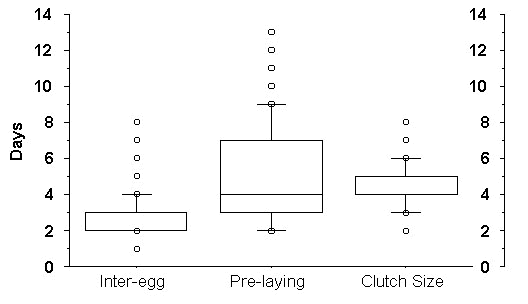
S44.4: Sperm storage, pair bondage, and genetic monogamy in hornbills
Mark Stanback1, Christian Boix2, David Richardson3, Tim Birkhead3, Bobbie Fletcher3 & John Mendelsohn4
1Department of Biology, Davidson College, PO Box 1719, Davidson, NC 28036, USA, e-mail mastanback@davidson.edu
; 2Percy FitzPatrick Institute, University of Cape Town, Rondebosch 7701, South Africa, e-mail cboix@botzoo.uct.ac.za; 3Department of Animal & Plant Sciences, The University, Sheffield, S10 2TN, UK; 4Research and Information Services of Namibia, PO Box 80044, Olympia, Windhoek, Namibia, e-mail mendelso@windhoek.alt.na Stanback, M., Boix, C., Richardson, D., Birkhead, T., Fletcher, B. & Mendelsohn, J. 1999. Sperm storage, pair bondage, and genetic monogamy in hornbills. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2657-2665. Johannesburg: BirdLife South Africa.Female hornbills are unique in that they seal themselves into their nest cavities prior to egg-laying and remain inside for lengthy egg-laying, incubation, and chick-rearing periods. Because some eggs are laid weeks after the female’s last copulation, females necessarily have the ability to store sperm for unusually long periods of time. Given that female birds generally prefer to mate with high quality males, one might expect some females to store sperm of extra-pair males in order to provide their offspring with especially high quality genetic fathers. Of course, the effectiveness of this female strategy is limited by multiple factors, including the potential for retaliation by the pair male. The outcome of this reproductive conflict is especially influenced by two things: the cost of male parental care and the dependence of the female. Male hornbills must supply the nutrients for a lengthy period of egg production, an equally lengthy incubation period, a complete moult of all female flight feathers, and the rearing of multiple chicks. Because of this exceedingly costly system of male uniparental care, males are expected to go to great lengths to avoid cuckoldry. For their part, females exist in a state of unparalleled dependence. Although a female can break out of the nest at any time, her moult renders her flightless --and easy prey. If a male suspected infidelity and withdrew parental care, both she and the brood would die. Hornbills thus exhibit the most extreme form of 'pair bondage' known in birds. As predicted by Gowaty's Constrained Female Hypothesis, DNA fingerprinting revealed no extra-pair offspring in a Namibian population of Monteiro's Hornbill Tockus monteiri.
INTRODUCTION
Most birds are now believed to have the ability to store sperm within blind-ended sperm storage tubules (SSTs) in the walls of the oviduct at the utero-vaginal junction (Bakst 1987). This ability acts to increase the length of the female’s fertile period, which in turn has far-reaching consequences for inter- and intra-sexual competition (Birkhead & Møller 1992, Birkhead 1994, Gowaty 1994). For species in which males and females form seasonal social relationships, the ability of a female to store sperm not only impacts the male's ability to control the reproductive behaviour of his mate (sensu Gowaty 1996), it also influences the costs and benefits of extra-pair copulations (EPCs) for both sexes. Indeed, the longer a female is able to store viable sperm, the stronger one might expect the effects on within-pair reproductive conflict to be.
The duration of sperm storage is most commonly measured as the interval between the last copulation and the laying of the last fertile egg (Birkhead & Møller 1992a, b). Unfortunately, even this conservative measure of sperm storage duration is of limited usefulness for most bird species, simply because most birds continue to copulate throughout the egg-laying period. Although females of certain pelagic seabirds are known to leave the colony for varying lengths of time after copulating in order to form eggs (Imber 1976, Birkhead et al. 1985), female hornbills (Bucerotiformes, Bucerotidae) are perhaps the only birds that are literally unable to engage in copulations during the egg-laying phase. For this reason, they are an ideal group with which to investigate reproductive conflict.
