S41.3: Dispersal studies in recently and historically fragmented forests: A comparison between Kenya and Belgium
Luc Lens1&2, Frank Adriaensen1 & Erik Matthysen1
1University of Antwerp, U.I.A., Department of Biology, B-2610 Antwerp, Belgium, fax 32 3 820 22 71, e-mail
llens@uia.ua.ac.be; fadria@uia.ua.ac.be; matthys@uia.ua.ac.b ; 2Department of Ornithology, National Museums of Kenya, PO Box 40658 Nairobi, Kenya Lens, L., Adriaensen, F. & Matthysen, E. 1999. Dispersal studies in recently and historically fragmented forests: A comparison between Kenya and Belgium. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2480-2491. Johannesburg: BirdLife South Africa.The highly threatened, primeval cloud forests of the Taita Hills in south-east Kenya presently cover less than 400 ha in a scatter of fragments. As these forests hold three endemic bird species as well as a suite of other endemic taxa, they are considered of exceptional conservation importance. In this paper we compare dispersal patterns, sex ratios and levels of fluctuating asymmetry of seven Taita forest birds, including two endemics, with similar data from various taxa in a historically-fragmented, Belgian landscape. The degree of interfragment dispersal was significantly lower, and the levels of asymmetry and of bias in sex ratio were orders of magnitude higher in the tropical site. These patterns are believed to result from differences in time scale (more recent fragmentation in the tropical site) and spatial scale (strict isolation from neighbouring populations and reduced interfragment dispersal in the tropical site).
INTRODUCTION
Habitat fragmentation, i.e. the reduction of large, continuous habitats to small, isolated remnants, affects the survival of natural populations in various and often complex ways (reviews by Wilcove et al. 1986; Saunders et al. 1991; Haila et al. 1993; Murcia 1995; Turner 1996). Understanding how populations respond to these complexities requires mechanistic studies focusing on aspects such as micro- and macro-habitat selection and behavioural aspects of movement in landscape mosaics.
An important issue in the study of the underlying mechanisms of the effects of habitat fragmentation is if and how the spatial configuration of the landscape affects patterns of dispersal. Dispersal has been widely identified as a key process in both population regulation and spatial distribution (Gaines & McClenaghan 1980; Greenwood 1980; Ebenhard 1991; Davis 1992). Being a prerequisite for gene flow, it also has substantial effects on the genetic structure of populations (Gillespie 1975; Barrowclough 1980; Slatkin 1985; Perrins 1990; Gilpin 1991). However, effects of habitat fragmentation on primary demographic aspects may be equally important (Lande 1988). For instance, how does reduction of disperser success affect the sex- and age-structure of populations (Matthysen & Currie 1996) ? A realistic integration of demographic and population genetic factors, applicable to species in natural environments, is therefore highly desired both from an evolutionary and conservation point of view (Lande 1988).
Unfortunately, current insights in the effects of tropical rain forest fragmentation tend to rely heavily on a limited number of localities (mainly Amazonian Brazil) and taxonomic groups (mainly birds) (see Turner 1996 for a review of studies on tropical rain forest fragmentation). Besides, consequences of fragmentation are rapidly becoming established as dogma in conservation biology, although perception of fragmentation effects often seems based on theoretical expectations rather than empirical evidence. As much of this evidence stems from studies in temperate regions, where forests remain as isolated patches following historical conversion to an agricultural landscape (Hunter 1991; Crome 1997), it can be questioned whether such evidence also applies to tropical areas, where forests continue to dominate the landscape but are currently subject to large-scale fragmentation.
We aim to contribute to the interpretation of this controversy through an integrated study on the genetic, demographic and dispersal characteristics of a suite of seven cloud forest birds, differing in the degree of habitat specialisation and dispersal capacity, in a tropical fragmented landscape in south-east Kenya. In this paper we compare preliminary findings on their extent of interfragment movement, bias in sex ratios and fluctuating asymmetry with case studies in a similarly fragmented forest 'archipelago' in a traditional Western European landscape, as a first evaluation of the suitability of such studies to predict effects of recent and large-scale tropical forest fragmentation. Fluctuating asymmetry (FA) represents random deviations from perfect symmetry in bilaterally-paired traits, and potentially gives information on environmental and/or genetic stress in individuals and populations (Parsons 1992, Palmer 1994, Møller & Swaddle 1997).
