S44.1: Female resistance to male control attempts and intraspecific variation in distributions of extra-pair paternity
Francisco Valera & Herbert Hoi
Konrad Lorenz-Institute, Savoyenstrasse 1a, A-1160, Vienna, Austria,
fax 43 1 486212128, e-mail f.valera@klivv.oeaw.ac.at, h.hoi@klivv.oeaw.ac.at Valera, F. & Hoi, H. 1999. Female resistance to male control attempts and intraspecific variation in distributions of extra-pair paternity. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2608-2621. Johannesburg: BirdLife South Africa.Female tactics to control paternity are usually cryptic and the underlying motivation is often difficult to assess. In this paper we focus first on female behavioural mechanisms to control paternity, namely on the basis of manipulation of basic information. In this regard we review two alternative strategies: female advertisement of her fertile status vs. hiding fertility. Females of some bird species try to attract males by special calls and conspicuous solicitation display to announce fertility and thereby incite male-male competition. On the other side egg covering during the fertile phase has been reported as a deceiving mechanism females may use to hide their fertility. A preliminary review of this behaviour in birds suggests that it can act more frequently than thought as a deceiving strategy to control paternity, mainly in species with a high intersexual conflict and with nests favouring such strategy (e.g. hole nesting species). The outcome of the intersexual conflict in species with such complex behavioural strategies may depend on a high degree on the quality of the combatants (either expressed in terms of experience or of physical condition), so that variation in the relative quality of both partners may account for intraspecific variation in distribution of extra-pair paternity. An alternative source of intraspecific variation in extra-pair paternity is habitat quality. Specific results in some study cases and the occurrence of a positive relationship between extra-pair paternity and average breeding success per year among species suggest that environmental conditions during the female fertile period may directly influence individual reproductive tactics, thus supporting the Female Constrained hypothesis (Gowaty 1996).
INTRODUCTION
Male and female interests are often different and such conflict results in a battle for the control of reproduction. Only about 25% of social monogamous bird species are also genetically monogamous, which suggests that male birds are often unable to control paternity (Gowaty 1996). Recent studies have shown that female birds have several tactics to control paternity. For instance, females may favour a specific male by means of physiological tactics (like selection of sperm within the female tract, Birkhead & M° ller 1993) or morphological mechanisms (like muscles in the cloaca to expel sperm, Davies 1983). Females can also determine the paternity of their offspring by means of behavioural mechanisms, namely the manipulation of male behaviour. For example, females may initiate and control intra-pair copulations and when involving in EPCs they may time both intra and extra-pair copulations to favour one specific male. Different degrees of control might be appropriate under particular circumstances. For instance, the mechanisms used by females before copulation will be largely behavioural, whereas in cases where the costs of behavioural control are too high, it may be appropriate to have some physiological control.
Intersexual conflicts frequently result in extra-pair paternity. The knowledge of the determinants of the level of extra-pair paternity is basic to understanding the factors promoting sexual selection. One approach to this problem is the analysis of inter and intra-specific variation in extra-pair paternity, which has been shown to be surprisingly marked (reviewed in Petrie & Kempenaers 1998). However our knowledge about the factors involved in such variation is still poor. Petrie & Kempenaers (1998) conclude that a behavioural approach at an individual level is necessary to understand variation in extra-pair paternity. As females of most bird species control paternity to a high degree it is important to consider variation in female behaviour as a way to understand variability in extra-pair paternity. Female tactics to control paternity are usually cryptic and the underlying motivation is often difficult to assess.
Birkhead & M° ller (1993) pointed out the acquisition of evidences showing the female manipulation of males as one of the mayor challenges facing behavioural ecology. Moreover, information on interindividual variability of female ability to manipulate males is needed. Thus, in the first part of this contribution we will focus on female behavioural mechanisms to control paternity, namely clear cases of female manipulation of males. We will offer some examples where the resolution of male-female conflict relies on the manipulation of basic information and will try to make clear the aim underlying the behaviour of each sex participating in the conflict. Then we will discuss some aspects which may explain intraspecific variation in distributions of extra-pair paternity in relation to female tactics, namely the quality of both partners and habitat quality.
