S44.3: Extra-pair paternity and paternal care: Differential male fitness via exploitation of variation among females
Patricia Adair Gowaty
Institute of Ecology, University of Georgia, Athens, Georgia 30602-2602, USA, fax 1 706 542 3344, e-mail gowaty@ecology.uga.edu
Gowaty, P.A. 1999. Extra-pair paternity and paternal care: Differential male fitness via exploitation of variation among females. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2639-2656. Johannesburg: BirdLife South Africa.The central organising principal of sexual dialectics theory is that males and females are in competition over the control of reproduction. This theory says that variation in female resistance to males’ efforts to influence, manipulate or control their reproductive decisions determines the mechanisms of competition among males and mating systems organisation itself. In socially monogamous birds with biparental care, males may exploit females’ needs for male help by 'trading' paternal provisioning for copulations, fertilisations, and faithfulness. This idea, the Constrained Female Hypothesis, says that variation in females’ abilities to resist manipulation via such 'helpful coercion' determines between-female patterns of multiple mating and male reproductive success variance. In this paper, I review and extend the Constrained Female Hypothesis emphasising how attention to variation in females may clarify unexplained variation in males. The Constrained Female Hypothesis predicts: probabilities of cuckoldry and sperm competition, variance in male reproductive success, probabilities of co-operative or conflicted day-to-day dynamics of socially monogamous pairs, and the basis of male choice of social mate. The model also predicts within- and between-species’ patterns of paternal care in relationship to extra-pair paternity. Resolution of the theoretical and empirical puzzles of the evolution of paternal provisioning and less than perfect genetic paternity may be a simple function of variation in females’ abilities to provision their offspring alone.
INTRODUCTION
Avian paternal provisioning characterises the mating systems of birds and it varies both between and within species -- geographically, locally, and intra-individually. Male parental care patterns are particularly important to questions about the evolution of social organisation because paternal care correlates to typical avian mating systems (Lack 1968, Orians 1969, Verner and Willson 1969, Emlen and Oring 1977, Clutton-Brock 1991, Gowaty 1981, 1996c, Wright in press): Most birds are socially monogamous and most have biparental care of young. This correlation motivates the most common explanation for social monogamy: Male parental care is essential for or enhances females' reproductive success and/or the survival of offspring. Accumulated observations provide equivocal support for the 'male parental care is essential' explanation for social monogamy, but these earlier studies implicate within-species between-individual variation among females in a theoretical resolution (Gowaty 1996b) to the monogamy puzzle. In this paper I review recent discussions (Gowaty 1996c, Wright in press) of a related question: What factors determine the functional relationship between realised genetic paternity and paternal provisioning?
Do Males Adjust Offspring Provisioning as a Function of Extra-pair Paternity?
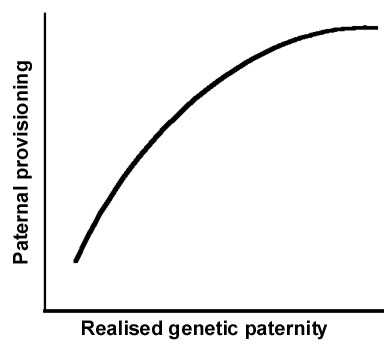
Molecular markers for genetic parentage testing (Avise 1996) have revealed that avian social monogamy is more likely than not associated with some extra-pair paternity, most often at non-trivial levels (see, e.g., summary in Gowaty 1996c). Williams (1966) and Trivers (1972) were probably the first to suggest theoretically that social monogamy would not equal genetical monogamy. 'In species where there has been strong selection for male parental care, it is more likely that a mixed strategy will be the optimal male course -- to help a single female raise young, while not passing up opportunities to mate with other females whom he will not aid (p 145).' Trivers’ (1972) logic predicted previously unobserved behaviour, though perhaps at levels even he did not expect. Nevertheless, his insight predicted previously unobserved behaviour likely to increase realised genetic paternity including mate-guarding. His intuitively satisfying evolutionary arguments suggested that the costs of male provisioning would select against males contributing to the care and feeding of offspring not theirs. Thus, the most basic prediction relating paternity to paternal care says that as paternity increases, paternal care should also increase; as realised paternity declines, paternal contributions also should decline (Wright in press).
The curve expressing the more modern formulation (Fig. 1) adapted from Wright (in press) incorporates two refinements to the most basic prediction of a positive correlation between the two variables: first, a threshold of effect below which adjustments to paternal care are not worth the effort; and, second, an asymptote in paternal care above which there would be no positive fitness gain for males. Despite the intuitively satisfying feel of this idea, observations designed to test it have seldom been so tidy (see, e.g., reviews in Wright in press; Kempaenaers & Sheldon 1997; Westneat and Sargent 1996).
For example, no statistically significant relationship exists between realised genetic paternity and paternal provisioning in some populations of Red-winged Blackbirds, Agelaius phoeniceus (Westneat 1995), Eastern Bluebirds (MacDougall-Shackleton et al. 1996), in Tree Swallows, Tachycineta bicolor, (Whittingham et al. 1993), Purple Martins, Progne subis (Wagner et al. 1996), House Martins, Delichon urbica (Whittingham & Lifjeld 1995), or Yellow Warblers, Dendroica petechia (Yezerinac et. al. 1996). These studies and presumably others unpublished failed to support the simple predicted declines in paternal provisioning as a function of increasing extra-pair paternity.
