S44.2: Sexual conflict in birds with biparental care: Share of care in small vs. large broods and late vs. early broods
Tore Slagsvold
Department of Biology, University of Oslo, PO Box 1050 Blindern, N-0316 Oslo, Norway
Slagsvold, T. 1999. Sexual conflict in birds with biparental care: Share of care in small vs. large broods and late vs. early broods. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2622-2638. Johannesburg: BirdLife South Africa.In altricial birds with biparental care, one of the parents may reduce its investment at the expence of its mate. The contribution of each sex would depend on the value of the current brood and the benefit gained from allocating time and energy to other activities. Here I review data from passerine birds on share of food provisioning in relation to brood size and time of breeding. This is because the value of the current brood would depend heavily on brood size, and the value of alternative behaviours would depend heavily on the time of the breeding season. Separate predictions were made according to whether alternative behaviours were available and beneficial to males and females. In case of early broods, males tended to reduce share of care in small compared to large broods. One explanation is that when brood size is small, males may invest in further mate attraction and extra pair copulations, forcing the female to take greater share. Males also tend to reduce their share of feeding in late compared to early broods. This may be related to a stronger priority of moult in males than in females. Great variation exists between species in share of care in current brood even when these are closely related and live in the same area. For instance, in one study area, male blue tits Parus caeruleus contributed very little to the feeding of second broods in contrast to male great tits Parus major. This may explain why second broods were much less common in blue tits than in great tits.
INTRODUCTION
In altricial birds with biparental care, one of the parents may reduce its investment at the expence of its mate (Trivers 1972; Beissinger 1987). Reduction of care by a parent is compensated to some extent by the other parent but often not completely so, and hence a parent may suffer some cost if reducing share of care, selecting for cooperation (Houston & Davies 1985; Winkler 1987; Wright & Cuthill 1989; Slagsvold & Lifjeld 1990). The level of investment in current brood would depend on the value of the brood, the significance of biparental care for offspring survival, and the opportunity for additional nestings (Maynard Smith 1977). Parents may reduce share of care to increase fitness by other means. For instance, males may engage in extra-pair copulations and try to attract additional mates (e.g. Westneat 1988; Carey 1990; Wright & Cuthill 1990), and they may improve own survival by starting to moult (Nilsson & Svensson 1996). Females may reduce care to start another breeding attempt (e.g. Oring 1986; Smith et al. 1987; Persson & Öhrström 1989). In a few altricial birds, mate desertion by either sex is part of the normal mating system (e.g. Beissinger & Snyder 1987; Persson & Öhrström 1989), whereas in other species it occurs only occasionally (e.g. Ezaki 1988; Eens & Pinxten 1995; Orell et al. 1996). If brood size is very small and there is still some opportunity for renesting, both parents may desert (Mock & Parker 1986).
Here I review data from passerine birds on male and female share of care in relation to brood size and time of breeding. The reproductive value of the current brood would depend heavily on brood size, and the benefit gained by allocating effort to alternative behaviours would depend heavily on time of the breeding season.
PREDICTIONS
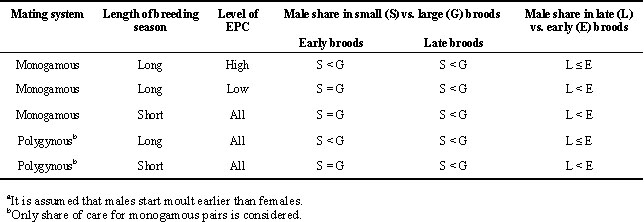
Table 1 summarises predictions on male and female share of care in monogamous and polygynous birds with biparental care.
Monogamy, long breeding season, early broods.
If the breeding season is long, there may be fertile females around in the nestling period of early broods. If the level of extra pair copulation (EPC) is high, early breeding males may invest in attempting EPCs also after hatching in their own nest (Westneat 1988), in particular if brood size of their own nest is small. This is because the value of the brood would be less if it is small than if it is large, and the female may also be better able to deal with the brood alone if the brood is small. Hence, we would expect male share of care to be less in case of small than in case of large, early broods. The prediction may hold true even if it is the females and not the males that are the primary seekers of EPC (Gowaty 1996). If males do not leave the territory they may still benefit from spending time and energy in mate attraction (song and visual displays).
An alternative hypothesis, giving the same prediction, is that if the sex ratio of the population is male biased, the current brood may be more valuable to the male than to the female. If so, males may take relatively greater share of care to large than to small broods (Richner et al. 1995; Christe et al. 1996).
If the level of EPC is low but there is a chance of a second brood laid by the same female, the male may invest heavily in the initial brood to enable the female to prepare for a second brood (labour division; e.g. Smith 1978; Edwards 1985; Weatherhead & McRae 1990; With & Balda 1990). The chance that the female would lay a second clutch size is greater if the size of the first brood is small (e.g. Smith et al. 1987; Lindén 1988; Verhulst & Hut 1996), and so males should also invest heavily in small first broods given that the pair-bond remains (Smith et al. 1987). If brood size is large, both parents may be needed for rearing the brood. We would predict similar male share of care in small and large early broods.
Monogamy, short breeding season, early broods.
If the breeding season is short, there would be no more fertile females around in the nestling period of early broods, and also no opportunity for a second brood. Hence, in case of early broods, male share of care in the nestling period would be independent of brood size.