RESULT AND DISCUSSION
Female behavioural mechanisms to control paternity.
Females can control paternity through a variety of mechanisms (reviewed in Birkhead & M° ller 1993). We will focus on behavioural tactics relying on manipulation of information, specifically in relation with males knowledge of the start and length of the female’s fertile period. Male awareness of female’s fertility is important for several reasons (e.g. initiation of paternity guards tactics, see Birkhead & M° ller 1992). Similarly females can get advantages when manipulating male’s knowledge of their fertility. We will review two female contrasting tactics: fertility signalling vs. hiding of the fertile phase.
Fertility signalling.
Recent papers have shown that females of some bird species may attract males by special calls and conspicuous solicitation displays to announce fertility and thereby incite male-male competition over copulatory access to her (Montgomerie & Thornhill 1989; Sheldon 1994; Stutchbury et al. 1994; Hoi 1997). For instance, in Chaffinches Fringilla coelebs, females have a very distinctive and frequent copulation solicitation display. Sheldon (1994) argued that females are in effect signalling their fertility to neighbouring males to increase male-male competition, and to choose potential fathers for their offspring. Similarly female Razorbills Alca torda incite male-male competition in mating arenas during their fertile period (Wagner 1998). These types of fertility signalling are not very costly for females, even more in comparison with the benefits they get, mainly clear evidences of the quality of possible partners. In contrast, fertility signalling is energetically very costly for female Bearded Tits Panurus biarmicus. Fertile females show a conspicuous form of pre-copulatory solicitation behaviour mediated by noisy calls designed to attract neighbouring males and enhance long-distance chasing flights. A similar tactic to control copulation partners, male chase flights solicited by females, has been described for House Sparrows Passer domesticus (Tost & Hoi, unpublished manuscript).
Such 'advertisement strategies' may occur more frequently than expected. Montgomerie & Thornhill (1989) described distinctive calls given by females in 18 species of birds and hypothesized that these females are in effect advertising their fertility to incite male-male competition. In fact this has been clearly shown for the Bearded Tit, where females, after inciting male chases, feign resistance (in an operational sense) in order to enhance competition between males and evaluate male quality (Hoi 1997). Two reasons argue in favour of 'feigning': females did not initiate chase flights when there was no other male available (see Hoi 1997), and when the males lose contact, females returned to them and initiated chase flights again. Pizzari, Hoi and Romero (unpublished manuscript) argue that in cases where the costs of female resistance to copulation are likely to exceed the costs of accepting a copulation, female resistance to copulations can function as a female mating strategy (feigning resistance) with two aims: selection of fit mates and signalling condition to potential mates. Thus, by advertising their fertile status and/or by feigning resistance females may get clear advantages like information for mate selection, male willingness to mate with her or indications of future parental commitment (see Hoi 1997).
Hiding the fertile phase.
On the other side females can get many advantages by hiding their fertile status. For instance, to escape from male mate guarding and get EPCs more easily (Birkhead & M° ller 1992), to avoid harassment (Birkhead & M° ller 1992), to time the copulations and favour a specific male (Hunter et al. 1993). However, even if females try to hide their fertile period, the appearance of eggs in the nest can be one cue that males may use to determine the fertile phase of the female (Birkhead 1982; M° ller 1987; Davies 1992; Hatchwell & Davies 1992). So the unavoidable egg laying makes it a difficult task to keep the male unaware of the female’s status.