METHODS
Study sites
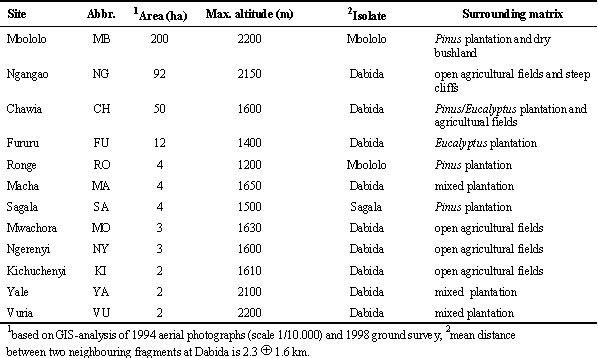
Kenya. The tropical study site lies at 03°20’S, 38°15’E, 150 km inland from the Indian Ocean and covers an area of 250 km². The Taita Hills are isolated from other mountainous areas and highland blocks - the closest of these 80 km away - by the semi-arid plains of Tsavo West National Park (at 600-700 m altitude), and are divided into three distinct isolates: Sagala Hill (1.450 m, separated ca. 20 km from the other isolates by a valley at 700 m), Mbololo Hill (2.209 m, separated ca. 5 km from the following by a valley at 900 m) and Dabida Hill (including the highest peak at 2.209 m) (Fig. 1). A detailed description of the physical geography of the study area is given in Brooks et al. (1998). The present landscape contains 12 cloud forest fragments scattered on hilltops and ridges and surrounded by a mosaic of human settlements, small-holder cultivation plots and plantations of Pinus, Eucalyptus, Cupressus and Juniperus (Table 1). Forest loss since the 1960’s has been very substantial, estimated at 99% for VU, 95% for SA, 85% for CH, 50% for NG and under 50% for MB (Beentje 1987). Based on a structural analysis of biomass, stem density, canopy cover, shrub density, stratification and extent of herbaceous ground cover (Wilder et al., in press), MB is the least disturbed and also the largest patch. NG has suffered from intermediate levels of disturbance while all other fragments, including CH, have been very heavily impacted.
Belgium. The temperate zone study site lies at 51°08’N, 04°32’E, on the south-eastern fringe of the city of Antwerp and covers an area of 200 km². It consists of a set of deciduous, coniferous and mixed secondary forest patches in a mosaic of traditional agricultural landscape and rapidly growing residential and industrial areas. Fragment sizes range from 0.25-55 ha, while some studies include reference plots in larger forests (>150 ha) at 5 to 30 km from the study area (Table 2). Distances between neighbouring fragments in the core study area vary from 100 to 600 m (overall mean for the entire study site = 567 m). In contrast to the afrotropical site, there has been no substantial forest loss for over two-hundred years (as shown by a comparison with historical maps). More details on the Antwerp study areas are given in Matthysen et al. (1995), Nour et al. (1998) and references therein.
Data collection
Kenya. Since July 1996, the following seven forest species have been captured with mistnets: Taita Thrush Turdus helleri (TT, endemic to the Taita Hills; Collar et al. 1994), Taita White-eye Zosterops silvanus (TW, endemic; Collar et al. 1994), White-starred Robin Pogonocichla helleri (WR), Stripe-cheeked Greenbul Andropadus milanjensis (SG), Cabanis’s Greenbul Phyllastrephus cabanisi (CG),Yellow-throated Woodland Warbler Phylloscopus ruficapillus (WW) and Olive Sunbird Nectarinia olivacea (OS). Upon capture, individuals were ringed, colour-ringed, radio-tagged (small numbers of Taita Thrush only) and aged. Biometrics were taken following standard procedures and each individual was blood sampled for the study of genetic variation and blood parasites. Two or more independent measurements of left and right tarsus length (measured to the nearest 0.1mm) were taken for the study of fluctuating asymmetry (FA) (Palmer, 1994). Unbiased population estimates of FA were obtained from a mixed regression model (Van Dongen et al., 1999). Taita thrushes and White-starred robins were sexed through analysis of the W-chromosome linked gene CHD-W and biometric discrimination functions based on these confirmed data (for details see Lens et al., 1998).