Like most other hornbills, members of the genus Tockus exhibit a peculiar breeding habit in which the female seals herself into the nest cavity prior to egg-laying by means of a nest plug made of mud and feces. Because further copulations are not possible once the female has entered the nest, all the eggs she lays must be fertilised by stored sperm. It is for this reason that we sought to explore the effects of sperm storage on reproductive conflict using hornbills. This required knowledge of not only the duration of sperm storage, but also the levels of extra-pair paternity (EPP) in natural nests.
METHODS
Study Site
We conducted field work for this study at the Daan Viljoen Nature Reserve near Windhoek, Namibia (22° 32’ S, 16° 58’ E). This part of Namibia is characterised as highland savanna thornveld and receives about 350 mm of rain annually, with most rain falling between November and February. Four species of hornbill, all members of the genus Tockus, breed regularly at Daan Viljoen. The most common species is Monteiro's Hornbill T. monteiri, a Namibian endemic. The other three are the Red-billed Hornbill T. erythrorhynchus, the yellow-billed hornbill T. flavirostris, and the Grey Hornbill T. nasutus. Although these birds normally nest in tree cavities (or cliff holes in the case of monteiri) (Kemp & Kemp 1972, Kemp 1976), they will also readily breed in artificial nestboxes (Riekert & Clinning 1985). Starting in 1991, MS and JM expanded the nestbox program initiated by Reikert and Clinning. By 1995 there were 130 nestboxes (interior dimensions = 25 cm x 21 cm x 50 cm, entrance diameter = 6 cm) in the study area. We attached these boxes to trees along a service road at intervals of approximately 300 m. Most boxes hang about 2 m off the ground.
Sperm Storage Duration: Timing of Egg-laying
To calculate the duration of sperm storage (see above), we monitored for a sample of females the number of days elapsing between their entering the nest cavity and the laying of their last egg. Three factors determine the length of this period: the length of the pre-laying interval (the number of days between the entrance of the female and the laying of the first egg), the clutch size, and the inter-egg interval. As the construction of the bulk of the nest plug usually takes several days, we considered females to have entered the nestbox when the nest entrance was sealed enough to prevent the exit of a bird inside. Once this stage was reached, we checked the nest daily for the appearance of the first egg. Because hornbills can lay eggs in the afternoon as well as the morning, we assigned exact lay dates using the following three criteria in the following order: the timing of the appearance of eggs, the pattern of inter-egg intervals, and hatch date. Because clutch sizes, pre-laying intervals, and inter-egg intervals do not differ among the four Tockus species breeding at Daan Viljoen (MS and CB, unpubl. data), we used data from all four species in some of the following analyses. However, monteiri is by far the most common species.
Sperm Storage Duration: Rates of Sperm Release
To address the rate at which sperm availability declines (and thus more closely approximate the maximum duration of sperm storage), we needed additional analyses. Although it is possible to quantify sperm stored in SSTs by microscopic examination of excised oviducal folds, a more informative and less invasive method exists - perivitelline (yolk membrane) analysis. Recent research has demonstrated for birds utilising stored sperm that the number of sperm penetrating the inner perivitelline layer of the freshly ovulated ova (as indicated by the number of microscopic holes visible in that layer of a sacrificed egg), provides a reasonable estimate of the overall numbers of sperm reaching the ovum at the time of fertilisation, which is in turn correlated with the number of sperm within SSTs at that time (Birkhead et al. 1993a, Birkhead et al. 1994). By counting sperm holes on multiple eggs within a clutch, one can approximate the rate at which sperm are released from SSTs (Birkhead et al. 1994, Birkhead and Fletcher 1994) -- and thus the duration of sperm storage. We removed fresh monteiri eggs (replacing them with dummy eggs), refrigerated them, and and later sent them to the University of Sheffield for perivitelline membrane analysis by BF (see Birkhead et al. 1994 for a description of methods).
Paternity Analysis
During the 1995 and 1996 breeding seasons, we obtained blood samples from a total of 38 monteiri families, representing a total of 135 individuals. We bled chicks about two weeks after hatching (earlier if their condition suggested they would not survive two weeks). We captured males using mist and bow nets. We stored blood samples in 100% ethanol and eventually shipped them to the laboratory of Dr. Terry Burke at the University of Leicester for paternity analysis by DR. Samples were analysed using multi-locus mini-satellite DNA fingerprinting. We excluded putative fathers if (a) there were two or more unattributable bands and (b) the bandsharing coefficient was less than 0.35.