Females of some species may have developed a strategy, namely egg covering, which under certain circumstances can make somehow 'avoidable' what it is thought to be 'unavoidable'. Egg covering during different phases of the breeding cycle is common in many bird species and may have several functions (to avoid predation, thermoregulatory reasons, to avoid intra and/or interspecific parasitism) (Fig. 1). However, egg covering as a deceiving mechanism to get advantages on the grounds of male-female conflict would require it to occur during the female fertile phase, i.e. during egg laying. This is the case of Penduline Tit Remiz pendulinus (Valera et al. 1997). Female Penduline Tits covering eggs during laying get important advantages:
This species performs uniparental care and both sexes try to become polygamous. Either sex may desert the brood during the egg-laying period. Which sex deserts is strongly influenced by the male detection of eggs during laying and hence by female egg burial behaviour. Egg covering behaviour is an important tactic for the female to increase her probability of becoming polyandrous, and this probability increases with the female’s ability to cover eggs and deceive the male. In summary, the longer the female keeps the eggs covered the more likely she is to become polyandrous.
A conflict between male and female over copulation frequency also occurs in this species. Overall, females get significantly less copulations than they solicit as males ignore solicitations and fail to copulate (Valera et al. unpublished manuscript). Egg-covering seems to be used by the female to control intra-pair copulations. We found that female solicitation and copulation rates do not differ during the pre-laying phase between females covering the eggs and females not covering them. However, during the egg-laying phase the former got more copulations whereas the later hardly copulate (Fig. 2).
In nests with extra-pair young the eggs remained buried for a longer time (more eggs buried) throughout the female cycle than in nests without extra-pair young (Schleicher et al. 1997). Thus, it is likely that females use this strategy not only to become polyandrous but also to get the opportunity for extra-pair copulations.
One may wonder if this is an extremely 'specialised' and isolated case of manipulation of information on the grounds of male-female conflict. However, the argument underlying such behaviour (the importance of the appearance of the eggs) has already been described. Hatchwell & Davies (1992) found that male Dunnocks Prunella modularis use the presence of an egg in the nest and the appearance or behaviour of females when carrying a developing egg to value their copulations (see also Jones 1986 and M° ller 1987 for similar results). Thus it is worth to review and interpret egg covering behaviour on the grounds of sexual conflict. Egg covering during laying occurs in many species and is a common feature of some birds (e.g. Parids). Two factors can contribute to the development of this uncommon behaviour as a deceptive strategy:
1) the existence of a high degree of male-female conflict,
2) the occurrence of favourable conditions to cover the eggs.
Let’s look for general patterns following these points.
One way males have to coerce females into mating is resource brokering (Gowaty 1996). Crucial resources that limit reproduction by female birds include territories, which may contain essential nutrients and nest sites. In this regard, we would expect a higher degree of intersexual conflict in cavity nesting species than in open-cup nesting species. This is theoretically so because cavity nest sites are rarer than open cup sites, making it easier for males to broker females┤ access to them. Thus the pair bond in cavity nesting species can be based on a high degree on coercion. Moreover, one favourable circumstance for egg covering is the lack of visibility in the nest, which is the case in species nesting in holes and domed nests. In this regard it is notorious that egg covering is a common behaviour in many of these species.
Following this reasoning, a review of egg covering behaviour in birds showed that egg burial during laying occurs in species with a high degree of intersexual conflict (ranked on the basis of the mating system, evidences of EPP and patterns of parental care) and that most of these species nest in holes (Fig. 3) (Valera et al. unpublished manuscript). These results are preliminary as no analysis to control for phylogenetic relationships has been made. However, they suggest that even if the original reason of egg covering may be different and though it may produce advantages on other grounds (predation avoidance, intraspecific parasitism avoidance) there are evidences that at least some females have 'learnt' to use this behaviour as a way to resist male control.
Factors accounting for intraspecific variation in distributions of extra-pair paternity.
Factors like breeding density, genetic variation in the population, intensity of sexual conflicts... have been argued to explain intraspecific variation in extra-pair paternity (see Petrie & Kempenaers 1998). However, factors like interindividual variability in quality and variation in habitat quality can influence male and female tactics. Following we will provide some examples on these regards.
Quality of the 'combatants'.