Belgium. The different case studies in the Antwerp archipelago were conducted during 1990-98 and included four passerines (Nuthatch Sitta europaea, Crested Tit Parus cristatus, Great Tit P. major, and Blue Tit P. caeruleus), one mammal (Eurasian red squirrel Sciurus vulgaris) and one butterfly (Winter moth Operophtera brumata). Study methods included nest box records (Great and Blue Tit), mistnetting/trapping with (passerines and Eurasian red squirrel) or without individual marking (Winter moth) and radio-telemetry (Eurasian red squirrel) (Table 2). For further details see Matthysen et al. (1995) and references therein.
RESULTS
Interfragment dispersal and distribution pattern
Kenya. Table 3 lists the number of local and dispersed juveniles per fragment. A total of 28 individuals were relocated after dispersal to another fragment (see also Fig. 1), while 81 individuals were relocated in their natal fragment. Given the isolation of the Taita Hills archipelago and the fact that the study area covers all suitable habitat patches, it is unlikely that a substantial proportion of the marked pool dispersed outside the area. Overall, 26% of the relocated birds emigrated to another fragment (calculated as the total number of emigrants over the total number of relocated individuals; Table 3).
Taita Hills species differed markedly in their distribution over the study area. Olive sunbirds and White-starred robins inhabited all 12 fragments. Taita thrushes and Yellow-throated woodland warblers, in contrast, were only present in the three largest patches (apart from four Taita thrushes that were recently captured in YA). Both greenbul species and Taita white-eyes additionally occurred in FU (largest of the smaller fragments, Fig. 1) and adjacent NY, but were absent in two-thirds of the smaller and more isolated remnants.
Belgium. Emigration rates of Great and Blue tits (calculated as the total number of second year birds breeding in a non-natal fragment over the total number of second year recruits, 60% emigrants ) and Nuthatches (calculations as in the Kenyan study, 80% emigrants) were significantly higher than these of the suite of Kenyan species (tits: c ²1= 43.09, P=0.0001; Nuthatches: c ²1= 24.02, P=0.0001). Eurasian red squirrels, in contrast, showed a comparable rate of emigration (calculations as in the Kenyan study: 12% emigrants, c ²1= 1.74, P=0.24) (Table 2).
A study on the frequency of occurrence of 18 forest species in the Antwerp archipelago revealed strong species-specific differences in susceptibility to forest fragmentation, while four single-species studies showed lower population densities in fragments compared to reference plots in ‘mainlands’ (Table 2).
Sex ratio
Kenya. The sex ratio of adult White-starred robins was positively skewed towards males in all fragments, while first year individuals consistently showed an even sex ratio (Table 4; age: c ²1= 8.1, P= 0.004; age*fragment: c ²2= 0.09, P= 0.96). Although not significantly different, this bias towards adult males tended to be stronger in smaller fragments (9 patches pooled, range 2-12 ha, 18% females) compared to larger ones (3 patches pooled, range 50-200 ha, 29% females). Adult Taita Thrushes showed a comparable skew in sex-ratio in the three larger fragments (Lens et al., 1998; see Table 4) but, in contrast to the previous species, male bias was inversely correlated with fragment quality (species*fragment: c ²2= 6.14, P= 0.04; Lens et al., 1998).