RESULTS
Sperm Storage Duration: Timing of Egg-laying
As mentioned above, the duration of the period between the last copulation (the entrance of the female into the nest cavity) and the laying of the last egg is dependent on the length of the pre-laying interval, the inter-egg intervals, and the clutch size. Although a pre-laying interval of 2-5 days is most common (Fig. 1), some females begin laying immediately after entering the nest cavity while others sit in their nest for nearly two weeks before they lay their first egg. Similarly, inter-egg intervals of 2-3 days are also most common (Fig. 1), though some females may lay eggs at intervals of up to 8 days. Because of this unusual variation in inter-egg intervals, clutch size variation has a profound effect on our measure of 'sperm storage duration'. While clutches of 4 and 5 were most common (Fig. 1), clutch size ranges from 2 - 8. The variance observed in all of these factors contributed to both the magnitude and variance of this traditional measure of sperm storage duration. As shown in Fig. 2, this period varies considerably--from less than a week to over 3 weeks.
Sperm Storage Duration: Rates of Sperm Release
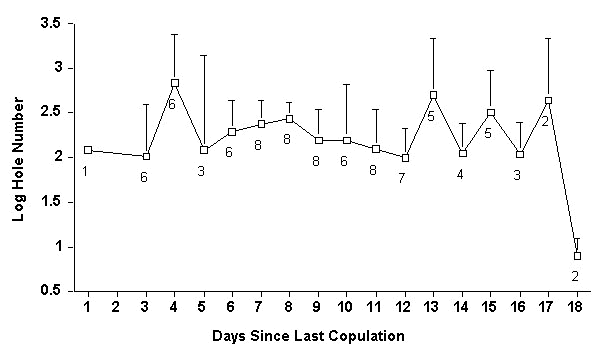
If passive leakage of sperm from SSTs results in an exponential decline in sperm availability over the course of laying, as it does in other species, one would expect the number of holes counted in perivitelline membranes to decrease monotonically when plotted against the number of days of sperm storage. As shown in Fig. 3, this was not the case: sperm appear to leak from SSTs at a constant but slow rate, resulting in an unusually long period of sperm storage.
Paternity Analysis
For our multi-locus minisatellite DNA fingerprinting, we scored an average of 16 bands for each chick/adult dyad. Bandsharing coefficients for putative father/offspring dyads differed significantly from those of presumably unrelated breeding adults (Z > 10.35, P < 0.0001). For these 135 putative father/offspring dyads, no chick had more than 2 unattributable bands, and only six had two unattributable bands. Moreover, no dyad had a bandsharing coefficient of less than 0.35, suggesting that putative fathers did indeed sire all tested offspring.
DISCUSSION
Sperm Storage Duration
The results of our analysis of the timing of egg-laying in hornbills suggests that the 'duration of sperm storage' (here defined as the interval between last copulation and last egg) is extremely variable, with some females completing clutches in as few as 6 or as long as 25 days. However, it is likely that even these longer durations represent minima.
First of all, it is unlikely that last laid eggs are fertilised only by sperm from copulations occurring on the day the female enters the nest. Although the phenomenon of sperm precedence (Birkhead et al. 1988a) elevates the 'value' of the final copulation, there is no reason to think that sperm from earlier copulations is incapable of fertilising eggs or that males should withhold copulations until the day the female enters the box. Females spend up to a week working on the nest plug before entering the cavity for the last time. At the very least, males should be expected to seek copulations with their mates as soon as construction of the nest plug begins.
Second, it is unlikely that sperm supplies are completely depleted following the laying of the last egg, even for those females laying 25 days after entering the nest cavity. However, even we were surprised to find such a slow rate of sperm depletion in our perivitelline analyses. The essentially flat line shown in Fig. 3 is in sharp contrast with the steep monotonic decrease Wishart (1987) found in chickens Gallus gallus. This suggests that the decline in sperm availability in hornbills is extremely gradual and, consequently, that hornbills are able to store sperm for unusually long periods of time. Although it would be imprudent to surmise that female hornbills can store sperm indefinitely, it seems probable that sperm storage may regularly exceed one month.
