S43.4: Bird community structure of a primary rain forest in Guiana: Changes with scale and disturbance
Jean-Marc Thiollay
Laboratoire d'Ecologie, E.N.S., 75230 Paris cedex 05, France, e-mail thiollay@biologie.ens.fr
Thiollay, J.-M. 1999. Bird community structure of primary rain forest in Guiana: Changes with scale and disturbance. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2580-2590. Johannesburg: BirdLife South Africa.Habitat heterogeneity and species rarity are critical factors explaining bird diversity and multispecies coexistence in tropical forests. The bird community structure of a continuous undisturbed rain forest in French Guiana was surveyed on successively larger areas from 102 to 105 ha. Species richness increased from 234 to 362 mainly through inclusion of new forest types (inselberg, river) and their associated habitat-specialist species, but also because of the low density, patchy distribution, and low detectability of many species. Most species were rare and had K-selected demography (low clutch size, high survival rates). Rarity took various forms, but had no significant and consistent ecological correlate. Habitat specialisation was a major determinant of both high diversity and vulnerability to disturbance. Constraints such as food availability, seasonality, predation pressure and interspecific competition were also probably responsible for low and irregular densities and contributed to the variation of community composition among sample areas and to the rate of species accumulation over increasing survey areas.
INTRODUCTION
Few complete bird population censuses and density estimates have been made in tropical forests and they were limited to 100-200 ha plots (see Terborgh et al. 1990, Thiollay 1994, Robinson & Terborgh 1997 for neotropical forests, and case studies in this symposium). Limitations come from the high number of species, often little known and very secretive, which require multiple complementary census techniques. Such relatively small sample plots are necessarily rather homogenous, whereas tropical forests are usually a mosaïc of different forest types harbouring different sets of habitat specialists. Therefore, comparative analyses are strongly dependent upon census scale and extending survey areas may provide new insights into species-area relationships and assessments of actual biodiversity.
Numerous avifaunal surveys have been carried out in the > 80000 km2 primary rain forest area of French Guiana since 1980 (Thiollay 1986, Tostain et al. 1992). Among them, different sites have been intensively studied from 1986-1997 within the 1000-km2 Nouragues Reserve, a vast tract of primary forest in NE Guiana that includes most of the typical lowland forest types represented in the interior of Guiana (Thiollay 1990, 1994). These sites will be used here to illustrate patterns and causes of species-area relationships and rarity from local to regional scales.
STUDY AREA
The core study site was a 100 ha plot at the Nouragues field station (4°05N-52°41W) where the density of all resident bird species was assessed (Thiollay 1994). Five natural forest types covered respectively 55, 26, 10, 4 and 5% of this plot: (1) 40-50 m high mature stands with open understory on well-drained slopes and plateaus; (2) lower stands with more irregular, moderately vine-loaded canopy; (3) areas frequently disturbed by tree falls, with thick vine-tangles, a dense undergrowth and a low broken canopy; (4) almost monospecific palm swamp forest in bottoms; (5) treefall gaps, recent or older, 50 to 1000 m2 each. Adjacent to this quadrat was a large granitic inselberg with bare rocky slopes, strewed with Clusia woodlots and surrounded by a belt of low dense forest on shallow soil. Further down (Saut Pararé and Camp Arataye), a river offered another set of semi-open habitats with new bird communities, notably the dense riparian vegetation, exposed rocks, fallen trees or branches overhanging water, seasonally flooded flats and low successional vegetation on disturbed waterbanks. Though patchily distributed, these local forest types, gaps and edges are typical features of Guianan primary forest landscapes.
METHODS
A complete census with density estimates was carried out in the 100 ha core plot from 1990 to 1992 over 8 months, using the spot-mapping method and 6658 independent species records from visual and auditory detections (Thiollay 1994, Appendix 1). Additionally, 1353 birds were caught within the same limits in mistnetting operations and many of them resighted or recaptured subsequently.