Belgium. Eurasian red squirrels showed an even sex ratio in large woodlands and a tendency to male bias in 8 small fragments (Table 2). However, this bias was not consistent and in some fragments males were absent during consecutive years. Nuthatches showed equal survival rates for adult males and females in all sites (Matthysen 1998), while this species and the three Parus species did not show evidence for a male surplus in the different populations. The latter only accounts for breeding birds, however, since we have no information on numbers, survival rates or sex ratios of non-territorial floaters which are extremely difficult to catch or even detect (e.g. Lens 1994).
Fluctuating asymmetry
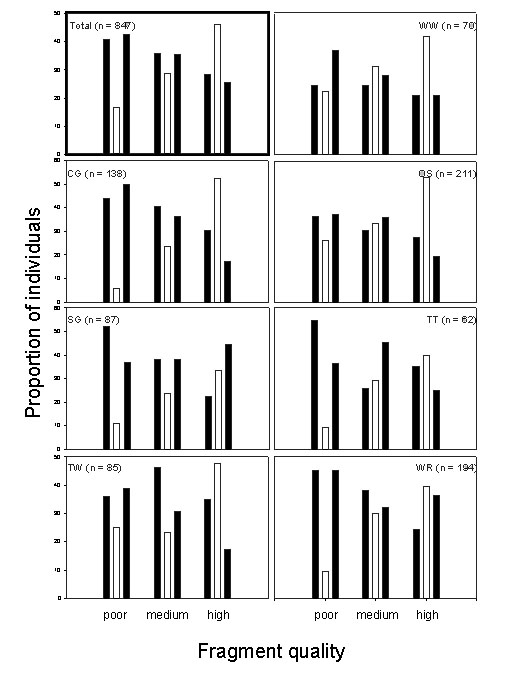
Kenya. The proportion of individuals with asymmetrical tarsi increased with decreasing fragment quality for all species (Fig. 2). An interspecific comparison of FA in tarsus length showed striking parallel patterns in relation to fragment quality: high FA in the low-quality fragment (CH), intermediate FA in the medium-quality fragment (NG) and insignificant FA in the high-quality fragment (MB) (L. Lens, S. Van Dongen, W. Wilder & T. Brooks, unpubl. data). Taita Thrushes and White-starred Robins further showed a highly significant sex-fragment interaction, whereby females were more asymmetrical than males in the poor- and medium-quality fragment but not in the high-quality one (L. Lens & S. Van Dongen, unpubl. data).
Belgium. Eurasian red squirrels showed higher levels of FA (hind feet length) in smaller fragments, whereas FA in Great tits (mass of regrown rectrices) and Winter moths (various wing measurements) did not show any relationship with fragmentation (Table 2). FA has not been studied so far in the other species.
DISCUSSION
The most significant result from our study in Kenya, so far, is the inverse relationship between the degree of fluctuating asymmetry and fragment quality in all seven study species. In both species that were sexed, females were more strongly affected than males, and the sex-dependent patterns in FA coincided with significant biases towards adult males. In three comparable case studies in Belgium, only one (Eurasian red squirrel) showed a slightly higher degree of FA in fragments compared to large forests. Interpopulation variation in the mean level of FA, however, did not correlate with woodland quality, and Wauters et al. (1996) concluded that the measure used in their study was too variable to be a useful indicator of stress differences between populations. Whether or not FA is an appropriate measure of stress remains a matter of much debate (e.g. Palmer & Strobeck 1992; Rowe et al. 1997), mainly because the predicted relationships between FA, fitness and environmental or genetic stress are often weak and highly heterogeneous, absent, or even opposite (Leung & Forbes 1996; but see Møller & Thornbill 1998). The apparent lack of correlation with FA might, however, in part be attributable to inadequate statistical procedures (S. Van Dongen, unpubl. data; see also Palmer & Strobeck 1986).