Polygyny, long breeding season, early broods.
If the breeding season is long, the male would trade mate attraction against feeding of chicks, in particular if brood size is small. We would expect male share of care to be less in small than in large early broods.
Polygyny, short breeding season, early broods.
If the breeding season is short, there would be no further females to attract after hatching of own brood, and so male share of care in case of early broods would be independent of brood size.
Late broods, all species.
Late in the breeding season parents may start moult while still feeding chicks. Moulting may reduce feeding effort (Ezaki 1988; Jenni & Winkler 1994; Orell et al. 1996; Svensson & Nilsson 1997), as shown when moult is experimentally induced by removing certain flight feathers (Slagsvold & Lifjeld 1988, 1990). In most species of passerine birds studied, males start moult of flight feathers some days before females (Jehl 1968; Orell & Ojanen 1980; Slagsvold & Lifjeld 1989; Jenni & Winkler 1994; Svensson & Nilsson 1997), e.g. two weeks earlier in great tits Parus major (Dhondt 1973). Male share of food provisioning may therefore be less if nesting is late than if nesting is early, except in cases when males invested heavily in mate attraction and EPC during the early nesting attempt.
Late in the breeding season, no fertile females would be available and no opportunity for renesting. Onset of moult is conditional because when brood size is large, moult may be delayed both in males and females (Bensch et al. 1985; Slagsvold & Lifjeld 1989; Siikamäki et al. 1994). Late in the breeding season males may therefore trade onset of moult against feeding of chicks, in particular if brood size is small. Hence, male share of care may be larger in large than in small broods in all species in which males start moult earlier than females.
METHODS
The predictions are tested by reviewing data from the literature on male and female food provisioning. Studies were selected according to the following rules: (1) Passerine birds only. (2) Monogamous pairs, or presumably so. (3) Field studies (with no extra food provided). (4) Only studies where separate data were available for both small and large brood sizes, and/or early and late broods. (5) Data were taken from as late in the nestling period as possible when chicks need less brooding. (6) Sample size of at least two broods in each group. When comparing parental share of care in small and large broods, only data for the extreme brood sizes were used (e.g. if data were available for brood sizes 3, 4 and 5, broods with 3 and 5 chicks were compared).
RESULTS
Large versus small broods
Table 2 shows data on male share of care (% of male contribution of total number of feeding visits) in relation to brood size. Most of these data were from first broods. The species have been divided in three groups according to breeding ecology. In further analysis of the data, mean values were calculated for each species when data were available from more than one locality. Mean values were also calculated for each of the three groups of species for the smallest and largest brood sizes in each study (Table 3).
For double-brooded species and long breeding season (group A, Table 2, 11 species), male share of care tended to be greater when brood size was large than when it was small (mean values of 45.6% and 41.5% respectievly, Table 3) though the difference was not statistically significant (Wilcoxon matched pairs test , Z = -1.34, P = 0.18; Fig. 1). Male share of care tended to be lower than female share of care for small broods (Z = -1.79, P = 0.074) but less so for large broods (Z = -1.43, P = 0.15).
For single-brooded species, monogamous, and short or moderately long breeding season (group B, Table 2, 6 species), male share of care averaged slightly above 50% and was about the same for large and small broods (53.2% and 54.2%, respectively, Table 3). Note that high mean value of male share of care in these species was largely due to the rook Corvus frugilegus. In this corvid, like in birds of prey, males and females have more specialized duties than in other passerines, females guarding the nest and males providing most of the food. Data were only available for two group C species (single brooded, occasionally polygynous, short and moderately long breeding season, Table 2). Male share of care was on average 47.5% and 46.0% for large and small broods, respectively (Table 3).
For group A species, male share of care was expected to increase with brood size, whereas this would not be the case for group B and C species. The results were in the predicted direction but were not statistically significant. For group A species male share of care was on average 4.1% higher for large broods than for small broods (Table 3) whereas the corresponding value for group B and C species combined was -0.4% (S.D. = 6.1, n = 8; Mann-Whitney U-test, Z = -1.41, P = 0.16).
It was predicted that for late broods male share of care would be positively correlated with brood size (Table 1). In the literature reviewed, data were only available in one study and the prediction was not supported. For late broods of eastern phoebes (Sayornis phoebe) male share of care was no higher for enlarged broods (34%, n = 6) than for reduced broods (35%, n = 8; for control broods it was 17%, n = 5; chicks 9 days old; from Table 1 in Conrad & Robertson 1993).
Late versus early broods
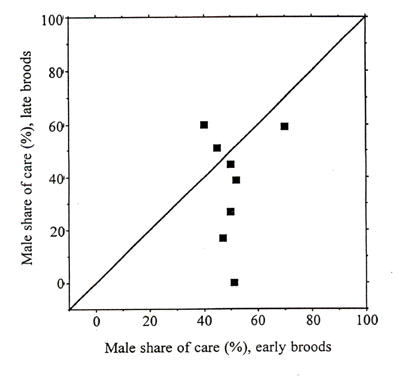
Male share of care tended to be lower for late than for early broods (mean values of 37.3% and 50.6%, respectively, Table 4; Fig. 2) though the difference was not quite statistically significant (Wilcoxon matched pairs test , Z = -1.54, n = 8, P = 0.12).
DISCUSSION
Are the results reliable?