The relative quality of the two combatants may play a major role in the definition of the scenario of the conflict and probably in its outcome. This is so because of two reasons:
- we would expect that females paired to low quality males should especially pursue EPCs to get access to genetically superior males whereas females paired to 'high quality' more attractive males may be more likely to be faithful (as shown by Kempenaers et al. 1992; Lifjeld & Robertson 1992; M° ller 1992; Johnsen & Lifjeld 1995).
- whether males are successful in their attempts to manipulate or control females depends critically on males’ quality and on females┤ intrinsic abilities to resist males┤ efforts. The relative quality of the two combatants is likely to greatly influence the outcome of the battle of the sexes.
Quality can be expressed in terms of experience (older birds are more experienced) or in terms of physical condition. Following we will explore the importance of quality on female tactics to resist male control by analysing the role of experience and physical condition. We will consider mainly the female point of view because female’s abilities to resist and retain control of their own reproduction is of central importance to the evolution of social behaviour in sexual species (Gowaty, 1996).
Differences in experience have been reported to cause differences among females in ability of discrimination (McDonald 1989) and in their own 'standards' of quality (Downhower & Lank 1994; Collins 1995). Experience may play an important role in those cases where female tactics to resist male control are based on manipulation of information. This seems to be the case of Penduline Tit. Egg covering behaviour in this species shows a high variability. Females vary in egg-covering behaviour (see Valera et al. 1997) so that some females do not cover the eggs or fail to keep them buried whereas others keep up to 4 eggs covered. Experience may account for these differences. We found that females covering the eggs during the first and second day tended to have larger and wider mask (mask width reflects age and dominance in the case of males, see Grubbauer 1995) and larger tarsus than females not covering eggs during first and/or second day. Though differences were not significant they suggest that females not covering the eggs may be younger, inexperienced birds.
Males probably also vary in their ability to detect covered eggs during the laying period. Some points suggest that males which desert earlier are the older, experienced and more attractive ones. The results of DNA-analyses show that the later the male deserts the nest and his female the more likely he is to get cuckolded (Schleicher et al. 1997). Furthermore, the occurrence and the frequency of extra-pair paternity may be explained by male attractiveness, as shown by a broader mask of non-cuckolded males (Fig. 4). Male mask is also related to male and female copulation behaviour as male mask width is significantly correlated to female copulation solicitations and to copulation rate (Valera et al. unpublished manuscript).
EPP in this species is very low (6,9% of young sired by an extra-pair male) and however females do have many opportunities for EPC due to the low intensity of different paternity guards. Hence, the low level of sperm competition does not seem to be due to efficient anti-cuckoldry strategies but rather to the fact that females are faithful to their mates. Female Penduline Tits engaging in EPC would neither gain nutritional benefits nor parental care from extra-pair males, thus the most likely benefits of this behaviour are genetic. We therefore would expect extra-pair chicks mainly in those cases where male and female quality mismatch. In fact, EPY appear in those nests where the male is of poor quality (narrow mask) and the female is experienced enough to cover the eggs and to get advantages of such behaviour (EPP, become polyandrous). Thus, quality in terms of experience of both partners is likely to affect the result of this conflict.
The Bearded Tit provides another example of the importance of female quality to resist male control and thus, of the explanation of intraspecific variation in distributions of extra-pair paternity. However, in this case quality refers to body condition. Females Bearded Tit apparently choose a permanent partner because of his parental ability but a copulation partner for other attributes. As said before, females incite male chases and feign resistance to enhance competition between males. In contrast to other studies, females paired to high-ranking males incited more chase-flights than females paired to low-ranking males (Hoi 1997). This indicates that chase-flights are energetically expensive. The number of chase-flights initiated depends on the condition (i.e. quality) of a female, so that the 'higher' quality females have access to more copulation partners and hence invest much more in energetically expensive chases.
Habitat quality.