While there is a clear need for further study of the above FA patterns and their suitability as estimates of homeostasis, the observed levels of asymmetry (and of bias in sex ratio) were at least an order of magnitude higher in Kenya than in the Antwerp archipelago, while the degree of interfragment dispersal was lower in the tropical site. How can we explain these differences? Firstly, there is the different forest history of both areas. Although fragmentation of the indigenous Taita Hills forests started early this century (Loveridge 1937), much habitat destruction occurred in the 1960s, with local forest losses as high as 99% between 1962-85 (Beentje 1987). In the Belgian study site, in contrast, the overall configuration of forest patches remained stable for over two hundred years. Hence, species have been confronted with fragmentation of their habitat for hundreds of generations, while those suffering from high levels of fluctuating asymmetry, or poor dispersal abilities, might already have disappeared from the Antwerp landscape (see Balmford 1996). Secondly, the structure of the landscape in which the forests are embedded, differs between the two sites. Interpatch distances are considerably larger in Kenya, while there is hardly any indigenous vegetation left in the surrounding matrix. The Taita Hills are further characterised by a series of undulating ridges and low-altitude valleys that may form supplementary barriers to dispersal (e.g. Whitcomb et al. 1981; Harris & Scheck 1991; Dale et al. 1994). In Belgium, fragments are largely surrounded by level agricultural and residential areas that contain a considerable amount of secondary vegetation. Thirdly, Taita Hills populations are spatially isolated from neighbouring ones by vast areas of ‘hostile’ habitat. Antwerp populations, in contrast, are characterised by very high turnover rates due to immigration from neighbouring areas. Hence, predicted differences in the amount of environmental and genetic stress between both areas resulting from differences in time scale (more recent fragmentation in Kenya) and spatial scale (isolation from neighbouring populations and reduced gene flow due to limited interfragment dispersal in Kenya) can be expected to cause differences in patterns of FA (as reviewed by
Møller & Swaddle 1997) and sex ratios in the direction as described above.Can we expect further insight in fundamental ecological mechanisms resulting from comparisons of such different ecosystems? Based on our preliminary findings, the Eurasian red squirrel seems an adequate species to model effects of forest fragmentation in the Taita Hills. Squirrel populations show weak patterns of FA and of skewed sex ratios in relation to forest fragmentation, and observations on radio-tagged subadults suggest relatively short dispersal distances and reluctance to cross open areas (G. Verbeylen, unpubl. data). Although based on limited data sets, the emigration rates of squirrels and of Taita species were of a comparable magnitude. Besides, increased local philopatry of squirrels in fragments appears insufficient to compensate for the loss of immigrants, which in turn is strongly correlated with reduced levels of genetic variation (Wauters et al. 1994). We therefore do believe that parallel studies on the demography and genetics of poor dispersers in traditional western landscapes (such as the Eurasian red squirrel in Antwerp) and common species in recently fragmented tropical landscapes (such as the White-starred Robin in the Taita Hills) can produce further insights into how genetic and population processes interact when determining minimum viable population sizes. Only with such information we will be able to conserve rare endemics such as the Taita thrush.
ACKNOWLEDGEMENTS
We would like to thank L. Bennun, T. Brooks, M. Githiru, E. Waiyaki, C. Wilder and the staff of the Ornithology Department (National Museums of Kenya) for invaluable assistance in data collection and discussion, and S. Van Dongen for statistical advice. LL is research assistant and EM research associate with the Fund for Scientific Research – Flanders (FSR). This study is supported by FSR research grant G.0111.97 to EM and cooperative contract 02/6/7-338-607 between the Flemish Interuniversity Council (VLIR) and W.N. Verheyen and EM.
REFERENCES
Balmford, A. 1996. Extinction filters and current resilience: the significance of past selection pressures for conservation biology. Trends in Ecology and Evolution 11: 193-196.
Barrowclough, G.F. 1980. Gene flow, effective population sizes, and genetic variance components in birds. Evolution 34: 789-798.
Beentje, H.J. 1987. An ecological and floristic study of the forests of the Taita Hills, Kenya. Utafiti 1: 23-66.
Brooks, T., Lens, L., Barnes, J., Barnes, R., Kihuria, J.K. & Wilder, C. 1998. The conservation status of the forest birds of the Taita Hills, Kenya. Bird Conservation Intern. 8: 119-139.