The main findings of the present study are that male share of care (1) tended to increase with increasing brood size, (2) in particular in species with multiple broods and long breeding season, and (3) male share of care tended to be less for late than for early broods. These results were constistent with the predictions made. However, none of the results were statistically signficant, perhaps because of small sample sizes and low statistical power. Are the results reliable?
(1) The present study is only a preliminary review of the literature and data from more species are needed. Effects of phylogeny should also be taken into account in the interspecific comparisons. In many cases, feeding records were often from only a short period of observation, few nests were studied, and often only in a single breeding season. Relative share of care by the sexes may differ between years (e.g. Zaias & Breitwisch 1989) and populations (e.g. McGillivray 1984).
(2) The review is based on provisioning rates as a measure of parental effort because such data were most readily available. The conclusions should therefore be treated with caution. For instance, prey type and load size may differ between males and females (Royama 1966; Biermann & Sealy 1982; Wittenberger 1982; Grundel 1987; Jones 1987; Moreno 1987) or be similar (Zaias & Breitwisch 1989; Wright & Cuthill 1990; Slagsvold 1997), and may vary with brood size (Biermann & Sealy 1982; Grundel 1987; Moreno 1987; Lifjeld 1988) or not (Wright & Cuthill 1990; Moreno et al. 1995). Provisioning rates may not necessarily correlate with weight of prey (Royama 1966; Biermann & Sealy 1982) or daily energy expenditure (Verhulst & Tinbergen 1997; but see Pärt et al. 1992 and point 5 below).
(3) In most passerine birds, females invest more in brooding than males, which may be one reason for high daily energy expenditure in females (Verhulst & Tinbergen 1997). Females may brood the chicks quite late in the nestling period (e.g. Smith et al. 1982; Slagsvold 1997). Need of brooding may vary in relation to brood size (e.g. be greater for smaller broods; Slagsvold 1997), and time of the breeding season.
(4) Males may invest more in brood defence than females (Zaias & Breitwisch 1989; Curio & Onnebrink 1995; Markman et al. 1995). Such defence may increase with brood size, though the increase may be similar in males and females (Curio & Onnebrink 1995). Consistent with the data on provisioning rates, male great tits seem to invest relatively less than females in defence of late broods compared to early broods (Onnebrink & Curio 1991; Curio & Onnebrink 1995).
(5) I found three studies that compared daily energy expenditure (DDE) of males and females with brood size (Delichon urbica, Hails & Bryant 1979; Parus major, Verhulst & Tinbergen 1997; Ficedula hypoleuca, Moreno et al. 1995). The same patterns were found in each species: (1) DDE was positively correlated with brood size in males but not in females; (2) DDE was lower in males than for females for small broods, and equal or lower for large broods. The first result is consistent with the conclusion derived above on male share of food provisioning and brood size. For discussion of the second result, see below.
(6) Typically polygynous species were excluded from the analysis. This was because male share of care would be more difficult to predict in case of polygyny than in case of monogamy because optimal male allocation would be strongly influenced by the time of the nestling period in relation to needs of guarding of extra mates and opportunities for further attraction of females. For instance, males may rarely feed the first hatched brood if they guard a second or third female (e.g. Nishiumi et al. 1996). However, if the breeding time of harem females is similar, males seem to invest most heavily in the brood with the larger number of chicks (e.g. Lifjeld & Slagsvold 1991).
Large versus small broods
Male share of care tended to increase with increasing brood size. This is consistent with predictions in Table 1 but does not necessary prove that the assumptions made are correct. Further studies are needed to find out what actually causes this relationship. In case of early broods and a long breeding season, males may spend effort in mate attraction and attempting EPC. Unfortunately, data on frequency of EPC are missing from most populations in which food provisioning was studied and data from other populations and other years may be inappropriate (cf. Bjørnstad & Lifjeld 1997).
Increasing male share of care with brood size is also consistent with the idea that current brood is of relatively higher value than future broods for males than for females (Richner et al. 1995; Christe et al. 1996). This is because the operational sex ratio may be male-biased due to a slightly higher survival in males than in females in most passerine birds, including great tits (Payevsky et al. 1997), e.g. because of male dominance in winter (Perrins 1979; Clobert et al. 1988). The idea was supported in a study of prevalence of malaria in great tits. When brood size was artificially increased by two chicks, males increased their share of care and suffered from increased prevalence of Plasmodium spp. Fewer infected than noninfected birds tended to survive to the next breeding season. Females did not increase provisioning rate significantly when brood size was enlarged, and they did not become more infected by malaria (Richner et al. 1995). In great tits males also tended to invest more heavily than females when the nest was infested experimentall with ectroparasites (Christe et al. 1996). However, the explanation offered by Richner et al. (1995) and Christe et al. (1996) for their results is not obvious for several reason.
(1) The study of parasite prevalence by Richner et al. (1995) was only conducted in a single year and in a single population. Norris et al. (1994) also manipulated brood size in great tits and also found that enlarging brood size increased parasite prevalance in males but not in females. However, in the latter study the result was far from clear-cut because reducing brood size also increased parasite prevalence in males. It should also be noted that prevalence of parasites was higher in females than in males. In a third study of great tits, the strength of the positive correlation between brood size and intensity of Haemoproteus blood parasite infection was similar in males and females (Ots & Horak 1996).
(2) If mortality is higher in females than in males outside the breeding season (Payevsky et al. 1997), females should invest heavily in current brood.