After censusing the 100 ha, three areas of approximately 1000 ha, 8-15 km from each other, were surveyed. The first one (Nouragues) was centred around the above 100 ha core plot and included , as a new habitat type, a 300 ha granitic inselberg and its belt of low dense forest. The second one (Saut Pararé) included a shallow river with rapids and wooded islets. The last one (Camp Arataye) was crossed by a deeper and wider part of the same river and included a sizeable area of seasonally flooded forest. Otherwise, the main habitats, i.e. the five natural forest types defined above, were essentially similar to those of the core plot and covered the largest part of all three 1000 ha plots. All natural forest habitats known in most northern Guiana were well represented in at least one of the 1000 ha quadrats and thus the area sampled was probably representative of the whole 100 000 ha Nouragues Reserve.
Within each of the larger sample plots, birds were searched during at least 15 full days at random along extensive transects in the understory, from dawn to dusk, walking slowly with frequent stops and crossing all forest types. I recorded all birds detected within 30 m on either side of foot trails. Open habitats and edges were also searched systematically, but not in a standardised way. Diurnal raptors (as well as swifts and swallows) were surveyed from large gaps and elevated look-outs mostly in late morning (Thiollay 1989a, b). Non-soaring raptors such as Forest Falcons Micrastur sp. were mostly detected when calling at dawn and dusk. Nocturnal species were also identified by their vocalisations at night. The 1000 ha areas were drawn from a central campsite (opportunistically located), using topographical maps and prominent landmarks. In spite of complete and repeated crossings of these areas, intensive searching effort and roughly similar time spent in each of them, such large plots could not be surveyed as fully as the 100 ha core plot and some species may have been missed.
The community structure was measured on the 100 ha plot where the actual abundance of all species was estimated. In the three successive 1000 ha sample areas, only the presence of additional species was taken into account because the spot-mapping method could not be used at this larger scale. Actual densities were not assessed and they would have been meaningless for patchy species, such as riverine birds, that can reach locally high densities. However I tallied the number of records per species on the fixed-width transects, whatever the number of individuals per record and excluding raptors, nocturnal et aerial species. This was used as an estimate of the overall relative abundance of species to assess differences in community structure between the larger plots. Ecological data were gathered during ornithological studies in the same and other areas of Guiana (e.g. Thiollay 1988, 1990, 1994).
The species alpha-diversity was assessed by the Shannon Index H' = - S pi loge pi and the species turnover, or beta-diversity, was calculated as g + l/N
1 + N2, where g was the number of species gained between sample plots, 1 and 2 and l the number of species lost and N1 and N2 the total number of species in the two plots (Wilson & Schmidt 1984).RESULTS
Species richness and community structure
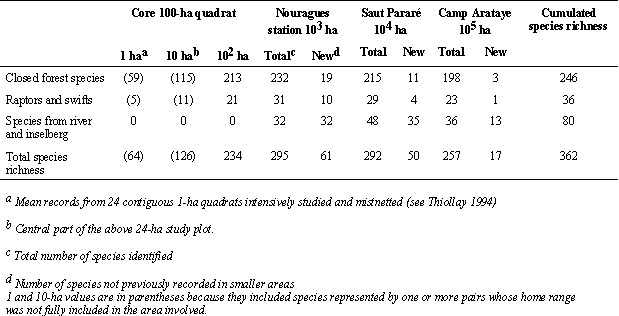
Excluding suspected non-breeding migrants, vagrants or doubtfully indentified taxa, 248 species were actually recorded within the 100 ha core plot (234 regular) and 362 on the three 1000 ha plots, which represents 81.7% of the 443 species known from the continuous primary forest tract of the interior of French Guiana. The 362 species were divided into 47 families and 239 genera, a high taxonomic diversity. However, the coexistence of ecologically and morphologically similar congeneric species was a striking feature: 57 pairs or trios and 7 groups of 4-6 congeners were found among forest birds alone (Appendix 1).
On the 100 ha plot, where abundance data were available, the diversity index was high (4.96), not only because of the high species richness (S), but also because of a high equitability (H’/log S = 0.90), a majority of species having low densities. Among all 362 species recorded, only 2 reached a density of 28-38 pairs /100 ha and 17 species were moderately abundant (8-18 pairs/100 ha), at least on the 100 ha core plot. Almost 95% of all species were thus rare by temperate forest standards (< 8 pairs/100 ha), though the overall bird density (829 breeding pairs/100 ha) was not lower than in most temeprate forests (Fig. 1, Thiollay 1994). Even in the most restrictive sense (species = 1 pair/100 ha and occurring on the 100 ha plot, according to Terborgh et al. 1990), 37% of species were still rare.