Collar, N.J., Stattersfield, A.J. & Crosby, M.J. 1994. Birds to watch 2. The world list of threatened birds. Birdlife Conservation Series No. 4. Cambridge; Birdlife International.
Crome, F.H.J. 1997. Researching Tropical Forest Fragmentation: Shall We Keep On Doing What We’re Doing? In: Laurance, W.F. & Bierregaard, R. Jr. (eds) Tropical Forest Remnants: Ecology, Management, and Conservation of Fragmented Communities; Chicago, London; The University of Chicago Press: 485-501.
Dale, V.H., Pearson, S.M., Offerman, H.L. & O’Neill, R.V. 1994. Relating patterns of land-use change to faunal biodiversity in the Central Amazon. Conservation Biology 8: 1027-1036.
Davis, G.J. 1992. Juvenile dispersal, limited breeding sites, and the dynamics of metapopulations. Theoretical Population Biology 41: 184-207.
Ebenhard, T. 1991. Colonisation in metapopulations - a review of theory and observations. Journal of the Linnean Society 42: 105-121.
Gaines, M.S. & McClenaghan, L.R. 1980. Dispersal in small mammals. Annual Review of Ecology and Systematics 11: 163-196.
Gillespie, J.H. 1975. The role of migration in the genetic structure of populations in temporarily and spatially varying environments I. Conditions for polymorphism. American Naturalist 109: 127-135.
Gilpin, M. 1991. The genetic effective size of a metapopulation. Biological Journal of the Linnean Society 42: 165-175.
Greenwood, P.J. 1980. Mating systems, philopatry and dispersal in birds and mammals. Animal Behaviour 28: 1140-1162.
Haila, Y., Saunders, D.A. & Hobbs, R.J. 1993. What do we presently understand about ecosystem fragmentation? In: Saunders, D.A., Hobbs, R.J. & Ehrlich, P.R. (eds) Nature Conservation 3: Reconstruction of fragmented ecosystems; Chipping Norton; Surrey Beatty & Sons: 45-55.
Harris, L.D. & Scheck, J. 1991. From implications to applications: the dispersal corridor principle applied to the conservation of biological diversity. In: Saunders, D.A., Hobbs, R.J. (eds) Nature Conservation 2: The role of corridors; Chipping Norton; Surrey Beatty & Sons: 189-220.
Hunter, M.L. Jr. 1991. Introductory remarks: bird conservation at a landscape scale: seeing the world from a bird’s-eye view. Acta XX Congressus Internationalis Ornithologici Vol. IV: 2283-2285.
Lande, R. 1988. Genetics and demography in biological conservation. Science 241: 1455-60.
Lens, L. 1994. Factors influencing crested tit Parus cristatus social organisation. PhD Thesis, University of Antwerp, Antwerp, Belgium.
Lens, L. & Dhondt, A.A. 1994. Effects of habitat fragmentation on the timing of Crested Tit Parus cristatus natal dispersal. Ibis 136: 147-152.
Lens, L., Galbusera, P., Brooks, T., Waiyaki, E. & Schenk, T. 1998. Highly skewed sex ratios in the critically endangered Taita Thrush as revealed by CHD genes. Biodiversity and Conservation 7 869-873.
Loveridge, A. 1937. Reports on the scientific results of an expedition to rain forest regions in Eastern Africa. IX. Zoogeography and itinerary. Bulletin of the Museum for Comparative Zoology 79: 479-541.
Leung, B. & Forbes, M.R. 1996. Fluctuating asymmetry in relation to stress and fitness: effects of trait type as revealed by meta-analysis. Ecoscience 3: 400-413.
Matthysen, E. 1998. The Nuthatches. London; T & AD Poyser.
Matthysen, E. & Currie, D. 1996. Habitat fragmentation reduces disperser success in juvenile nuthatches Sitta europaea: evidence from patterns of territory establishment. Ecography 19: 67-72.
Matthysen, E., Lens, L., Van Dongen, S., Verheyen, G.R., Wauters, L.A., Adriaensen, F. & Dhondt, A.A. 1995. Diverse effects of forest fragmentation on a number of animal species. Belgian Journal of Zoology 12: 175-183.