(3) The value of the current brood may be greater for females than for males because the cost of renesting may be greater for females (Rytkönen et al. 1993). This is because the female has to produce the clutch and in most passerines she also builds the nest. Moreover, during both periods of breeding females may be at higher risk of predation than males (Slagsvold & Dale 1996).
(4) If the assumption of higher value of current brood to males than to females is correct, males should in general invest more heavily than females. Males may take more risk during brood defence than females (Zaias & Breitwisch 1989; Curio & Onnebrink 1995; Markman et al. 1995). However, they do not seem to spend more energy overall. As mentioned above, in three species of passerine birds, including great tits studied by Richner et al. (1995), DDE tended to be lower in males than in females when brood size was small, and no higher in males when brood size was large (Hails & Bryant 1979; Moreno et al. 1995; Verhulst & Tinbergen 1997).
(5) If males suffer more than females when increasing brood size, this should be reflected in subsequent survival following manipulation of brood size. Such an effect has not been found in studies of great tits (Boyce & Perrins 1987; Pettifor et al. 1988), nor in some other studies (e.g. Nur 1984, 1988; Gustafsson & Sutherland 1988; Orell & Koivula 1988). In fact, in great tits and blue tits, brood size may have stronger influence on survival in females than in males (Nur 1984; Boyce & Perrins 1987; Dhondt 1987), as also number of broods (Verhulst 1995; McCleery et al. 1996).
An alternative interpretation of increasing male share of care with brood size is that females are closer to a maximum working load at any brood size and so are less able than males to respond when brood size is increased. The data on daily energy expenditure mentioned above are consistent with this view.
Consequences of male and female investments patterns
Males may invest relatively more in food provisioning if current brood is large and allocate relatively more effort to attempting EPC and further mate attraction if current brood is small. In such cases it may pay the female to have a larger brood to increase male contribution. We may therefore predict that in species with high frequency of EPC and in species with some probability of polygyny, clutche size early in the breeding season may be relatively higher than in species with no male opportunities for extra matings. The prediction from this hypothesis would be difficult to test across species, however, because clutch size may be related to a number of other factors. For instance, male food provisioning may be negatively related to level of EPC (Westneat & Sherman 1993) which may counteract any selection for larger broods in such cases. A female counter tactic to ensure male assistance would be to behave aggressively towards intruding females. A problem with such behaviour is that it would compete with effort allocated to chick brooding and feeding. In general, resident females are less aggressive during the nestling period than during earlier stages of breeding (Slagsvold & Lifjeld 1994). Another female tactic may be to ensure synchronous hatching of the brood. Then there would be many chicks to feed of similar ages, and lack of male care may put the whole brood in danger, forcing the male to participate (Slagsvold et al. 1994, 1995).
Male share of care tended to be lower for late than for early broods. This may help to explain why many passerine birds are single brooded, or only occasionally have two broods. For instance, in Northern Europe, blue tits Parus caeruleus seldom have second broods (Perrins 1979). I have studied four cases of second breeding near Oslo, Norway, and the males never fed the chicks (Table 3). Apparently, this was because in blue tits males give higher priority to moult than females (Svensson & Nilsson 1997).
Late in the breeding season females may face a dilemma with regard to optimal brood size. Then males may allocate little effort to parental duties because of moult. Hence, females may benefit from laying a small clutch. On the other hand, as argued above (cf. Table 1), males may still contribute more to a large than to a small brood late in the breeding season because of higher reproductive value of a larger brood. Consequently, in species in which males start moult earlier than females, it would be difficult to predict what the optimal size of late clutches would be from a female perspective. In the present preliminary review, I found only data on one species to compare male share of care with brood size late in the breeding season; no correlation was found (Conrad & Robertson 1993).
Which consequences would the patterns of male and female provisioning rules have for the survival prospects of parents? In most species of passerines studied, males increased their feeding rate more than females with increasing brood size. This was also reflected in a stronger increase of energy expenditure with increasing brood size in males than females. We might therefore expect survival to be more affected by brood size manipulation in males than in females. However, this prediction is not necessarily true. When brood size is small, males may allocate effort to other activities than feeding, such as nest defence (e.g. Markman et al. 1995), mate attraction and attempting EPCs, and territorial defence. Such activities may be costly in terms of survival (e.g. injuries from fighting with other males, increased risk of predation). Feeding of nestlings scores high on an energy budget because of the high energetic cost of flight. However, it also involves foraging, where the bird can self-feed. Measuring DDE is therefore not equivalent with measuring total parental investment. For instance, in many altricial birds the male provides almost all the food to the family during the early stages of breeding. He may therefore have higher DDE than the female and so apparently paying a higher cost. However, in such cases the female would be very dependent on the male for obtaining food and she may also suffer from predation when sitting on the nest, whereas the male may visit rich patches of food where he can fulfill his own needs before returning to the nest.
CONCLUSION
In a number of passerine birds, a sexual conflict may exist over parental investment. This conflict may have important influence on male and female share of care in current brood and also on optimal brood size. Males may force females to invest heavily by spending effort in other activities, like mate attraction and onset of moult, which would increase their own reproductive fitness and survival prospects but not those of the mate. Females, on the other hand, seem to have fewer such options for manipulating the partner´s contribution. However, they may use such as brood size, willingness to lay another clutch with the same male, and sexual faithfullness in case of repeat nesting. The latter tactics are poorly understood. Also, we know very little of why males start moult before females. Is this because they need to be prepared for competition among males for breeding territories, mates, and dominance positions in winter flocks, or is it because of higher residual reproductive value in males than in females?
