero-Arctostaphyletum callunetosum, 22 = Caricetum fuscae, 31 = Polygono-Trisetion, 39 = Poion alpinae, 43 = Nardion. In vegetation unit 14 (Alnetum viridis / Adenostylo-Cicerbitetum) no prey sampling has been done.