Møller, A.P. & Swaddle, J.P. 1997. Asymmetry, developmental stability, and evolution. Oxford; Oxford University Press.
Møller, A.P. & Thornbill, R. 1998. Bilateral symmetry and sexual selection: a meta-analysis. American Naturalist 151: 174-192.
Murcia, C. 1995. Edge effects in fragmented forests: implications for conservation. Trends in Ecology and Evolution 10: 58-62.
Nour, N. 1997. Ecological and behavioural effects of habitat fragmentation on forest birds. PhD Thesis, University of Antwerp, Antwerp, Belgium.
Nour, N., Currie, D., Matthysen, E., Van Damme, R. & Dhondt, A.A. 1998. Effects of habitat fragmentation on provisioning rates, diet and breeding success in two species of tit (great tit and blue tit). Oecologia 114: 522-530.
Palmer, A.R. 1994. Fluctuating asymmetry analysis: a primer. In: Markow, T.A. (ed.) Developmental Instability: its origins and evolutionary implications; Dordrecht; Kluwer: 335-364.
Palmer, A.R. & Strobeck, C. 1992. Fluctuating asymmetry as a measure of developmental stability: implications of non-normal distributions and power of statistical test. Acta Zoologica Fennica 191: 57-72.
Parsons, P.A. 1992. Fluctuating asymmetry: a biological monitor of environmental and genomic stress. Heredity 68: 361-364.
Perrins, C.M. 1990. Concluding remarks: dispersal and gene flow. In: Blondel, J., Gosler, A., Lebreton, J.-D. & McCleery, R. (eds) Population biology of passerine birds. NATO ASI Series, vol. G24; Berlin; Springer: 475-480.
Rowe, l., Repasky, R.R. & Palmer, R. 1997. Size-dependent asymmetry: fluctuating asymmetry versus antisymmetry and its relevance to condition-dependent signalling. Evolution 51: 1401-1408.
Saunders , D.A., Hobbs, R.J. & Margules, C.R. 1991. Biological consequences of ecosystem fragmentation: a review. Conservation Biology 5: 18-32.
Slatkin, M. 1985. Gene flow in natural populations. Annual Review of Ecology and Systematics 16: 393-430.
Turner, I.M. 1996. Species loss in fragments of tropical rain forest: a review of the evidence. Journal of Applied Ecology 33: 200-209.
Van Dongen, S., Backeljau, T., Matthysen, E. & Dhondt, A.A. 1994. Effects of forest fragmentation on the population structure of the winter moth Operophtera brumata L. (Lepidoptera, Geometridae). Acta Oecologica 15: 193-206.
Van Dongen, S., Molenberghs, G. & Matthysen, E. 1999. Statistical analysis of fluctuating asymmetry: REML estimates of a mixed regression model. Journal of Evolutionary Biology 12: 94-102.
Wauters, L.A. & Dhondt, A.A. 1990. red squirrel (Sciurus vulgaris Linnaeus, 1758) population dynamics in different habitats. Zeitschrift für Säugetierkunde 55: 161-175.
Wauters, L.A., Dhondt, A.A., Knothe, H. & Parkin, D.T. 1996. Fluctuating asymmetry and body size as indicators of stress in red squirrel populations in woodland fragments. Journal of Applied Ecology 33: 735-740.
Wauters, L.A., Hutchinson, Y., Parkin, D.T. & Dhondt, A.A. 1994. The effects of habitat fragmentation on demography and on the loss of genetic variation in the red squirrel. Proceedings of the Royal Society of London, Series B 255: 107-111.
Whitcomb, R.F., Robbins, C.S., Lynch, J.F., Whitcom, B.L., Klimliewcz, M.K. & Bystrak, D. 1981. Effects of forest fragmentation on avifauna of the eastern deciduous forest. In: Burgess, R.C. & Sharpe, D.M. (eds) Forest island dynamics in man-dominated landscapes; Springer-Verlag; New York: 125-205.