REFERENCES
Alatalo, R.V., Lundberg, A. & Ståhlbrandt, K. 1982. Why do pied flycatcher females mate with already-mated males? Animal Behaviour 30: 585-593.
Bédard, J. & Meunier, M. 1983. Parental care in the savannah sparrow. Canadian Journal of Zoology 61: 2836-2843.
Beissinger, S.R. 1987. Mate desertion and reproductive effort in the snail kite. Animal Behaviour 35: 1504-1519.
Beissinger, S.R. & Snyder, N.F.R. 1987. Mate desertion in the snail kite. Animal Behaviour 35: 477-487.
Bensch, S., Gezelius, L., Grahn, M., Hasselqvist, D., Lindström, Å. & Ottosson, U. 1985. Influence of brood size on moult in female willow warblers. Ornis Scandinavica 16: 151-152.
Best, L.B. 1977. Nestling biology of the field sparrow. Auk 94: 308-319.
Biermann, G.C. & Sealy, S.G. 1982. Parental feeding of nestling yellow warblers in relation to brood size and prey availability. Auk 99: 332-341.
Bjørnstad, G. & Lifjeld, J.T. 1997. High frequency of extra-pair paternity in a dense and synchronous population of willow warblers Phylloscopus trochilus. Journal of Avian Biology 28: 319-324.
Boyce, M.S. & Perrins, C.M. 1987. Optimizing great tit clutch size in a fluctuating environment. Ecology 68: 142-153.
Breitwisch, R., Merritt, P.G. & Whitesides, G.H. 1986. Parental investment by the northern mockingbird: male and female roles in feeding nestlings. Auk 103: 152-159.
Carey, M. 1990. Effects of brood size and nestling age on parental care by male field sparrows (Spizella pusilla). Auk 107: 580-586.
Christe, P., Richner, H. & Oppliger, A. 1996. Begging, food provisioning, and nestling competition in great tit broods infested with ectoparasites. Behavioral Ecology 7: 127-131.
Clobert, J., Perrins, C.M., McCleery, R.H. & Gosler, A.G. 1988. Survival rate in the great tit Parus major in relation to sex, age, and immigration status. Journal of Animal Ecology 57: 287-306.
Conrad, K.F. & Robertson, R.J. 1993. Patterns of parental provisioning by eastern phoebes. Condor 95: 57-62.
Curio, E. & Onnebrink, H. 1995. Brood defense and brood size in the great tit (Parus major): a test of a model of unshared parental investment. Behavioral Ecology 6: 235-241.
Dhondt, A.A. 1973. Postjuvenile and postnuptial moult in a Belgian population of great tits, Parus major, with some data on captive birds. Gerfaut 63: 187-210.
Dhondt, A.A. 1987. Reproduction and survival of polygynous and monogamous blue tits Parus caeruleus. Ibis 129: 327-334.
East, M. 1981. Aspects of courtship and parental care of the European robin Erithacus rubecula. Ornis Scandinavica 12: 230-239.
Edwards, P.J. 1985. Brood division and transition to independence in blackbirds Turdus merula. Ibis 127: 42-59.
Eens, M. & Pinxten, R. 1995. Mate desertion by primary female European starlings at the end of the nestlig stage. Journal of Avian Biology 26: 267-270.
Ezaki, Y. 1988. Mate desertion by male great reed warblers Acrocephalus arundinaceus at the end of the breeding season. Ibis 130: 427-437.
Gowaty, P.A. 1996. Multiple mating by females selects for males that stay: another hypothesis for social monogamy in passerine birds. Animal Behaviour 51: 482-484.
Grundel, R. 1987. Determinants of nestling feeding rates and parental investment in the mountain chickadee. Condor 89: 319-328.
Gustafsson, L. & Sutherland, W.J. 1988. The costs of reproduction in the collared flycatcher Ficedula albicollis. Nature (London) 335: 813-815.
Hails, C.J. & Bryant, D.M. 1979. Reproductive energetics of a free-living bird. Journal of Animal Ecology 48: 471-482.
Hegner, R.E. & Wingfield, J.C. 1987. Effects of brood-size manipulations on parental investment, breeding success, and reproductive endocrinology of house sparrows. Auk 104: 470-480.
Houston, A.I. & Davies, N.B. 1985. The evolution of cooperation and life history in the dunnock, Prunella modularis. In: Sibly, R.M. & Smith, R.H. (eds) Behavioural ecology: the ecological consequences of adaptive behaviour. Oxford; Blackwell: 471-487.
Jehl, J.R.J. 1968. The breeding biology of Smith´s longspur. Wilson Bulletin 80: 123-149.
Jenni, L. & Winkler, R. 1994. Moult and ageing of European passerines. London; Academic Press: 217pp.
Jones, G. 1987. Parental foraging ecology and feeding behaviour during nestling rearing in the swallow. Ardea 75: 169-174.
Leffelaar, D. & Robertson, R.J. 1986. Equality in feeding roles and the maintenance of monogamy in tree swallows. Behavioral Ecology and Sociobiology 18: 199-206.