The range of body masses was wide and log normally distributed: 7% of very small species (> 8 g), 59% of small to medium-sized species (8-64 g), 25% of larger species (65-510 g) and 9% of big species (> 550 g). This suggests a fairly comprehensive use of resources by the bird community. Likewise, the vertical distribution of the 282 true forest species was roughly correlated with the distribution of foliage density, structural complexity and overall productivity derived from light intensity: 10.5% of the species were terrestrial or low above the ground, 42% were associated with mid to upper levels of understory, 37.5% were mostly restricted to the upper canopy and 10% to treefall gaps and disturbed areas (Appendix 1). The division of species between trophic categories was less clearcut because many specific diets were generalised, e.g. many species consumed arthropods and fruits or nectar.
Species-area relationships and habitat selection
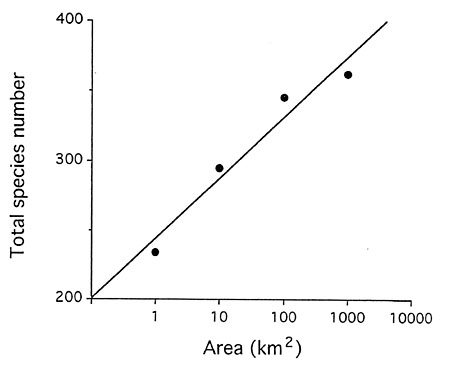
There was a steep and continuous increase of the number of species recorded (S) with increasing sample area (A) from 1 to 3000 ha (Fig. 2). The slope of the regression line of LogS/LogA ranged from 0.021 (forest species) to 0.199 (river and inselberg species) and was most significant for the total species richness (R
2 = 0.927, P = 0.037). From 102 to 105 ha, the total bird community increased by 62%, but the bird species number was still not levelling off in our third 1000 ha sample quadrat. Indeed, at least 81 additional species have been identified in 13 other 1000 ha plots, surveyed so far in similar primary forest tracts of French Guiana, spread over an area almost 80 times larger than that of the 100000 ha Nouragues Reserve.The increase in species richness, however, mostly came from raptors and edge species (95/116 species gained above 100 ha vs 33/246 for closed forest species, Table 1). The species turnover between 1000 ha plots was 17.5-20.8%, a high value, given the proximity of plots and homogeneity of forest habitats, even if changes may have been overestimated because of species missed in the last two, less intensively surveyed plots. The regional forest bird community (362 species) was divided into four categories. The first one was the minimal richness of any 100 ha plot and the next three ones represented the main origins of the new species added with increasing sampling area, i.e. low density, patchy distribution and local habitat specialisation: (1) 148 regular forest species (40.9%) had a density > 1 pair/100 ha and occurred on all study plots; (2) 52 intrinsically rare forest species (14.4%) had a mean density = 1 pair100 ha, but occurred on all three 1000 ha plots; (3) 82 patchily distributed species (22.7%), that were present only in 1 or 2 1000 ha plots: this category included only true forest species (continuous or little fragmented habitat); (4) 80 large natural gap species (22.1%) i.e. restricted to 1-2 plots because they were associated with patchy successional habitats (forest edge, river side or rocky outcrop). All four categories included a wide range of families, body sizes, microhabitat selection and food habits.
Patterns and ecological correlates of rarity
Categories 2-4 above involve different forms of rarity: low density but rather continuous distribution, uneven or checkerboard distribution within suitable habitats and association with patchily distributed habitats. All imply multiple constraints and, above all, habitat heterogeneity at different scales, arising from edaphic conditions or seasonal variability.and disturbance regimes.
