S41.1: Movement of songbirds in fragmented forests: Can we 'scale up' from behaviour to explain occupancy patterns in the landscape?
André Desrochers1, Susan J. Hannon2, Marc Bélisle1 & Colleen C. St. Clair2
1 Centre de Recherche en biologie forestière, Faculté de foresterie et de géomatique, Université Laval, Sainte-Foy, Québec, G1K 7P4, Canada, fax 418 656 3551, e-mail Andre.Desrochers@sbf.ulaval.ca; 2Department of Biological Sciences, University of Alberta, Edmonton, Alberta, T6G 2E9, Canada, e-mail Sue.Hannon@ualberta.ca
Desrochers, A., Hannon , S.J., Bélisle, M. & St. Clair, C.C. 1999. Movement of songbirds in fragmented forests: Can we 'scale up' from behaviour to explain occupancy patterns in the landscape? In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2447-2464. Johannesburg: BirdLife South Africa.A recognition of spatial scale is of paramount importance in avian ecology and is usually the landscape ecologist's arena. However, recent studies by behavioural ecologists have started bridging the gap between landscape-scale patterns and individual-scale processes. The disruption of dispersal in birds caused by forest loss and fragmentation provide an opportunity for behavioural ecologists to investigate processes with potential landscape-scale consequences. Here we review inferential studies of the effects of forest fragmentation on bird movement, focusing on patterns of species occurrences and relocations of marked individuals. Then we review studies that describe how birds move or flow through specific landscape elements. We finally propose and illustrate with examples two behavioural approaches to understand the effect of forest fragmentation. The first approach is the study of bird trajectories in relation with landscape elements they encounter during dispersal. The second approach is the study of individuals' responses to changing landscape elements when experimentally attracted to a remote location. Simple hypotheses from trajectory and response studies can be made to explain species’ distribution at the landscape scale. We illustrate this approach qualitatively using indices of species reluctance to cross gaps and their presence in isolated patches. Further and more rigorous testing of such hypotheses in the field will determine whether behavioural work can be 'scaled-up' and thus contribute to explain bird occupancy patterns in the landscape.
INTRODUCTION
The importance of explicitly recognising scale in ecological studies has been emphasised numerous times (e.g. Addicott et al. 1987; Wiens 1989; Kotliar & Wiens 1990). Ecological systems are organised hierarchically and processes acting at one scale may or may not explain phenomena at other scales (Allen & Starr 1982; Wiens 1989). Behavioural and landscape ecology operate on different spatial scales and thus historically scientists in these disciplines have operated independently. Recently, Lima & Zollner (1996) and others emphasised the importance of behavioural phenomena (e.g. movement and habitat selection) in explaining population dynamics and patterns of occupancy of species across patchy landscapes and called for the development of a 'behavioural ecology of ecological landscapes'. The potential link between behaviour and landscape processes is but one illustration of the importance of behavioural ecology in solving conservation problems, as highlighted in a recent book (Clemmons & Buchholz 1997).
One of the conservation problems that affects birds most pervasively is forest fragmentation (e.g. Wilcove et al. 1986; Saunders et al. 1991; Andrén 1994; Askins 1995, but see Andrén 1994; Fahrig 1997; Bender et al. 1998). Although birds are more vagile than other vertebrate taxa, several studies have shown that landscape-level fragmentation of forests may constrain the ability of birds to explore and colonise forest patches (e.g. Villard et al. 1995). Studying the effects of forest fragmentation on forest songbirds thus provides an excellent opportunity for linking behavioural and landscape perspectives. However, very few studies have addressed the relationship between forest patch occupancy and concomitant dispersal and exploration behaviours, even though these may be important for the acquisition of suitable territories (Brewer & Harrison 1975; Morton 1992; Matthysen & Currie 1996; Morton 1997), nesting success (Badyaev et al. 1996) and survival outside the breeding season.
Studies of the impacts of forest fragmentation on birds have typically been conducted at the patch and landscape scales and have focused on descriptions of patterns of occupancy and/or breeding success in patches of varying sizes and isolation. More recently, investigators have focused on movements of marked individuals across fragmented landscapes, especially during dispersal or the post-fledging period, in order to explain occupancy patterns and investigate metapopulation dynamics. The major weakness of these studies is that the pathway or trajectory of movement has not been measured, only the start and end points. Thus, relative 'permeability' of landscape elements to movement can only be inferred. Patch occupancy patterns by some species may be determined by the cost of exploratory movements (discussed by Bernstein et al. 1991; Plissner & Gowaty 1996). For other species, however, exploration of landscapes, fragmented or not, may be less costly and they may use the degree of forest fragmentation as an indicator of habitat quality (i.e. level of nest predation (e.g. Wilcove 1985; Robinson et al. 1995) or food availability (Burke & Nol 1998)).
In order for behavioural ecologists to make a contribution to explaining effects of forest fragmentation, we must first understand the responses of species at smaller spatial scales (i.e. their perceptual range, propensity to cross gaps or to use corridors, relative movement rates across different habitat types, responses to barriers and so on; see Wiens 1992 for a conceptual model). Second we must determine whether such responses help predict patterns at the landscape scale. If so, it should be possible to predict the cumulative effect of a large number of short-term, small-scale movement decisions on bird occurrence in forest fragments. Use of detailed conceptual models (e.g. Wiens 1992) and individual-based, spatially explicit models (e.g. Schippers et al. 1996; Tyler & Hargrove 1997) will aid us choosing which behaviours to measure and allow us to test predictions empirically in the field.