Lifjeld, J.T. 1988. Prey choice and nestling hunger: an experiment with pied flycatchers, Ficedula hypoleuca. Animal Behaviour 36: 134-139.
Lifjeld, J.T. & Slagsvold, T. 1991. Sexual conflict among polygynous pied flycatchers feeding young. Behavioral Ecology 2: 106-115.
Lindén, M. 1988. Reproductive trade-off between first and second clutches in the great tit Parus major: an experimental study. Oikos 51: 285-290.
Markman, S., Yom-Tov, Y. & Wright, J. 1995. Male parental care in the orange-tufted sunbird: behavioural adjustments in provisioning and nest guarding effort. Animal Behaviour 50: 655-669.
Maynard Smith, J. 1977. Parental investment - a prospective analysis. Animal Behaviour 25: 1-9.
McCleery, R.H., Clobert, J., Julliard, T. & Perrins, C.M. 1996. Nest predation and delayed cost of reproduction in the great tit. Journal of Animal Ecology 65: 96-104.
McGillivray, W.B. 1984. Nestling feeding rates and body size of adult house sparrows. Candian Journal of Zoology 62: 381-385.
Mock, D.W. & Parker, G.A. 1986. Advantages and disadvantages of egret and heron brood reduction. Evolution 40: 459-470.
Moreno, J. 1987. Parental care in the wheater Oenanthe oenanthe: effects of nestling age and brood size. Ornis Scandinavica 18: 291-301.
Moreno, J.L., Cowie, R.J., Sanz, J.J. & Williams, R.S.R. 1995. Differential response by males and females to brood manipulation in the pied flycatcher: energy expenditure and nestling diet. Journal of Animal Ecology 64: 721-732.
Nilsson, J.Å. & Svensson, E. 1996. The cost of reproduction: a new link between current reproductive effort and future reproductive success. Proceedings of the Royal Society of London, Series B 263: 711-714.
Nishiumi, I., Yamagishi, S., Maekawa, H. & Shimoda, C. 1996. Paternal expenditure is related to brood sex ratio in polygynous great reed warblers. Behavioral Ecology and Sociobiology 39: 211-217.
Norris, K., Anwar, M. & Read, A.F. 1994. Reproductive effort influences the prevalence of haematozoan parasites in great tits. Journal of Animal Ecology 63: 601-610.
Nur, N. 1984. The consequences of brood size for breeding blue tits I. Adult survival, weight change and the cost of reproduction. Journal of Animal Ecology 53: 479-496.
Nur, N. 1988. The cost of reproduction in birds: an examination of the evidence. Ardea 76:155-168.
Onnebrink, H. & Curio, E. 1991. Brood defense and age of young: a test of the vulnerability hypothesis. Behavioral Ecology and Sociobiology 29: 61-68.
Orell, M. & Koivula, K. 1988. Cost of reproduction: parental survival and production of recruits in the willow tit Parus montanus. Oecologia 77: 423-432.
Orell, M. & Ojanen, M. 1980. Overlap between breeding and moulting in the great tit Parus major and willow tit P. montanus in northern Finland. Ornis Scandinavica 11: 43-49.
Orell, M., Rytkönen, S., Koivula, K., Ronkainen, M. & Rahiala, M. 1996. Brood size manipulations within the natural range did not reveal intragenerational cost of reproduction in the willow tit Parus montanus. Ibis 138: 630-637.
Oring, L.W. 1986. Avian polyandry. Current Ornithology 3: 309-351.
Ots, I. & Horak, P. 1996. Great tits Parus major trade health for reproduction. reproduction: a new link between current reproductive effort and future reproductive success. Proceedings of the Royal Society of London, Series B 263: 1443-1447.
Payevsky, V.A., Vysotsky, V.G., Yefremov, V.D., Markovets, M.Y., Morozov, Y.G. & Shapoval, A.P. 1997. Sex-specific survival rates in birds. Journal of General Biology (Moscow) 58: 5-20.
Perrins, C.M. 1979. British tits. London; Collins: 304pp.
Persson, O. & Öhrström, P. 1989. A new avian mating system: ambisexual polygamy in the penduline tit Remiz pendulinus. Ornis Scandinavica 20: 105-111.
Pettifor, R.A., Perrins, C.M. & McCleery, R.H. 1988. Individual optimization of clutch size in great tits. Nature (London) 336: 160-162.
Pinkowski, B.C. 1978. Feeding of nestlings and fledgling eastern bluebirds. Wilson Bulletin 90: 84-98.
Power, H.W. 1980. The foraging behavior of mountain bluebirds. Ornithological Monographs 28: 1-72.
Pärt, T., Gustafsson, L. & Moreno, J. 1992. 'Terminal investment' and sexual conflict in the collared flycatcher (Ficedula albicollis). American Naturalist 140: 868-882.
Richner, H., Christie, P. & Oppliger, A. 1995. Parental investment affects prevalence of malaria. Proceeding of the National Academy of Science USA 92: 1192-1194.
Royama, T. 1966. Factors governing feeding rates, food requirement and brood size of nestling great tits Parus major. Ibis 108: 313-347.
Rytkönen, S., Orell, M. & Koivula, K. 1993. Sex-role reversal in willow tit nest defence. Behavioral Ecology and Sociobiology 33: 275-282.
Røskaft, E. 1983. Sex-role partitioning and parental care by the rook Corvus frugilegus. Ornis Scandinavica 14: 180-187.