It is evident that habitat quality can influence male and female tactics and habitat quality is an aspect of the Female Constrained hypothesis. One way males may attempt to manipulate or control females is by action on females bodies directly through conditioning or force. However males may also try to control their partners indirectly through brokering females access to crucial resources. When females are able to resist direct control mechanisms, males should be selected to attempt to coerce females into mating for access to male-brokering resources. This may be especially common in birds because, as we have seen, female birds seem able to resist many mechanisms of direct male control (Gowaty 1996). The question then is: how does environmental quality affect female’s options?
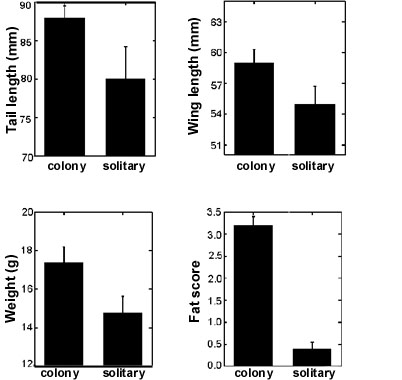
In the case of the Bearded Tit, where female quality plays an important role in the strategy of 'resistance as a ploy', the breeding situation was shown to influence male and female tactics. This species is non territorial, breeding both in colonies and isolated places. The absence of territories probably precludes males to control females by resource brokering. Female Bearded Tits choose the nesting place on the basis of their own quality so that colonial breeding females are larger and in better condition than solitary females (Fig. 5, see also Hoi & Hoi-Leitner 1997). The outcome is that 'higher' quality females in colonies have access to more copulation partners. Similarly, poor quality females prefer to breed in solitary places, thus reducing males possibilities to engage in EPCs.
The relation between variation in territory quality and extra-pair paternity can also be seen in Serins Serinus serinus. Serin is a social monogamous, semi-colonial breeding cardueline finch. It has loose territories, which serve for courtship, feeding and nesting. Nevertheless birds also forage out of the territory. We could find a close relationship between habitat quality (food availability) during the fertile phase and the percentage of EPP (Fisher exact test, P = 0.015), so that more nests with extra-pair youngs were found in good places than in poor territories (Hoi-Leitner et al. unpublished manuscript).
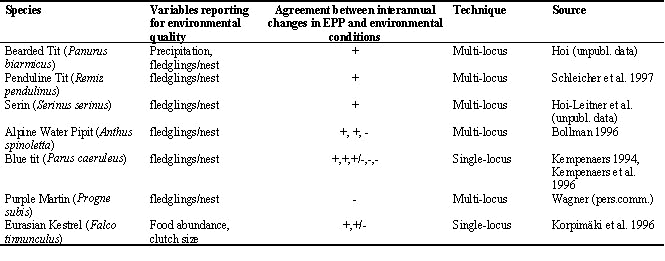
Intraspecific interannual variation in EPP has been reported in several studies (Gowaty & Bridges 1991; Graves et al. 1993; Dunn et al. 1994; Weatherhead et al. 1994; Whittingham & Lifjeld 1995; Yezerinac et al. 1995). Moreover, there seems to exist a positive relationship between extra-pair paternity and average breeding success per year (Table 1), which indicates that environmental conditions during the female fertile period may directly influence individual reproductive tactics, as predicted by the Female Constrained hypothesis (Gowaty 1996). Alternatively, environmental factors may indirectly affect extra-pair behaviour. Ecological variables can influence breeding density, sex-ratio, breeding synchronisation, which are known to be important for the extension of extra-pair paternity (Birkhead & M° ller 1992; Stutchbury & Morton 1994; Petrie & Kempenaers 1988).