In contrast, observations of Reed Buntings Embiriza shoeniclus (Dixon et al. 1994) showed that males adjusted their provisioning as a direct function of their degree of realised genetic paternity within broods. This is the only within-species study of a socially monogamous passerine to date that clearly supports the simple prediction that males should vary their paternal contributions as a function of their genetic paternity. Notably, two studies of socially polyandrous birds, one on Dunnocks Prunella modularis (Davies et al. 1992) and the other on Pukeko Porphyrio porphyrio (Jamieson et al. 1994) showed that in polyandrous groups males adjusted their provisioning as a function of their realised genetic paternity. The success of the prediction in socially polyandrous species stands in intriguing contrast to its frequent apparent failure in socially monogamous birds.
My own data on Eastern Bluebirds, Sialia sialis, are ambiguous. In one study, using methods reported in Gowaty and Bridges (1991a, b) we ordered males by the proportion of provisioning they provided and examined the likelihood of caring for offspring not theirs between subpopulations of the best and worst provisioners. Males that provisioned the most were statistically less likely to be caring for offspring not theirs than were males that provisioned the least. This result is consistent with the simple prediction (Fig. 1) of the Standard Adaptive Hypothesis, but because we were only able to reliably test males at the ends of the continuum of paternal provisioning, we can say nothing about factors affecting provisioning by most males.
In a second study (Gowaty et al. unpublished) there was no statistically significant relationship among males between realised genetic paternity and paternal care. Impressive variation in paternal provisioning for uncuckolded males existed in this sample of 59 Eastern Bluebird families: Males caring only for their own young provisioned between 0 to 100% of the food deliveries to nestlings. However, for males with both extra-pair and within-pair young in their nests, a different picture emerged. Realised genetic paternity and paternal care were significantly negatively related. In our study population extra-pair paternity had effects on paternal provisioning, but additional factors had important effects too. Thus, our studies provide only moderately less ambiguous support for the Standard Adaptive Hypothesis than those reporting no statistically significant relationship.
One conclusion from the empirical tests is that the real world is more complex than the simple prediction of the Standard Adaptive Hypothesis.
'Paternity must be considered in the context of the mating system as a whole, including patterns in extra-pair matings' (Wright, in press, p 1).
Indeed, these seeming empirical complexities have lead to far more complex theories (Westneat and Sherman 1993; Whittingham et al. 1992). So, that Wright says,
'...paternal care in any particular system will therefore depend upon the precise shape of age-dependent fitness effects and trade-offs between parental effort (PE), mating effort (ME), and somatic effort (SE)' (Wright in press, p 10).
'For any one male, there is a range of possible reproductive options, expressed in terms of various combinations of investment in PE, ME and SE. The life history decision for males is therefore to optimise the proportions of investment in PE, ME and SE in successive breeding attempts such that they maximise the overall fitness returns over their lifetime (Wright 1998, p 4).'
For example, consider Westneat and Sherman (1993). They modelled how trade-offs in mating effort, parental effort, and somatic effort might shape social behaviour in biparental species under a series of varying assumptions including whether provisioners could assess their relatedness to nestlings and whether provisioning behaviour was fixed or facultative. Predictions from their ideas depend on exactly specified models of the relationships between genetic paternity in current and future broods as well as to genetic paternity associated with additional matings. To directly test their ideas researchers must evaluate the availability to provisioners of information about paternity, the per male between-brood likelihood of caring for nonkin, as well as the trade-offs between mating effort and parental effort for each breeding attempt a male makes. As they note, it is not clear exactly what behaviour(s) counts as mating effort or parental effort, and they also caution that sometimes components of mating effort may also be components of parental effort. Nevertheless, their model seems to mimic life and their theoretical conclusion converges on empirically derived wisdom: Genetic 'parentage and parental care are intimately linked, but ...their interrelationship is complex' (Westeneat and Sherman 1993, p 76).
Debate among theorists has bubbled over to debate among field workers, perhaps because to predict patterns of extra-pair paternity and paternal care, it seems we must know everything else about the mating system! Rejection of the more complex theories even in application to within-species variation is unlikely without very long-term data. Will this theory be able to simultaneously explain either the between-species variation represented by Reed Buntings, Tree Swallows, and Eastern Bluebirds or the within-species, between population, and within population, between individual variation of Eastern Bluebirds? Indeed, what are appropriate ways to test the simple and more complex theories? Even well-designed field tests seem too leaky to satisfy some sceptics, who apparently, as I do, find it difficult to believe that there is no adaptive relationship between paternity and paternal care (see Kempenaers & Sheldon 1997, 1998; Lifjeld et. al. 1998; Wagner et al. 1998).
Have We Left Something Out?
Is it possible that the stated and unstated assumptions of these earlier theories are not met? For that matter, what do we know about our most basic assumptions? Are offspring survival and 'parental effort' positively correlated? Does selection favour parental care because of offspring or parental fitness benefits? What are the tradeoffs between mating effort and parental effort for males and females, and how big are they? Do males suffer the costs of such tradeoffs more than females? Do data capable of rejecting or clearly supporting these assumptions exist?
Might some critical simplifying variables straight-forwardly -- i.e., without a complete empirical description of almost everything else -- predict novel relationships? Might some simple overlooked variable explain observed relationships between realised genetic paternity and paternal provisioning?