Røskaft, E. & Slagsvold, T. 1985. Differential mortality of male and female offspring in experimentally manipulated broods of the rook. Journal of Animal Ecology 54: 261-266.
Seel, D.C. 1969. Food, feeding rates and body temperature in the nestling house sparrow Passer domisticus at Oxford. Ibis 111: 36-47.
Siikamäki, P., Hovi, M. & Rätti, O. 1994. A trade-off between current reproduction and moult in the pied flycatcher - an experiment. Functional Ecology 8: 587-593.
Slagsvold, T. 1997. Brood division in birds in relation to offspring size: sibling rivalry and parental control. Animal Behaviour 54: 1357-1368.
Slagsvold, T. 1997. Is there a sexual conflict over hatching asynchrony in American robins. Auk 114: 593-600.
Slagsvold, T., Amundsen, T. & Dale, S. 1994. Selection by sexual conflict for evenly spaced offspring in blue tits. Nature (London) 370: 136-138.
Slagsvold, T., Amundsen, T. & Dale, S. 1995. Costs and benefits of asynchronous hatching in blue tits Parus caeruleus. Journal of Animal Ecology 64: 563-578.
Slagsvold, T. & Dale, S. 1996. Disappearance of female pied flycatchers in relation to breeding stage and experimentally induced molt. Ecology 77: 461-471.
Slagsvold, T. & Lifjeld, J.T. 1988. Ultimate adjustment of clutch size to parental feeding capacity in a passerine bird. Ecology 69: 1918-1922.
Slagsvold, T. & Lifjeld, J.T. 1989. Hatching asynchrony in birds: the hypothesis of sexual conflict over parental investment. American Naturalist 134: 239-253.
Slagsvold, T. & Lifjeld, J.T. 1990. Influence of male and female quality on clutch size in tits (Parus spp.). Ecology 71: 1258-1266.
Slagsvold, T. & Lifjeld, J.T. 1994. Polygyny in birds: the role of competition between females for male parental care. American Naturalist 143: 59-94.
Smith, H.G., Källander, H., Fontell, K. & Ljungström, M. 1988. Feeding frequency and parental division of labour in the doble-brooded great tit Parus major. Behavioral Ecology and Sociobiology 22: 447-453.
Smith, H.G., Källander, H. & Nilsson, J.-Å. 1987. Effect of experimentally altered brood size on frequency and timing of second clutches in the great tit. Auk 104: 700-706.
Smith, J.N.M. 1978. Divison of labour by song sparrows feeding fledged young. Canadian Journal of Zoology 56: 187-191.
Smith, J.N.M., Yom-Tov, Y. & Moses, R. 1982. Polygyny, male parental care, and sex ratio in song sparrows: an experimental study. Auk 99: 555-564.
Svensson, E. & Nilsson, J.-Å. 1997. The trade-off between molt and parental care: a sexual conflict in the blue tit? Behavioral Ecology 8: 92-98.
Trivers, R.L. 1972. Parental investment and sexual selection. In Campbell, B. (ed) Sexual selection and the descent of man, 1871-1971. Chicago; Aldine: 136-179.
Verhulst, S. 1995. Reproductive decisions in great tits. Thesis. Groningen; University of Groningen: 184pp.
Verhulst, S. & Hut, R.A. 1996. Post-fledging care, multiple breeding and the costs of reproduction in the great tit. Animal Behaviour 51: 957-966.
Verhulst, S. & Tinbergen, J.M. 1997. Clutch size and parental effort in the great tit Parus major. Ardea 85: 111-126.
Weatherhead, P.J. & McRae, S.B. 1990. Brood care in American robins: implications for mixed reproductive strategies by females. Animal Behaviour 39: 1179-1188.
Westneat, D.F. 1988. Male parental care and extrapair copulations in the indigo bunting. Auk 105: 149-160.
Westneat, D.F. & Sherman, P.W. 1993. Parentage and the evolution of parental behavior. Behavioral Ecology 4: 66-77.
Winkler, D.W. 1987. A general model for parental care. American Naturalist 130: 526-543.
With, K.A. & Balda, R.P. 1990. Intersexual variation and factors affecting parental care in Western bluebirds: a comparison of nestling and fledgling periods. Canadian Journal of Zoology 68: 733-742.
Wittenberger, J.F. 1982. Factors affecting how male and female bobolinks apportion parental investments. Condor 84: 22-39.
Wright, J. & Cuthill, I. 1989. Manipulation of sex differences in parental care. Behavioral Ecology and Sociobiology 25: 171-181.
Wright, J. & Cuthill, I. 1990. Manipulation of sex differences in parental care: the effect of brood size. Animal Behaviour 40: 462-471.
Zaias, J. & Breitwisch, R. 1989. Intra-pair cooperation, fledgling care, and renesting by northern mockingbirds (Mimus polyglottos). Ethology 80: 94-110.