Sperm Storage and Opportunities for EPCs
If a female can lay a fertile egg weeks after entering the box, this also suggests that a female could copulate weeks before entering the box and use this sperm to fertilise at least some of her eggs (sensu Birkhead and Biggins 1998). Long-term sperm storage thus gives female hornbills an unusually long fertile period. A fertile period extending one month prior to egg-laying gives a female unprecedented opportunities to engage in EPCs and enjoy benefits such as fertility insurance, genetic diversity, and genetic quality (Birkhead and Møller 1992). Indeed, in an environment where the choice of male partners is constrained by the choices of earlier-pairing females, long-term sperm storage could potentially liberate females to the extent that they could separately pursue social and genetic mates.
This scenario, however, assumes that male hornbills have little influence on the mating behaviour of their mates. According to Gowaty’s (1996) Constrained Female Hypothesis, females should always attempt to be fertilised by males of high genetic quality, whereas males can generally be expected to try to control their mate's choice of sexual partners. Whether a male is successful in controlling the sexual activities of his mate depends on two things: (1) the female’s intrinsic abilities to resist male control (female quality) and (2) the environmental potential for resistance by the female (Gowaty 1996). The observed rate of EPP should therefore reflect this battle of the sexes. According to Gowaty’s (1996) model, females that are able to raise chicks with minimal male assistance (either because of their own inherent quality or a superabundance of resources) are free to pursue EPCs. One should therefore observe high levels of EPP within the broods of such females. However, if females cannot breed successfully without male assistance or access to male-brokered resources, males can be said to be winning the battle of the sexes. In such circumstances, males will demand, and presumably receive, a greater degree of fidelity: monogamy via coercion. In other words:
'if, as a consequence of a female engaging in extra-pair copulations, a male partner ... withholds all care resulting in reproductive failure, it will pay females to remain faithful and retain their partner's full assistance.' (Birkhead & Møller 1996).
Where do hornbills fall in the spectrum of male control and female resistance? Given the potential for female EPCs, one might expect to find them well toward the female resistance end. However, other aspects of their breeding biology introduce an element of male control into the equation. In fact, Gowaty (1996) singles out hornbills as the group of birds exhibiting the greatest degree of male control. What then is responsible for the presumably strong male control observed in hornbills, and how does this fit with the results of our paternity analyses?
Male control in hornbills is the product of two phenomena: the dependence of the female and the high cost of male parental care. These are in turn a product of the very thing that makes hornbill breeding biology unique – the nest plug. This nest plug provides excellent protection against the many predators that would otherwise threaten a cavity nester. Dependable protection is quite necessary, for female hornbills remain sealed inside the nest for two months. Yet it is her food requirements during this time that appears to result in both the extreme female dependence ('pair bondage') and the high cost of male parental investment observed in these birds: everything the female gets comes from the male through a small slit in the nest plug. Indeed, female quality may be of little importance in a system in which food availability is filtered almost exclusively through the male.
Moreover, female energetic needs are far greater than simply basal metabolic requirements. The female produces clutches of up to 8 eggs from the food provided by her mate. At entry, her follicles are only partially 'yolked up' (MTS, unpubl. data). Additionally, food brought by the male fuels an incubation period that can last up to 6 weeks. Another energetic expense imposed on the male is female moult. After the completion of egg-laying, the female undergoes a simultaneous moult of all flight feathers (remiges and retrices). These feathers regrow over the following 5 weeks, again fuelled by food provided by the male. Finally, and probably most demanding of all, the male alone must provide all the food for the growing nestlings. In fact, even after the female breaks out, the male continues to supply most of the food for the chicks (MTS unpubl. data). Male hornbills thus not only exhibit male uniparental care, they also pay many of the costs of reproduction for females. Thus the cost of reproduction in male hornbills is arguably among the highest in any bird. This high cost necessarily makes cuckoldry prohibitively expensive.
Our paternity data demonstrate that males do successfully avoid cuckoldry. How? Frequent copulation, as observed in many birds of prey (Birkhead and Møller 1992) appears not to be utilised. Indeed, we never observed any copulations in our population, despite intensive observations. Mate-guarding, though probably utilised to some degree, is neither intense nor long-lasting (unpubl. data) – certainly not sufficient to accommodate the extended fertile period of female hornbills. Moreover, the effectiveness of overt mate-guarding is questionable: mate-guarding is observed in many species in which EPP is relatively common.