Wilcove, D.S., McLellah, C.H. & Dobson, A.P. 1986. Habitat fragmentation in the temperate zone. In: Soulé, M.E. (ed.) Conservation biology: the science of scarcity and diversity; Sunderland MA; Sinauer Associates Inc.: 237-256.
Wilder, C., Brooks, T. & Lens, L. In press. Vegetation structure and composition of the Taita Hills forests. Journal of the East African Natural History Society.
Table 1. Kenya: characteristics of the twelve remaining Taita Hills cloud forest fragments.

Table 2. Belgium: summary of the area set-up, methodology and main results of the case studies conducted in the Antwerp ‘archipelago’
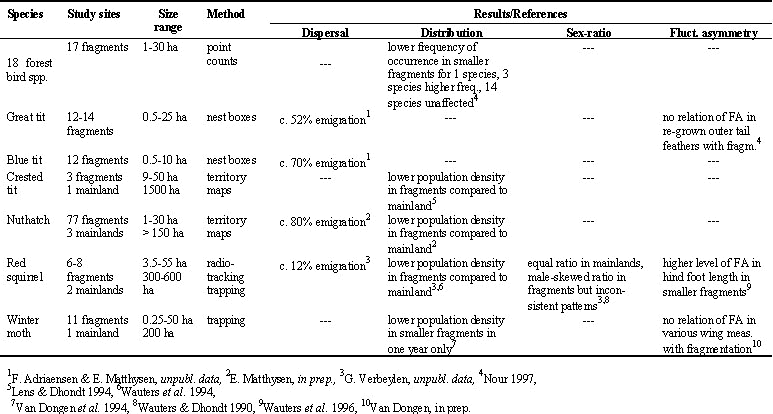
Table 3. Kenya: number of juveniles that were relocated in their natal fragment (locals, n= 81) or in a different fragment (emigrants, n= 28), and the distribution of dispersers among the fragments after settlement (immigrants).
Table 4. Kenya: number of females:males captured per fragment (with percentage of females in brackets).
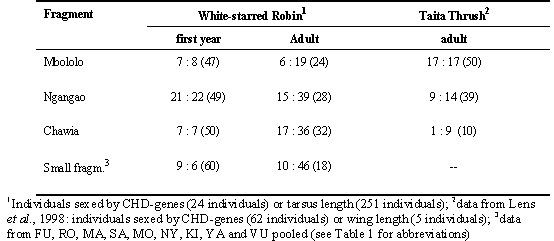
Fig. 1. Kenya: between-fragment dispersal by juveniles. Lines connect sites of first capture and recapture/resighting, and therefore do not necessarily match dispersal routes. Figures above lines indicate number of dispersal cases.
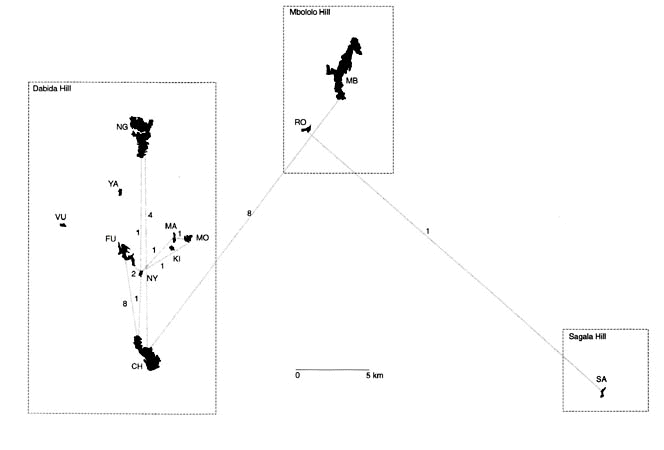
Fig. 2. Kenya: proportion of individuals with asymmetrical tarsi in relation to fragment quality. First black bars represent individuals with shorter left tarsus, second black bars represent individuals with longer left tarsus, white bars represent individuals with equally-sized tarsi, n represents sample size. See text for species abbreviations and for details on fragment quality.