Here I describe a theory that emphasises sources of variation in genetic paternity and in paternal provisioning from the perspectives of females (Gowaty 1997b). It focuses on a generally overlooked variable, namely within-population, between-individual variation among females in their abilities to feed their offspring without the contributions of males (Gowaty 1996b, c; 1997a). Although this theory rests on the fulcrum of variation in females, it predicts critical variation in males including probabilities of cuckoldry and sperm competition, variance in male reproductive success, day-to-day social dynamics of individual males with their socially monogamous mates, the basis of male mate choice, and the relationship between realised genetic paternity and paternal provisioning. Like other recent paternal care-genetic paternity theories, it is conceptually simple, but unlike others, it is easily tested.
THE CONSTRAINED FEMALE HYPOTHESIS
Variation in Extra-pair Paternity and Paternal Provisioning
Here I will argue that between-female variation in abilities to raise their offspring alone predicts males' responses to less than perfect realised paternity. Within- and between-species variation (Gowaty 1996b, c; 1997a) in the ability of avian females to raise their offspring without male help inspired me to begin with this question.
The Constrained Female Hypothesis emphasises variation in females’ abilities to feed offspring alone and qualitatively predicts functional relationships between extra-pair paternity in broods and provisioning by mothers’ social mates. It departs from other discussions in some crucial ways. 1). The Constrained Female Hypothesis does not assume, as did some earlier theories relating female need to male parental care and social monogamy, that the functional value of paternal care is to increase the reproductive success of females (Wittenberger and Tilson 1980). 2). It does not even assume, as earlier genetic paternity-paternal care theories did, that the function of parental care is to increase the fitness of offspring. 3). It does not assume as have some static optimisation and ESS models (reviewed most recently in Wright in press) that females compensate for withdrawals of male provisioning. 4). The Constrained Female Hypothesis leaves open to question whether what parents do to their offspring is beneficial, hurtful, or null to the fitnesses of those offspring (Gowaty 1996c). 5). It assumes that whatever a parent does to offspring serves the fitness interests of the parent. 6). In contrast to the emerging 'whole mating systems' approach, it predicts rather than depends upon, distributions of and rates of extra-pair paternity among females. 7). It predicts a multifactoral basis for and hierarchical organisation of female mate choice. 8). It does assume that freely expressed female choice favours the viability component of offspring fitness (Gowaty in press).
The Constrained Female Hypothesis says that non optimal males prevent some females from achieving their best gametic partner -- in terms of the viability component of their offspring’s fitnesses -- by persuading, manipulating, or coercing females into mating with them instead of with more optimal mates (Gowaty 1996). Non-optimal males are most likely 'to change females' minds' when females are vulnerable to persuasive or manipulative efforts. It follows from the consequences of anisogamy argument (Williams 1966; Trivers 1972; Parker et al. 1972; Gowaty 1997a) that says that for typical vertebrates in which males are limited primarily by their access to females and in which females are limited primarily by their access to nutrients, that males and females will be under selection to manipulate (influence, effect, control) for their own advantage the reproductive capacities of their limiting resources (Gowaty 1997a). Therefore, we can expect females to attempt to control access to their limiting nutrients by 'farming' or other manipulations of nutritional resources and males to attempt to control the reproductive capacities of females, exploiting for their own interests available variation in females. Crucial to the Constrained Female Hypothesis is the argument that, whenever the fitnesses of resources (living nutrient sources or females) suffer at the hands of others including resource users, they will be under selection to resist the influence of others on their reproductive decisions. That is, whenever females' fitnesses suffer because of male influences on females' reproductive decisions, females should experience selection to resist males’ efforts. Thus, the Constrained Female Hypothesis emphasises that the sexes are in conflict over the control of reproduction. It assumes that sexual conflict, rather than unadulterated sexual co-operation, drives the evolution of social behaviour -- even in socially monogamous unions characterised by often extreme levels of behavioural co-operation.
Figure 2 is a graphical model of the Constrained Female Hypothesis based on a mechanism males may use to attempt to persuade females -- who might not otherwise do so -- to mate with them. Male parental care is something for which females 'trade' copulations and fertilisations. Therefore, male parental 'care' serves not the fitness interests of offspring, but the fitness interests of males who persuade females to mate with them through 'trades'. This hypothesis predicts that needy females will be less likely to seek or accept extra pair copulations, and that females able to raise offspring successfully without male provisioning will be most likely to seek or accept extra-pair copulations. It says selection will favour males that exploit variation among females in their vulnerabilities to persuasion, manipulation, or coercion. It predicts that offspring of even 'helpfully coerced' females in socially monogamous unions will have lower viabilities than non coerced females.
On the horizontal axis of Figure 2 is female 'quality' and its interaction with environmental quality. 'Female quality' indicates traits of females that result in variation in their abilities to raise their offspring alone. 'Environmental quality' means characteristics of the habitat that facilitate provisioning and other care of nestlings by females. Operational expressions of the horizontal axis may include females' foraging efficiencies, their intrinsic metabolic rates, and the availability of near-by food resources. On the vertical axis is an independent measure of female fitness such as number of nestlings fledged. The exact shapes of the two curves do not matter, but their relationship to one another does. The top curve represents a female's fitness when her social mate 'helps' her; the bottom curve is her fitness when her social partner does not help. The two curves are congruent on the right-hand side of the graph because in some combinations of female intrinsic ability and environmental quality, female fitness will be at a maximum whether or not she has male help.