Large but more or less contiguous home ranges are found in large species (e.g. Cracidae), top predators (e.g. Accipitridae), permanent multispecies flock members (e.g. Myrmotherula, Philydor, Xiphorhynchus), ant followers (e.g. Pithys, Gymnopithys), upper canopy frugivores (e.g. Cotingidae), and group-living or gregarious species (e.g. Psittacidae). Smaller territories with substantial distributional gaps are common and often involve specific habitat requirements (e.g. tree fall or successional habitat species). Seasonal or nomadic movements seem to be frequent, notably among frugivores (e.g. Ramphastidae).
The well known inverse relationship between body size and density was generally confirmed here, both among insectivores (r = 0.27, P < 0.001) and frugivores (r = 0.31, P < 0.05). Conversely, the proportions of rare species (categories 2-4 above) among taxa with different diets, social behaviours or habitat requirements were not significantly different from the proportion in the overall community used as the null hypothesis in two-tailed binnonial tests with
a = 0.05. In mature forest however, the proportion of rare species increased from near ground (12.5%) to mid (32.2%) and canopy (43.8%) levels (P < 0.05).Many species had a markedly different abundances between study sites, both within the Nouragues Reserve and between current plots and other Guianan localities. The most striking difference was found between very similar and closely coexisting congeners, one of which was more abundant than the other, suggesting competitive or dominance relationships (e.g. Ramphastos, Veniliornis, Philydor, Cercomacra, Formicarius, Platyrinchus, Polioptila), but opposite examples abounded of such permanent species pairs where both members were almost equally common (e.g. Thamnomanes, Myrmotherula, Cyanerpes).
DISCUSSION
The bird species richness of this neotropical forest community was equivalent to that measured in South American primary rain forest plots, i.e. 220-260 resident species on 1-2 km2 (Robinson et al. 1990, Terborgh et al. 1990). This is consistently 30-40% higher than in similar lowland Old World tropical forests (Brosset 1990) and five times higher on average than in temperate forests (Ferry & Frochot 1968, Karr 1971, Tomialojc et al. 1984, Holmes et al. 1986). Although the intensive 100 ha study plot included the main forest types, it harboured only 64% of the resident forest species known from the Nouragues Reserve and 53% of those identified in the primary forest tract of the interior of French Guiana. This may have important consequences on the sampling methodology of such rich communities where plots smaller than 100 or even 1000 ha are inappropriate for surveying beta species diversity.
The composition of this forest bird community must be looked at three scales: (1) The spatial variability, at the local level, was due to species specific habitat selection and high habitat heterogeneity (Terborgh 1985, Gentry 1990). A substantial proportion of species was associated with edges and gaps of various sizes, either from edaphic, topographical or disturbance origin. Upper canopy species, which often come down in gaps, may be added to this category. Among them, were vine tangle, bamboo or dense riparian forest specialists and those associated with low dense vegetation. There are many such species throughout Amazonia and they contribute significantly to the total species richness (Remsen & Parker 1983, Kratter 1997, Robinson & Terborgh 1997). Together they represented here 59% of the total bird community. The highly structured vertical distribution of species was also an important aspect for species co-existence in this 50 m tall vegetation; (2) The regional variability involved marked differences in the community composition and relative abundance of species between localities, either within the Nouragues Reserve, or within the interior of French Guiana, let alone between Guiana and neighbouring countries (Thiollay unpubl. obs.). Such differences in community structure sometimes occurred in spite of apparently similar forest types. Therefore species richness drawn from a single area may not be generalised, and (3) The temporal variability of tropical forest bird communities and food sources is still often underestimated, though seasonal and year to year changes have been repeatedly documented (Fogden 1972, Greenberg 1981, Leigh et al. 1983, Sabatier 1985, Boinski & Fowler 1989) and confirmed in Guiana. In addition to seasonal, but irregular movements of some frugivores, the sudden increase or disappearance of even insectivorous species in the supposedly stable primary forest, as well as occasional sightings of probably vagrant birds (not included in this study) suggested population fluctuations and large scale dispersal of canopy as well as understory birds.
The primary tropical forest bird communities exhibit a typical structure , i.e. a prevalence of rare species with few dominants, found both at the community scale and within each ecological guild. This structure changed in secondary and logged forests (Thiollay 1992) towards an increasing proportion of abundant species and fewer rare species. It also changed in the same direction within semi-open habitats (gaps, edges, riversides) and it may be used as an index of forest maturity, closeness or degree of disturbance.