Table 1. Predictions on male share of food provisioning in passerine birdsa

Table 2. Male share of food provisioning in passerine birds
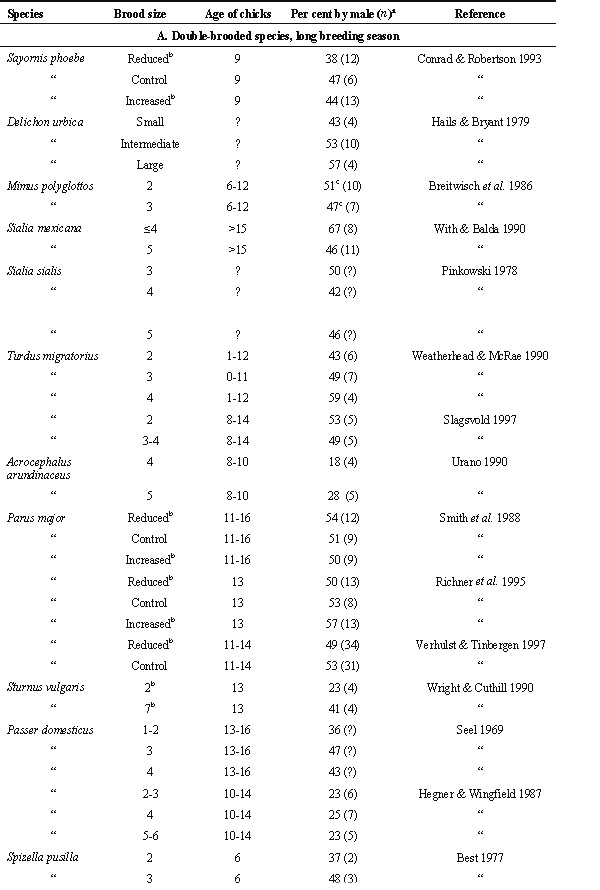
Table 2. continued
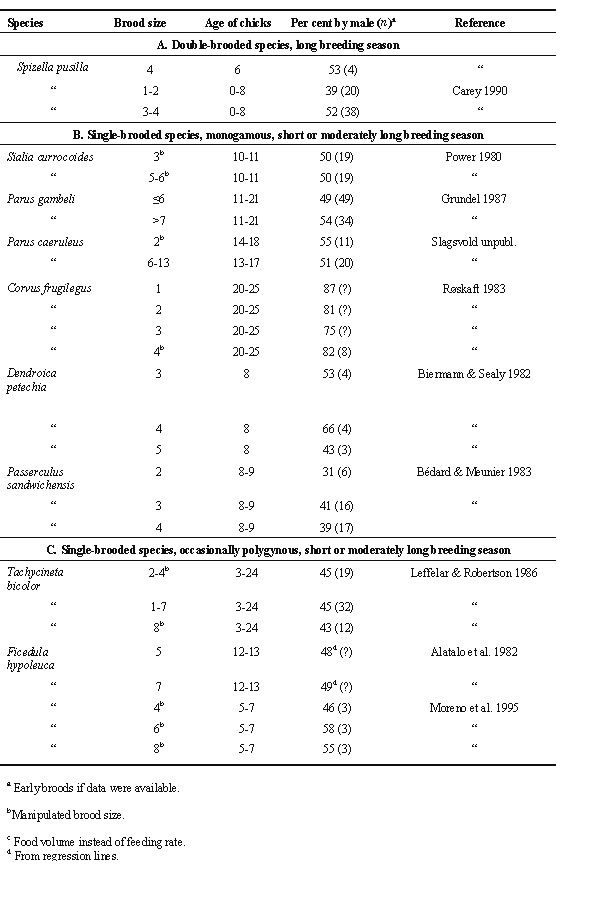
Table 3. Male share of food provisioning in passerine birds in large and small broods. Data from Table 2
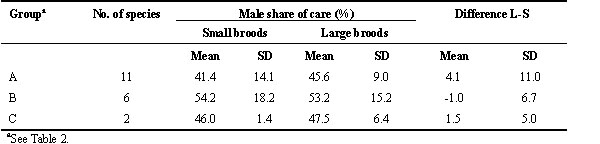
Table 4. Male share of food provisioning early and late in the breeding season in passerine birds
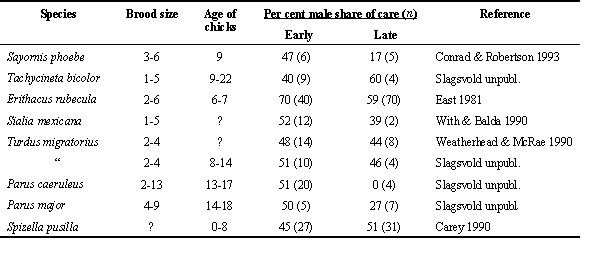
Fig. 1. Male share of food
provisioning to small and large broods in passerine birds. Filled circles: group A species
(n = 11), open circles: group B species (n = 6), open squares: group C species (n = 2).
Fordefinition of groups, see Table 2. A symbol above the y = x line means that male share
of care was greater for large than for small broods in that species.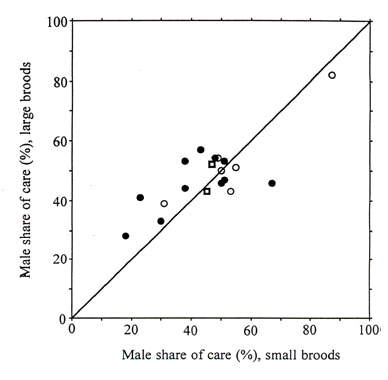
Fig. 2. Male share of food provisioning to early and late broods in passerine birds. A symbol above the y = x line means that male share of care was greater for late than for early broods in that species.