To sum up, first we have reviewed some poorly known behavioural mechanisms females may use to control paternity. Then we have seen how the performance and success of such mechanisms may rely on a high degree on the quality of both partners. Finally we have shown some instances of how environmental parameters influence female chances. The Constrained Female Hypothesis predicts that high quality females or females in permissive environments are more likely to produce extra-pair offspring than low quality females or females in less permissive environments (Gowaty 1996). Female Bearded Tits and Penduline Tits are able to resist direct control mechanisms by means of behavioural tactics. Moreover, male control by means of resource brokering is unlikely as both species live in rich, permissive environments. In these two species we find a freely expressed female choice mating system. Serin exemplifies how females breeding in good territories suffer less risk of being controlled by male through resource brokering. This is supported by data showing a relationship between extra-pair paternity and average breeding success per year.
ACKNOWLEDGEMENTS
During the elaboration of this paper FV benefited from a grant from the Spanish Ministry of Education and Culture (Programa de Formaciˇn de Personal Investigador, Becas en el Extranjero) and HH was funded by a grant from the Ísterreichische Fonds zur F÷rderung der Wissenschaftlichen Forschung (No: P9143-Bio).
REFERENCES
Birkhead, T.R. 1982. Timing and duration of mate guarding in Magpies, Pica pica. Animal Behaviour 30: 277-283.
Birkhead, T.R. & M° ller, A.P. 1992. Sperm competition in birds. Evolutionary causes and consequences. London; Academic Press: 282 pp.
Birkhead, T.R. & M° ller, A.P. 1993. Female control of paternity. Trends in Ecology and Evolution 8: 100-104.
Bollman, K. 1996. The mating system of the Alpine Water Pipit in a variable environment: ecological, demographic and fitness aspects. PhD. Thesis, Univ. of ZŘrich, ZŘrich.
Collins, S.A. 1995. The effect of recent experience on female choice in Zebra finches. Animal Behaviour 49: 479-486.
Davies, N.B. 1983. Polyandry, cloaca pecking and sperm competition in Dunnocks. Nature 302: 334-336.
Davies, N.B. 1992. Dunnock Behaviour and Social Evolution. Oxford University Press, 272 pp.
Downhower, J.F. & Lank, D.B. 1994. Effect of previous experience on mate choice by female Mottled Sculpins. Animal Behaviour 47: 369-372.
Dunn, P.O., Robertson, R.J., Michaud-Freeman, D. & P.T. Boag. 1994. Extra-pair paternity in Tree Swallows: why do females mate with more than one male?. Behavioural Ecology and Sociobiology 35: 273-281.
Gowaty, P.A. 1996. Battles of the sexes and origins of monogamy. In: Black, J.M. (ed). Partnerships in Birds: The Study of Monogamy. Oxford University Press: 21-52.
Gowaty, P.A. & Bridges, W.C. 1991. Nest box availability affects extra-pair fertilizations and conspecific nest parasitism in Eastern Bluebirds. Animal Behaviour 41: 661-675.
Graves, J., Ortega-Ruano, J. & Slater, P.J.B. 1993. Extra-pair copulations and paternity in Shags: do females choose better males?. Proceedings of the Royal Society of London (Serie B) 253: 3-7.
Grubbauer, P. 1995. The importance of the nest in reproductive success and mate choice for an uniparental incubator. A study in the Penduline Tit (Remiz pendulinus). MA Thesis, Univ. of Vienna, Vienna.
Hatchwell, B.J. & Davies, N.B. 1992. An experimental study of mating competition in monogamous and polyandrous Dunnocks Prunella modularis: I. Mate guarding and copulations. Animal Behaviour 43: 595-609.
Hoi, H. 1997. Assessment of the quality of copulation partners in the monogamous bearded tit. Animal Behaviour 53: 277-286.
Hoi, H. & Hoi-Leitner, M. 1997. An alternative route to coloniality in the bearded tit: females pursue extra-pair fertilizations. Behavioural Ecology 8: 113-119.
Hunter, F.M., Petrie, M., Otronen, M., Birkhead, T.R. & M° ller, A.P. 1993. Why do females copulate repeatedly with one male?. Trends in Ecology and Evolution 8: 21-26.