Reduction of paternal provisioning can affect female fitness -- perhaps dramatically for females on the left-hand side of the graph, but not at all on for females on the right. Thus, males may influence some females' reproductive decisions through a process of 'helpful coercion' (Gowaty 1996b, c; 1997), and some females' options -- their probabilities of immunity from the constraints of 'helpful coercion' -- will depend on their own skills, intrinsic abilities, and the quality of the environments in which they find themselves.
The Female Constraint Hypothesis predicts the dynamics of individuals within socially monogamous mating systems. It predicts which females most likely will take options to mate multiply. It predicts which males will engage in extra-pair mate-searching behaviour. It predicts among-male variance in reproductive success as a function of males’ social mates. It predicts which males within populations most likely will vary their paternal contributions as a function of realised genetic paternity and which will not. It predicts between-female variation in the trait basis of females’ mating choices for both extra-pair partners and social partners. It also predicts between male variation in the trait basis of males’ mating choices. It predicts among-female variances in offspring produced and offspring surviving. Note that this simple hypothesis predicts within population, between female variation in offspring, females and males.
The Female Constraint Hypothesis also predicts between species variation in mean frequencies of extra-pair paternity; relative advantages for females engaging in extra-pair fertilisations; and distributions of variance in male reproductive success due to extra-pair mating. Most important for this paper, it predicts species in which male provisioning will and will not vary as a function of extra-pair offspring in their nests.
PREDICTIONS
Well endowed and lucky females multiply mate more
Well endowed and lucky females (Fig. 2) -- represented on the right hand side of the graph -- will more often take options for extra-pair copulations and will have more extra-pair offspring than less well endowed or unlucky females on the left. This is the central prediction of the Constrained Female Hypothesis; it follows from the idea that females vary in their vulnerability to persuasion, influence, or manipulation of their reproductive decisions by males.
Frequencies of extra-pair paternity within broods
The second prediction about distributions of sires among broods with extra-pair offspring follows similar logic. An extra-pair male will more often sire all the offspring of extra-pair mating females on the right hand side of the graph. Both extra-pair and within-pair males will sire offspring when females on the left hand side engage in extra-pair matings. In other words, skilled and lucky females will more often be socially monogamous with a male whose territory she shares, while simultaneously genetically monogamous with an extra-pair male. Relatively less skilled and unlucky females with extra-pair offspring will more often be genetically polyandrous, i.e., they will produce some offspring for the territorial male partner and some with extra-pair males.
Males with skilled and lucky social mates have more time for extra-pair activities
Males socially paired with relatively more skilled or lucky females will have more time to seek extra-pair matings themselves than males socially paired to relatively less skilled or unlucky females. Therefore, the Constrained Female Hypothesis predicts male variance in reproductive success will be greater among those paired with skilled and lucky females than among those mated to less skilled or unlucky females. This logic leads to the surprising prediction that reproductive success variance among males may be a function of the quality of their social mates, perhaps as much as functions of their own intrinsic qualities.
Between-sex cooperation can occur all along the continuum of female variation
If males are distributed at random with respect to females’ intrinsic characteristics and environmental quality, the social dynamics of pairs will be a simple function of their likelihood of producing highly viable offspring. This means that in a population in which females vary in their abilities to raise offspring alone, if males are distributed randomly, by chance some females all along the continuum will pair with males optimal for them in terms of offspring viability. These pairs may experience relatively small amounts of behavioural conflict because their fitness prospects in their mutual offspring will be congruent and perhaps considerable. Other females, however, will pair with less optimal males and it is these females that may or may not opt for extra-pair fertilisations as a function of their dependence on male parental care. Whether these females have multiply sired broods or not, individuals in these unions will continue to have non-congruent fitness interests and will be most likely to evidence continuing behavioural conflicts.
Male choice matters too
If between-sex social dynamics vary in the way that the Constrained Female Hypothesis says, selection will favour males -- and not just females -- that discriminate among potential mates. If offspring viability selection favours female mate choice, whenever two females prefer a given male, selection will favour those males that discriminate among them (see, e.g., Altmann 1997). Selection will favor such males when they prefer more skilled, more competent females over less skilled, less competent females.
Basis of male mate choice under different probabilities of extra-pair mating by females
One might expect that males would always prefer the most competent, highly skilled females as the mothers of their offspring (Hardy 1997, Altmann 1997). But, what might males prefer when they themselves are non-optimal for particular females with whom they potentially would socially pair? Given the option, females may extra-pair mate, in which case, paternity certainty cues of potential mates may serve male fitness interests better than female skill or competence. In this case, males that are able to monopolise resources or provide skills to females who need them, may be able to trade access to these resources or skills for increased paternity certainty (Gowaty 1997a, 1996a, c). Males aid and abet sexual selection among females by exploiting female variation for their own benefit. Thus, males choosing among females for whom they are non-optimal should prefer to mate with females whose reproductive decisions they can influence or control. Selection should favour males that are sensitive to and can exploit variation in female vulnerability to trades; and, males should devalue a female’s competence or skill as a function of her likelihood of opting for extra-pair fertilisations. Under the threat of loss of genetic paternity, males will prefer females vulnerable to male persuasion, influence, or control. This prediction leads to a novel question about the basis of male mate choice. What traits -- behavioural, physiological, or morphological -- provide cues of variation in females’ vulnerabilities to persuasion, influence peddling, manipulation, or physical coercion? Could females’ responses to males’ attempts to 'courtship feed' provide information to males about females’ vulnerabilities to their influence?