What prevents females from making the most of their sperm storage capabilities and obtaining extra-pair sperm from males of higher quality than their own mates? Although paternity analyses have demonstrated that EPCs are quite common in birds, it should be remembered that EPCs have costs as well as benefits for females. Birkhead and Møller (1992) list seven potential costs: unsexy sons, male retaliation, risk of injury, harassment from extra-pair males, foraging cost of nearby male, disease or parasite transmission, and increased risk of predation. Following the logic of Gowaty’s (1996) Constrained Female Hypothesis, the only cost of EPCs sufficient to explain genetic monogamy in hornbills is male retaliation.
Such retaliation can potentially take many forms, the most likely being the withholding of parental care. The most extreme form of resource withholding, at least for a hornbill, is desertion. If a male hornbill suspected infidelity and withheld food, the chicks would die. Unlike the females of many other species, female hornbills are unable to compensate for deficiencies in male food deliveries. Indeed, females are unable to deliver any food at all. Withdrawal of male assistance would result in the death of all the offspring, including those fathered by the male. This, however, is not an evolutionarily viable strategy (Whittingham et al. 1992, Westneat and Sherman 1993), at least at first consideration. Yet the situation presented by hornbills is quite different from those presented by most other biparental species. In hornbills, the withdrawal of male parental care would result in the death of both the chicks and, more importantly, the breeding female. Why would the female die? Although the nest plug is formidable, her departure from the nest as the chicks complete their growth is a normal part of the breeding cycle. Why would it be deadly at other times of the breeding cycle? In a word -- moult. Because of her simultaneous moult of flight feathers, the female is quite unable to fly until her feathers are completely grown in. Indeed, our data suggests that females normally emerge from the nest cavity as soon as their feathers can support them. But is flightlessness necessarily a death sentence? Perhaps not always, but often enough: a flightless female hornbill would be easy prey for a variety of common avian and mammalian predators. For example, at a nest from which the male disappeared, a flightless female flavirostris cannibalised her youngest chick, broke out of the box, and was never seen again (MTS, unpubl data).
Is fatal male retaliation a commonly observed event? No. In fact, the above case probably represents depredation of a male. Retaliation is a response to suspected infidelity; it is reactive. Coercion is proactive: given that the male literally has control over the life of the female, we argue that the threat of retaliation is sufficient to ensure fidelity. As long as the male has the upper hand, the risk to a female of seeking EPCs is prohibitive. Recent years have seen a spate of experimental studies of the relationship between paternity and paternal care (Wright and Cotton 1994, Sheldon et al. 1997, Kempenaers et al. 1998). In all of these studies, reactive retaliation is the predicted response to perceived threats to paternity. In no bird other than a hornbill would the withdrawal of male parental care result in the death of the breeder female. Thus proactive coercion should be especially, and perhaps uniquely effective in producing genetic monogamy in hornbills. Indeed, as long as male hornbills enjoy the potential for retaliation, they need not exercise it regularly. Overt mate-guarding may simply be a means of controlling females in species that lack the 'upper hand' that male hornbills enjoy.
ACKNOWLEDGMENTS
The Daan Viljoen hornbill project has been supported by the following grants to MS: National Geographic Research Grant, Fulbright Fellowship, National Science Foundation International Postdoctoral Fellowship, American Philosophical Society research grant, and a Chicago Zoological Society research grant. Special thanks go to Phoebe Barnard, Terry Burke, Jordan Karubian, Nancy Popkin, Rob Simmons, the Namibian Ministry of the Environment, and the staff of the Daan Viljoen Nature Reserve.
REFERENCES
Bakst, M.R. 1987. Anatomical basis of sperm-storage in the avian oviduct. Scanning. Microscopy. 1: 1257-1266.
Birkhead, T.R. 1994. Enduring sperm competition. Journal of Avian Biology 25: 167-170.
Birkhead, T.R. & Biggins, J.D. 1998. Sperm competition mechanisms in birds: models and data. Behavioral Ecology 9: 253-260.
Birkhead, T.R., Johnson, S.D. & Nettleship, D.N. 1985. Extra-pair matings and mate guarding in the Common Murre Uria aalge. Animal Behaviour. 33: 608-619.
Birkhead, T.R. & Fletcher, F. 1994. Sperm storage and the release of sperm from the sperm storage tubules in Japanese Quail Coturnix japonica. Ibis 136: 101-105.
Birkhead, T.R. & Møller, A.P. 1992. Sperm competition in birds: evolutionary causes and consequences. San Diego; Academic Press: 282pp.