Johnsen, A. & Lifjeld, J.T. 1995. Unattractive males guard their mates more closely: an experiment with Bluethroats (Luscinia s. svecica). Ethology 101: 200-212.
Jones, G. 1986. Sexual chases in sand martins (Riparia riparia): cues for males to increase their reproductive success. Behavioural Ecology and Sociobiology 19: 179-185.
Kempenaers, B. 1994. The social mating system and behavioural aspects of sperm competition in the blue tit (Parus caeruleus). PhD Thesis, Univ. of Antwerpen, Antwerpen, Belgium.
Kempenaers, B., Verheyen, G.R., van den Broeck, M., Burke, T., Van Broeckhoven, C. & Dhondt, A.A. 1992. Extra-pair paternity results from female preference for high-quality males in the Blue Tit. Nature (London) 357: 494-496.
Kempenaers, B., Verheyen, G.R. & Dhondt, A.A. 1997. Extra-pair paternity in the Blue Tit (Parus caeruleus): female choice, male characteristics and offspring quality. Behavioural Ecology 8: 481-492.
Korpimńki, E., Lathi, K., May, C.A., Parkin, D.T., Powell, G.B., Tolonen, P. & Wetton, J.H. 1996. Copulatory behaviour and paternity determined by DNA fingerprinting in Kestrels: effects of cyclic food abundance. Animal Behaviour 51: 945-955.
Lifjeld, J.T. & Robertson, R.J. 1992. Female control of extra-pair fertilization in Tree swallows. Behavioural Ecology and Sociobiology 31: 89-96.
McDonald, D.B. 1989. Correlates of male mating success in a lekking bird with male-male cooperation. Animal Behaviour 37: 1007-1022.
M° ller, A.P. 1987. Mate guarding in the Swallow Hirundo rustica: an experimental study. Behavioural Ecology and Sociobiology 21: 119-123.
M° ller, A.P. 1992. Frequency of female copulations with multiple males and sexual selection. American Naturalist 139: 1089-1101.
Petrie, M. & Kempenaers, B. 1998. Extra-pair paternity in birds: explaining variation between species and populations. Trends in Ecology and Evolution 13: 52-58.
Schleicher, B., Hoi, H., Valera, F. & Hoi-Leitner, M. 1997. The importance of different paternity guards in the polygynandrous Penduline tit (Remiz pendulinus). Behaviour 134: 941-959.
Sheldon, B.C. 1994. Sperm competition in the Chaffinch: the role of the female. Animal Behaviour 47: 163-173.
Stutchbury, B.J., Rhymer, J.M. & Morton, E.S. 1994. Extra-pair paternity in Hooded Warblers. Behavioural Ecology 5: 384-392.
Valera, F., Hoi, H. & Schleicher, B. 1997. Egg burial in Penduline tits, Remiz pendulinus: its role in mate desertion and female polyandry. Behavioural Ecology 8: 20-27.
Wagner, R.H. 1991. The use of extra-pair copulations for mate appraisal by Razorbills, Alca torda. Behavioural Ecology 2: 198-203.
Wagner, R.H. 1998. Hidden leks: sexual selection and the clustering of avian territories. In: Parker, P.G. & Burley, N. (eds). Extra-Pair Mating Tactics in Birds. Ornithological Monographs, American Ornithologists┤ Union, Washington, D.C.
Weatherhead, P.J., Montgomerie, R., Gibbs, H.L. & Boag, P.T. 1994. The cost of extra-pair fertilizations to female red-winged blackbirds. Proceedings of the Royal Society of London (Serie B) 258: 315-320.
Westneat, D.F., Sherman, P.W. & Morton, M.L. 1990. The ecology and evolution of extra-pair copulation in birds. Current Ornithology 7: 331-369.
Whittingham, L.A. & Lifjeld, J.T. 1995. Extra-pair fertilizations increase the opportunity for sexual selection in the monogamous House Martin. Journal of Avian Biology 26: 283-288.