THE EXTENDED FEMALE CONSTRAINT HYPOTHESIS
So far, I have emphasised the following points: 1. In socially monogamous birds variation in extra-pair paternity is a function of variation in females. 2. Extra-pair paternity occurs because of advantages for females rather than just for males. 3. To evolve male provisioning need only enhance male fitness rather than offspring or female fitness. 4. Predictions about frequencies and distributions of extra-pair paternity, the effects of extra-pair paternity on paternal provisioning, and on male and female mate choice are fine-grained, focusing on within-population, between-individual variation that is frequently unaddressed and unexplained by other theories. 5. The simplicity of the ideas rendering them vulnerable to rejection by fairly straight-forward tests. Now, I turn to an extension of these ideas that predicts functional relationships between provisioning by males and genetic paternity.
To predict the relationship between paternal care and genetic paternity I extended the horizontal axis (Fig. 3) on the left to include cases where females have no success at all without male help (panel A) and on the right to those cases where male 'help' is detrimental to females' fitnesses (panel D). I will begin by considering females from Figure 2, the Panel B and C females.
In panel B, males enhance female fitness, even though male help is not essential; the upward curve of the slope indicates that the degree of fitness enhancement is a function of increasing female intrinsic abilities and the richness of her environment. Thus, panel B females are vulnerable to the withdrawal of paternal provisioning and it is among a group of such females -- that vary in the degree of enhancement provided by males -- that we should expect to see empirical match to the intuitive, simple prediction of Figure 1. Because these females are vulnerable and have a higher probability of a 'trade-basis' for their mating decisions, paternal provisioning should vary as a function of genetic paternity.
In panel C male help cannot enhance female fitness; therefore, panel C females are invulnerable to 'helpful coercion', and females should have a lower probability of a 'trade-basis' for their mating decisions. Thus, here, male provisioning should seldom or never vary systematically with realised genetic paternity.
In panel D female fitness declines with 'male help'; females are invulnerable to 'helpful coercion'. Once again the Constrained Female Hypothesis predicts no systematic relationship between genetic paternity and paternal provisioning of males socially paired to Panel D females. The theoretical possibility that male help or presence may decrease female fitness, begs the question of benefits for males of staying (Gowaty 1996a). Pairs with panel D females will experience especially acute behavioural conflicts.
In panel A, females have no fitness without male help -- paternal provisioning is essential to females, and all mating should have a 'trade-basis' component. Strong selection should favour males who perceive and respond to less than perfect genetic paternity making females especially vulnerable to threats of withdrawal of their provisioning. Thus, little or no extra-pair paternity should occur. When these most constrained females do mate with extra-pair males, it will most likely be in trade for current or future nutritional benefits. The likelihood of extra-pair mating for so-called 'direct' benefits raises other interesting points. 1. Despite females’ universal dependence on males, there will be variation between pairs in their abilities to provision their offspring such that extra-pair mating by females could enhance the fitness of their social mates! If the quality of maternal care is a function of resource availability and if variation in maternal care correlates with offspring survival, and if mothers can increase their access to essential resources by extra-pair mating, male 'encouragement' of their mates’ extra-pair mating seems not-too-farfetched. 2. Only very minor modifications of this logic provide a novel theoretical pathway to the origin of social polyandry, perhaps of the sort observed in Dunnocks and Pukekos (Jamieson et. al 1994). In challenging environments a pathway to social polyandry will open when females trade extra-pair copulations and fertilisations for direct benefits. I call this explanation for the origin of social polyandry the 'Benefits of Cuckoldry Hypothesis'.
Tests of the predictions of the Constrained Female Hypothesis, for most insectivorous passerine species require tissue samples for DNA typing from socially paired females and males as well as their putative offspring, data on female foraging skill, a measure of availability of insects in foraging territories, and a measure of relative contributions to parental care by social mates.
The Constrained Female Hypothesis predicts little extra-pair paternity among the broods of females for whom male help is essential (A), relatively more among females who do not require help from their social mates (B), and the most among females for whom male help has no fitness benefits (C and D). Figure 5a displays these predictions.
Figure 4 and Figure 5 b show predicted patterns of male provisioning to offspring of Panel A, B, C and D females, when genetic paternity is less than perfect. The Constrained Female Hypothesis predicts that the only time that males decrease their paternal provisioning is when they 'traded' paternal provisioning for fertilisations. Thus, males mated to panel C or D females should not change their provisioning as a function of multiple mating by females.