Rarity took many forms according to patterns of species distribution, abundance and stability in space and time. Rarity probably results mostly from environmental constraints (Gaston 1994), such as habitat heterogeneity, low availability and unpredictable distribution of food resources, interspecific competition and predation pressure that were all identified in our study area as critical factors limiting the distribution or abundance of many species (Thiollay 1990, 1991). A major factor in understanding the dynamics of these tropical forest birds in their K-selected demographic traits, i.e. low productivity, high survival rates, delayed reproduction and importance of floating individuals (Jullien 1996). The patchy distribution of many species and the seemingly high dispersal rates suggested that populations of rare species may be maintained by a metapopulation dynamic process, a promising hypothesis to investigate in future studies.
The species-area curve over a large sample area and the low density or patchy distribution of most species also have important implications for conservation. The size of viable populations is still a matter of conjecture and accurate estimates of demographic parameters are still not available for any tropical forest bird population. A minimum size of 100 pairs would be in the lower range of viable isolated populations (Soulé 1987). An area of 10
4 to 105 ha should be necessary to accommodate a 100 pair population of 60% of species in our study community. Such an area should include a mosaic of all main forest types and patches of a sufficient size to maintain in them the natural disturbance regime that is probably a key factor to the conservation of a significant proportion of bird species.REFERENCES
Boinski, S. & Fowler, N.L. 1989. Seasonal patterns in a tropical lowland forest. Biotropica 21: 223-233.
Brosset, A. 1990. A long-term study of rain forest birds in M’Passa, Gabon. In: A. Keast (ed.) Biogeography and ecology of forest birds communities. The Hague; SPB Academic Publ.: 259-274.
Ferry, C. & Frochot, B. 1968. Recherches sur l’écologie des oiseaux forestiers de Bourgogne. Trois années de dénombrement des oiseaux nicheurs sur un quadrat de 16 ha en forêt de Citeaux. Alauda 36: 68-82.
Fogden, M.P.L. 1972. The seasonality and population dynamics of equatorial forest birds in Sarawak. Ibis 114: 307-343.
Gaston, K.J. 1994. Rarity. London; Chapman and Hall.
Gentry, A.H. (ed.) 1990. Four neotropical rain forests. New Haven; Yale University Press.
Greenberg, R. 1981. The abundance and seasonality of forest canopy birds on Barro Colorado Island, Panama. Biotropica 13: 241-251.
Holmes, R.T., Sherry, T.W. & Sturges, F.W. 1986. Bird community dynamics in a temperate deciduous forest: long-term trends at Hubbard Brook. Ecological Monographs 56: 201-220.
Jullien, M. 1996. Signification adaptative de la vie en groupe chez les oiseaux: le cas des oiseaux forestiers néotropicaux.Doctorate thesis, Paris VI University, Paris, France.
Karr, J.R. 1971. Structure of avian communities in selected Panama and Illinois habitats. Ecological Monographs 41: 207-233.
Kratter, A.W. 1997. Bamboo specialisation by amazonian birds. Biotropica 29: 100-110.
Leigh, E.G., Rand, A.S. & Windsor, D.M. (eds) 1983. The ecology of a tropical forest. Seasonal rythms and long-term changes. Oxford; Oxford University Press.
Remsen, J.V. Jr & Parker, T.A. III 1983. Contribution of river-created habitats to bird species richness in Amazonia. Biotropica 15: 223-231.
Robinson, S.K. & Terborgh, J. 1997. Bird community dynamics along primary successional gradient of an Amazonian Whitewater river. In: J.V. Remsen (ed.) Studies in Neotropical ornithology honoring Ted Parker. A.O.U. Monograph 48; Washington D.C.; A.O.U.: 641-672.
Robinson, S.K., Terborgh, J. & Munn, C.A. 1990. Lowland tropical forest bird communities in Western Amazonia. In: A. Keast (ed.) Biogeography and Ecology of forest bird communities. The Hague; SPB Academic Publ.: 229-258.
Sabatier, D. 1985. Saisonnalité et déterminisme du pic de fructification en forêt guyanaise. Revue d’Ecologie (Terre et Vie) 40: 289-320.