Birkhead, T.R. & Møller, A.P. 1996. Monogamy and sperm competition in birds. In: Black, J. (ed.) Partnerships in birds: the study of monogamy. New York; Oxford University Press: 323-343.
Birkhead, T.R., Pellatt, E.J. & Fletcher, F. 1993. Selection and utilization of spermatozoa in the reproductive tract of the female Zebra Finch Taeniopygia guttata. Journal of Reproduction and Fertility 99: 593-600.
Birkhead, T.R., Pellatt, J.E. & Hunter, F.M. 1988. Extra-pair copulation and sperm competition in the Zebra Finch. Nature 334: 60-62.
Birkhead, T.R., Sheldon, B.C. & Fletcher, F. 1994. A comparative study of sperm-egg interactions in birds. Journal of Reproduction and Fertility 101: 353-361.
Gowaty, P.A. 1994. Architects of sperm competition. Trends in Ecology and Evolution 9: 160-162.
Gowaty, P. A. 1996. Battle of the sexes and origins of monogamy. In: Black, J. (ed.) Partnerships in birds: the study of monogamy. New York; Oxford University Press: 21-52.
Imber, M.J. 1976. Breeding biology of the Grey-faced Petrel Pterodroma macroptera gouldi. Ibis 118: 51-64.
Kemp, A.C. 1976. A study of the ecology, behaviour and systematics of Tockus hornbills (Aves: Bucerotidae). Transvaal Museum Memoir 20.
Kemp, A.C. & Kemp, M.I. 1972. A study of the biology of Monteiro's Hornbill. Annals of the Transvaal Museum 27: 255-268.
Kempenaers, B., Lanctot, R.B. & Robertson, R.J. 1998. Certainty of paternity and paternal investment in Eastern Bluebirds and Tree Swallows. Animal Behaviour 55: 845-860.
Riekert, B.R. & Clinning, C.F. 1985. The use of artificial nest boxes in the Daan Viljoen Game Park. Bokmakierie 37: 84-86.
Sheldon, B.C., Rasanen, K. & Dias, P.C. 1997. Certainty of paternity and paternal effort in the Collared Flycatcher. Behavioral Ecology 8:421-428.
Westneat, D.F. & Sherman, P.W. 1993. Parentage and the evolution of parental behavior. Behavioral Ecology 4:66-77.
Whittingham, L.A., Taylor, P.D. & Robertson, R.J. 1992. Confidence of paternity and male parental care. American Naturalist 139: 1115-1125.
Wishart, G.J. 1987. Regulation of the length of the fertile period in the domestic fowl by numbers of oviductal spermatozoa as reflected by those trapped in laid eggs. Journal of Reproduction and Fertility 80: 493-498.
Wright, J. & Cotton, P.A. 1994. Experimentally induced sex differences in parental care: an effect of certainty of paternity? Animal Behaviour 47: 1311-1322.
Fig. 1. Factors influencing sperm storage duration in Daan Viljoen hornbills: (A) Inter-egg interval (days): n = 493 eggs, (B) Pre-laying interval (days): n = 129 clutches, (C) Clutch size (eggs): n = 198 clutches. Box plots display 10th, 25th, 50th, 75th, and 90th percentiles.
Fig. 2. Total duration of sperm storage, as measured by the length of the period between the last possible copulation and the laying of the last egg. Frequency refers to number of clutches exhibiting sperm storage durations of stated length.
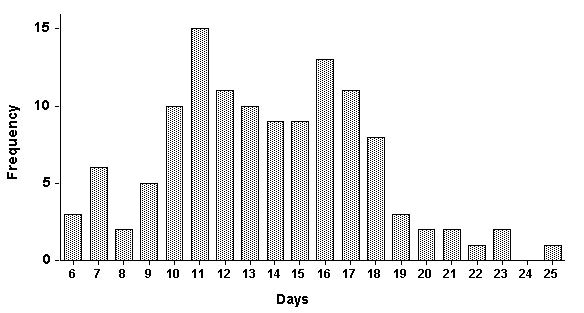
Fig. 3. Sperm availability over time. Numbers of sperm holes (log transformed) in the perivitelline membrane of eggs laid at different points during the period of sperm storage. Sample sizes (below each data point) represent the numbers of sampled eggs laid 'x' days since the female entered the box. The sharp decline at day 18 is presumably an artifact of small sample sizes – these birds lay fertile eggs up to 25 days after the last copulation (see Fig. 2).