PREDICTIONS FROM COMPETING HYPOTHESES
The Constrained Female Hypothesis also facilitates tests of the predictions of competing hypotheses. Under the Standard Adaptive Hypothesis that male help serves to increase females' or offspring fitness, when male help is costly to males, males should withdraw their help when their genetic paternity is less than perfect. This hypothesis predicts (Fig. 6a) decreases in paternal provisioning with numbers or proportion of extra-pair offspring for panel A, B, and C females. When the Shareable Paternal Care Hypothesis, withdrawal reduces offspring survival (Maynard Smith 1977), so no benefit accrues; so, there should be no declines for panel A and B females (Fig. 6b). The Constrained Female Hypothesis predicts that males should reduce paternal provisioning when females defect from implied bargains (Fig. 4 and Fig. 5b). Figure 7 shows the predictions of these three hypotheses relative to females’ abilities to feed their offspring without male help. Figure 7 demonstrates that conformance with any one pattern of variation across female types would reject two of the patterns, while supporting a third.
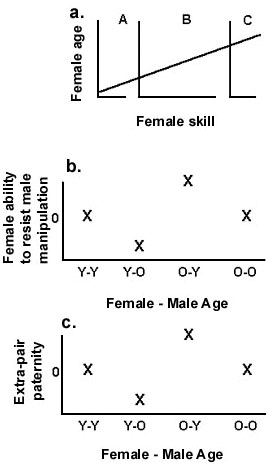
The Constrained Female Hypothesis also makes predictions about the interactions of male and female age variation with extra-pair offspring in males’ nests and paternal provisioning (Fig. 8). Assuming constant resource availability and that females’ foraging and breeding success depend at least partly on experience, younger females will be more vulnerable than older females. In within-population comparisons, younger females will occur thus: Panel A > B > C females (Fig. 8a). If experience is important to females’ relative vulnerabilities and males’ abilities to coerce females’ reproductive decisions, within populations, Panel B (Fig. 8b) females should preferentially socially pair with younger, rather than older males. Put another way: Older females socially paired to younger males will have lower vulnerabilities to their social mates than younger females mated to older or same-aged males, so older females should have a higher probability of taking options for extra-pair fertilisations (Fig. 8c).
Within-population tests of these predictions require data about (1) Intrinsic variation in females (e.g., foraging skills); (2) Availability to females of food resources (e.g., insect abundances); and (3) Frequency and distribution of extra-pair paternity among females; and (4) Paternal provisioning patterns (expressed as the % of provisioning provided by males).
Interspecific tests require (1) Estimates of the species-average dependence of females on paternal provisioning; (2) Estimates of the species-average rates of extra-pair paternity; (3) Estimates of species-average paternal provisioning patterns.
For several species some data are available. For instance, among the Great Tits Parus major male parental care seems useful, but not essential (Sasvari 1986; Bjorklund and Westman 1986), to females suggesting that Great Tit females fit into Panel B. Male Great Tits decrease provisioning as a function of the percentage of extra-pair paternity in their broods (Lubjuhn et al. 1993), consistent with the prediction. Among Eastern Bluebirds in Clemson, South Carolina male parental care is often useful to females and occasionally at least, females who are alone do just as well as paired females in raising their offspring (Gowaty 1983). In other words, Clemson Eastern Bluebird females seem to be panel B and C females, so that both the lack of relationship overall in one data set and the observed decrease in paternal provisioning with decreases in genetic paternity in the subsample of males tending multiply-sired broods are consistent with the Constrained Female Hypothesis. Tree Swallow females seem to be panel C females because females alone do as well as paired females in all populations studied so far (Dunn and Robertson 1992), so that the observed lack of relationship between genetic paternity and paternal provisioning (Whittingham et. al 1993) is also consistent with the Constrained Female Hypothesis. ‘Secondary female’ success indicates that male parental care is not essential in Red-winged Blackbirds. In many cases it may not be even helpful to females, so that the lack of decline in paternal provisioning as a function of genetic paternity is also consistent with the Constrained Female Hypothesis.
Of course, each of these results may be consistent with other explanations and only tests of alternatives will solve these puzzles. I hope that the fine-grained predictions of the Constrained Female Hypothesis will help bring hasty resolution to the problems of males in socially monogamous birds.
SUMMARY
I have emphasised that tests of the idea that paternal provisioning 'helpfully coerces' females into reproductive decisions they might not otherwise make may be productive in our efforts to understand the selective dynamics on social systems with bi-parental care. A perspective on male versus female contests over control of reproduction may solve emerging theoretical and empirical difficulties with more complex hypotheses. The most important take-home message is that variation among females is understudied, particularly since variation among females is the theoretical fulcrum on which interesting alternatives for both females and males may rest.
ACKNOWLEDGMENTS
For comments and discussion I thank Stephen P. Hubbell. I thank Jon Wright for provocative and collegial conversations as well as for sharing his in press review. I thank Doug Booher, Cathy Carver, Jill Goldstein, Judy Guinan, Jason Lang, and Melanie Rathburn for comments on the manuscript.
REFERENCES
Altmann, J. 1997. Mate choice and intrasexual reproductive competition: contributions that go beyond acquiring more mates. In: Gowaty, P. A. (ed.). Feminism and evolutionary biology: boundaries, intersections, and frontiers. New York: Chapman and Hall. pp. 320-333.
Avise, J. 1996. Molecular Markers, Natural History, and Evolution. Chapman & Hall: New York
Bjorklund, M. & Westman, B. 1986. Adaptive advantages of monogamy in the great tit (Parus major): An experimental test of the polygyny threshold model. Animal Behaviour 34: 1436-1440.
Clutton-Brock, T. 1991. The Evolution of Parental Care, Princeton University Press. Princeton, New Jersey.