Yezerinac, S.M., Weatherhead, P.J. & Boag, P.T. 1995. Extra-pair paternity and the opportunity for sexual selection in a socially monogamous bird (Dendroica petechia) Behavioural Ecology and Sociobiology P.T. 37: 179-188.
Table 1. Relationship between extra-pair paternity and environmental conditions. Extra-pair paternity is measured in terms of % nests with extra-pair youngs. If there is agreement in the direction of the interannual change between extra-pair paternity rate and environmental conditions a + sign is used, otherwise a - sign is used.

Fig. 1. Reported functions of egg covering behaviour in birds.
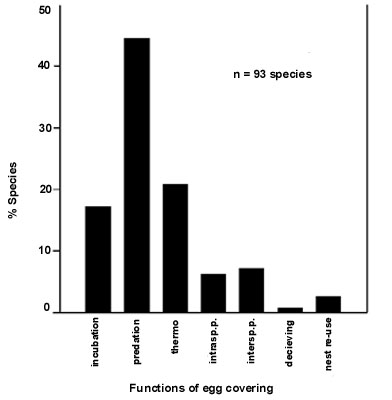
Fig. 2. Penduline tits copulation rate (number of successful copulations/20 min) during the pre-laying (days -5 to -1) and laying phase (days 0 to +3, day 0 = day the first egg is laid) in nests with covered eggs (filled bars, n = 27 and 35 nests respectively) and nests with uncovered eggs (open bars, n = 18 and 19 nests respectively).Significance values (P) are due to a Students t-test (two-tailed).
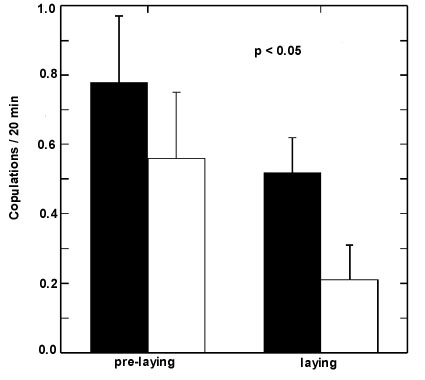
Fig. 3. Egg covering behaviour in birds in relation to the degree of intersexual conflict (ranked on the basis of the mating system, evidences of EPP and patterns of parental care) and type of nest (open vs. cavity nests).
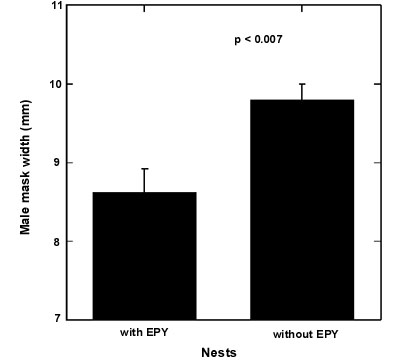
Fig. 4. Mask width of male Penduline Tits in relation to the occurrence of extra-pair youngs.
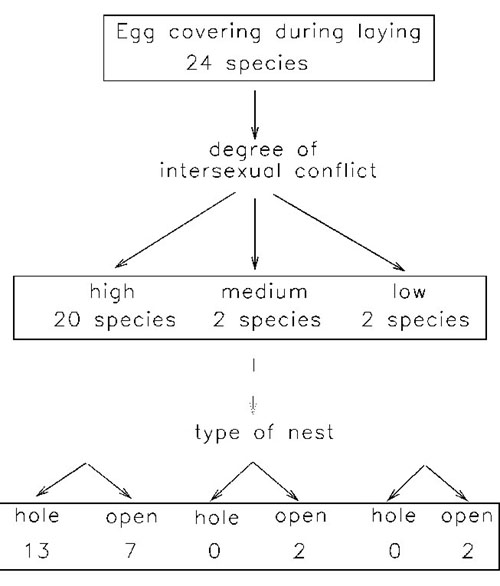
Fig. 5. Morphology for colonial and solitary breeding female Bearded Tits.