Soulé, M.E. (ed.) 1987. Viable populations for conservation. Cambridge; Cambridge University Press.
Terborgh, J. 1985. Habitat selection in Amazonian birds. In: M.L. Cody (ed.) Habitat selection in birds. New-York, N.Y.; Academic Press: 311-338.
Terborgh, J., Robinson, S.K., Parker, T.A. III, Munn, C.A. & Pierpont, N. 1990. Structure and organization of an Amazonian bird community. Ecological Monographs 60: 213-238.
Thiollay, J.M. 1986. Structure comparée du peuplement avien dans trois sites de forêt primaire en Guyane. Revue d’Ecologie (Terre et Vie) 41: 59-105.
Thiollay, J.M. 1988. Diversité spécifique et coexistence en forêt tropicale: l’occupation des milieux par une guilde d’oiseaux insectivores en Guyane. Revue d’Ecologie (Terre et Vie) 43: 59-92.
Thiollay, J.M. 1989a. Area requirements for the conservation of rain forest raptors and game birds in French Guiana. Conservation Biology 3: 128-137.
Thiollay, J.M. 1989b. Censusing of diurnal raptors in a primary rain forest: comparative methods and species detectability. Journal of Raptor Research 23: 72-84.
Thiollay, J.M. 1990. Comparative diversity of temperate and tropical forest bird communities: the influence of habitat heterogeneity. Acta Oecologica 11: 887-911.
Thiollay, J.M. 1991. Tropical rain forest bird communities: emerging patterns and processes of organization. Acta XX Cong. Intern. Ornith., Christchurch, New Zealand: 1489-1496.
Thiollay, J.M. 1992. Influence of selective logging on bird species diversity in a Guianan rainforest. Conservation Biology 6: 47-63.
Thiollay, J.M. 1994. Structure, density and rarity in a Amazonian rain forest bird community. Journal of Tropical Ecology 10: 449-481.
Tomialojc, L., Wesolowski, T. & Walankiewicz, W. 1984. Breeding bird community of a primaeval temperate forest (Bialowieza National Park, Poland). Acta Ornithologica 20: 241-310.
Tostain, O., Dujardin, J.L., Erard, C. & Thiollay, J.M. 1992. Oiseaux de Guyane. Brunoy, France; Société d’Etudes Ornithologiques.
Table 1. Number of resident species identified in successively larger areas in the Nouragues Nature Reserve. The 100-ha quadrat was inside the Nouragues field station 1000-ha study area. The next two 1000 ha sample surveys (Pararé and Arataye) were 8 and 16 km away and were part of concentric areas of 10000 and 100000 ha respectively around the field station.

Fig. 1. Abundance distribution of bird species within the Nouragues Nature Reserve. Below the 1000 ha mark mean abundances were drawn from densities of true forest birds on the 100 ha core study plot. Rarer species were entered in the first of the three 1000 ha plots where they appeared (last 3 bars), but this underestimate their actual density.
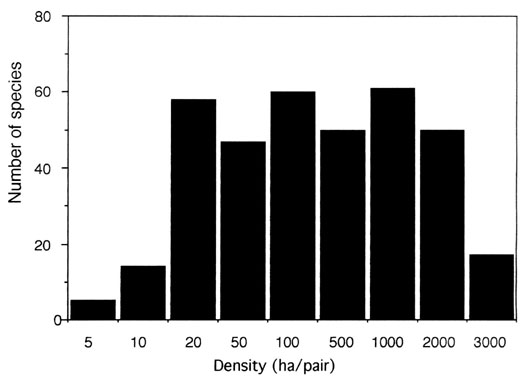
Fig.2. Species/area regression of the bird community sampled in the Nouragues Nature Reserve.
Appendix 1: Composition of the forest bird community censused in the Nouragues Nature Reserve in three 1,000 ha plots.

Appendix 2: Bird species restricted to marginal forest habitats: I = granitic inselberg and associated woodlands: R = river, islets and seasonally flooded forest alongside. For each species are given the habitat type and the number of 1000 ha plots where the species was recorded.