Davies, N.B., Hatchwell, B.J., Burke, T. and Robson, T. 1992. Paternity and parental effort in dunnocks Prunella modularis: how good are male chick-feeding rules? Animal Behaviour. 43: 729-745.
Dixon, A., Ross,D., O’Malley, C., & Burke, T. 1994. Parental investment inversely related to degree of extra-pair paternity in the reed bunting. Nature (London) 371: 698-700.
Dunn, P.O. & Robertson, R. J. 1992. Geographic variation in the importance of male parental care and mating systems in tree swallows. Behavioural Ecology 3: 291-299.
Emlen, S.T. & Oring, L.W. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197: 215-223.
Gowaty, P.A. 1981. An extension of the Orians-Verner-Willson model to account for mating systems besides polygyny. American Naturalist 118(6):581-589.
Gowaty, P.A. 1983. Male parental care and apparent monogamy among Eastern Bluebirds (Sialia sialis). American Naturalist 121:149-157.
Gowaty, P. A. 1996a. Multiple mating by females selects for males that stay: A novel hypothesis for monogamy in birds. Animal Behaviour 51: 482-484.
Gowaty, P.A. 1996b. Battles of the sexes and origins of monogamy: IN: J. L. Black (Ed.)Partnerships in Birds. Oxford Series in Ecology and Evolution. Oxford University Press: Oxford pp 21-52.
Gowaty, P.A. 1996c. Field studies of parental care in birds: New data focus questions on variation in females. In: Advances in the Study of Behaviour. (ed. by: C. T. Snowdon and J. S. Rosenblatt). Academic Press, New York pp 476-531.
Gowaty, P.A. 1997a. Sexual dialectics, sexual selection, and variation in mating behaviour. In: Feminism and Evolutionary Biology (ed. P. A. Gowaty). Chapman Hall: New York, pp 351-384.
Gowaty, P.A. 1997b. Females' Perspectives in Avian Behavioural Ecology. Journal of Avian Biology. 28: 1-8.
Gowaty, P.A. 1998. Mate Choice. Encyclopedia of Reproduction. Academic Press, New York.
Gowaty, P.A. & Bridges, W.C. . 1991a. Nest box availability affects extra-pair fertilizations and conspecific nest parasitism in eastern bluebirds, Sialia sialis. Animal Behaviour 41:661-675.
Gowaty, P.A. & Bridges, W.C. 1991b. Behavioural, demographic, and environmental correlates of uncertain parentage in eastern bluebirds. Behavioural Ecology 2: 339-350.
Hrdy, S.B. 1997. Raising Darwin's Consciousness: Female sexuality and the prehominid origins of patriarchy. Human Nature 8:1-49.
Jamieson, I.G., Quinn, J.S., Rose, P.A. & White, B.N. 1994. Shared paternity among non-relatives is a result of an egalitarian mating system in a communally breeding bird, the pukeko. Proc. Roy. Soc. Lond. B. 257: 271-277.
Kempenaers, B. & Sheldon, B.C. 1997. Studying paternity and paternal care: pitfalls and problems. Animal Behaviour 53: 423-427.
Kempenaers, B. & Sheldon, B.C. 1998. Confounded correlations: a reply to Lifjeld et al. and Wagner et al. Animal Behaviour 55: 241-244.
Lack, D. 1968. Ecological Adaptations for Breeding in Birds. Chapman and Hall, London.
Lifjeld, Jan T., Anthonisen, K., Blomqvist, D., Johnsen, A., Krokene, C. & Rigstad, K. 1998. Studying the influence of paternity on paternal effort: a comment on Kempenaers & Sheldon. Animal Behaviour 55: 235-238.
Lubjuhn, T., Curio, E., Muth, S.C., Brün, J. & Epplen, T.J. 1993. Influence of extra-pair paternity on parental care in great tits (Parus major). In: DNA Fingerprinting: State of the Science. (Ed by Pena, S.D.J., Chakraborty, R., Epplen, J.T. and Jefferys, A.J.), pp. 379-385. Birkhäuser Verlag, Bern.
MacDougall-Shackleton, E.A., Robertson, R.J. & Boag, P.T. 1996. Temporary male removal increases extra-pair paternity in eastern bluebirds. Animal Behaviour 52:1177-1183.
Maynard Smith, J. 1977. Parental investment: A prospective analysis. Animal Behaviour 25:1-9.
Orians, G.H. 1969. On the evolution of mating systems in birds and mammals. American Naturalist 103:589-603.
Parker, G.A., Baker, R.R. & Smith, V.G.F. 1972. the origin and evolution of gametic dimorphism and the male-female phenomenon. Journal of Theoretical Biology 36:529-533.
Sasvari, L. 1986. reproductive effort of widowed birds. Journal Animal Ecology 55:553-564.
Trivers, R.L. 1972. Parental investment and sexual selection. In: Sexual Selection and the Descent of Man, B. C. Campbell (ed), pp. 136-179. Aldine, Chicago.
Verner, J. and Willson, M. 1969. Mating systems, sexual dimorphism, and the role of male North American passerine birds in the nesting cycle. Ornithological Monographs 9: 1-
Wagner, R.H., Schug, M.D. & Morton, E.S. 1998. Studying paternity and paternal care: the value of negative results. Animal Behaviour 55:239-240.
Westneat, D.F. 1995. Paternity and paternal behaviour in the red-winged blackbird, Agelaius phoeniceus. Animal Behaviour 49: 21-35.
Westneat, D.F. & R. Craig Sargent. 1996. Sex and parenting: the effects of sexual conflict and parentage on parental strategies. Trends in Ecology and Evolution 11: 87-91.
Westneat, D.F. & Sherman, P.W. 1993. Parentage and the evolution of parental behaviour. Behavioural Ecology 4: 66-77.
Whittingham, L.A., Dunn, P.O. & Robertson, R. J.. 1993. Confidence of paternity and male parental care: an experimental study in tree swallows. Animal Behaviour 46:139-147.
Whittingham, L.A. & Lifjeld, J.T. 1995. High paternal investment in unrelated young: extra-pair paternity and male parental care in house martins. Behavioural Ecology Sociobiology 37:103-108.
Williams, G.C. 1966. Natural selction, costs of reproduction, and a refinement of Lack’s principle. American Naturalist 100:697-690.
Wittenberger, J.F. & Tilson, R.L. 1980. The evolution of monogamy: hypotheses and evidence. Annual Review of Ecology and Systematics 11:50-68.
Wright, J. 1998. Paternity and paternal care. in press
Yezerinac, S.M., Weatherhead, P. J. & Boag, P.T. 1995. Extra-pair paternity and the opportunity for sexual selection in a socially monogamous bird (Dendroica petechia). Behavioural Ecology Sociobiology 37:179-188.
Fig. 1. The prediction from the Standard Adaptive Hypothesis of the relationship between genetic paternity and male parental care from Wright (in press).

Fig. 2. Model of the Constrained Female Hypothesis from Gowaty (1996b).
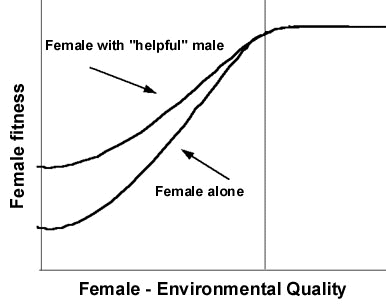
Fig. 3. Model of the Extended Female Constraint Hypothesis from Gowaty (1996c).
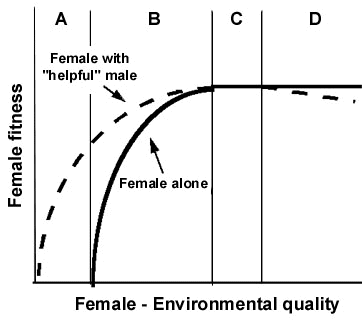
Fig. 4. Predicted variation in paternal provisioning as a function of extra-pair paternity depends on females’ abilities to feed their offspring alone. Figure 3 contains descriptions of panel A, B, C, and D females.
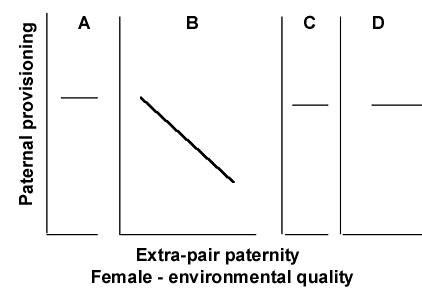
Fig. 5. Summary predictions of a. extra-pair paternity, b. paternal provisioning, and c. 'background' mate-guarding intensity under the extended Constrained Female Hypothesis (see Fig. 3 for descriptions of panel A, B, C, and D).
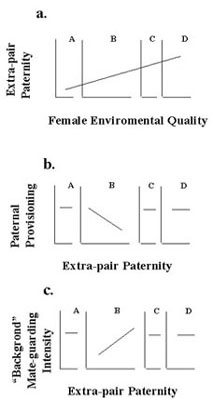
Fig. 6. The Standard Adaptive Hypothesis predicts (Fig. 6a) decreases in paternal provisioning with numbers or proportion of extra-pair offspring for panel A, B, C, and D females. When withdrawal of shareable paternal care reduces offspring survival (Maynard Smith 1977), no benefit of withdrawal accrues; thus, there should be declines for C and D females, but not for A and B females (Fig. 6b).
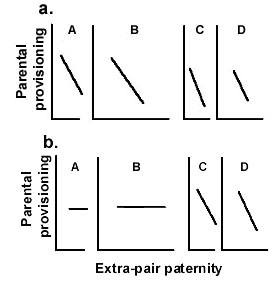
Fig. 7. Comparative predictions of three hypotheses relating paternal provisioning to genetic paternity for A, B, and C panel females. Predictions from a. the Standard Adaptive Hypothesis; b. the Shareable Paternal Care Hypothesis; and c. the Constrained Female Hypothesis.
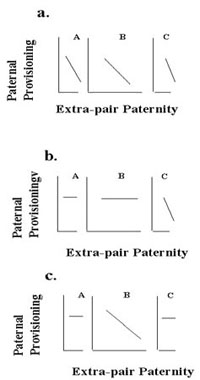
Fig. 8. Predictions from the Constrained Female Hypothesis concerning a. female age and variation in female skill; b. female resistance ability as a function of relative age of females and males; c. extra-pair paternity as a function of the interaction of female age and male age.