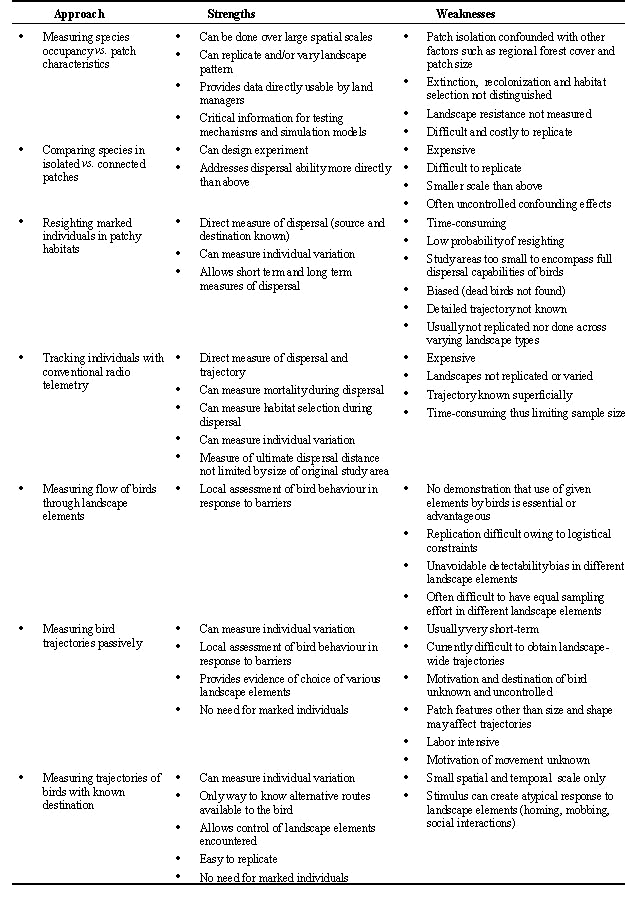
In this paper we review empirical studies that have looked at dispersal as a species-specific or an individual-based process, but with varying degrees of precision. (For a review of theoretical models, see Lima and Zollner (1996)). Based on this diversity of approaches, we assess ways to better predict the patterns of the occurrence of birds in fragmented landscapes. We have integrated this disparate literature by grouping the studies into three methodological categories and by evaluating the strengths and weaknesses of each. The first category of studies infer dispersal limitations from species occupancy of forest patches. The second category of studies measures endpoints or points along the paths of marked individuals' movements between forested patches. The third category of studies describe trajectories of individuals across landscapes with various degrees of forest fragmentation.
Our focus is on the third category which we illustrate with some of our own studies. Finally, we highlight the unexplored potential of behavioural data as a tool to predict patterns of occupancy on the landscape.
Inferring constraints on dispersal from patch occupancy
Because birds are relatively vagile organisms, they may perceive a fragmented landscape as more connected than species with poorer dispersal abilities. In particular, the large-scale seasonal movements of migratory birds, invite one to question whether gaps between patches on the order of hundreds of metres would deter bird movement across landscapes. Migration and dispersal, however, are very different processes with different motivations and costs/benefits to movement (Villard et al. 1995). Perceptions of landscape connectivity to a bird likely differ seasonally, due partly to its reproductive state. One way of inferring a lack of landscape connectivity (thwarted dispersal) to birds is to describe their pattern of occupancy in patches which vary in their isolation and connectedness across fragmented landscapes.
Both patch isolation and matrix 'resistance' to movement could affect the probability of a species arriving at a patch, yet only the former has received substantial attention. Moreover, because most studies have been done in agricultural landscapes, the influence of resistance of different matrix types remains poorly documented (Opdam 1991). Isolated patches may be unoccupied by a species for the following reasons: (1) 'psychological' aversion to crossing openings (Lynch & Whigham 1984); (2) physical limitations on vagility; (3) high site tenacity (Matthysen et al. 1995); (4) mortality during movement; (5) low detectability of the patch (i.e. may be beyond the species perceptual range (relating to patch size, orientation and distance; Temple 1991; Gutzwiller & Anderson 1992; Zollner & Lima 1997)); or (6) high extinction rate in the patch due to changes in patch suitability associated with fragmentation (e.g. changes in land use within patches). Of course, it is possible that patch occupancy results from an interaction between the latter factors. Whatever the mechanism, the end result is that isolated patches have lower rates of colonisation, occupancy and/or abundance than closer patches (all other factors held constant). If this is not the case, then we can infer that fragmentation does not thwart dispersal among patches.
Occupancy of isolated patches
There has been a plethora of studies examining impacts of forest fragmentation on bird communities. Here, we discuss those studies which have explicitly examined the effect of patch isolation on the probability of colonisation or occupancy of the patch and have limited our discussion to studies done in forest. Van Dorp & Opdam (1987) and Opdam (1991) point out the difficulties in comparing studies that have used different measures of isolation, done in different regions which vary in the type of landscape and matrix, the size and isolation range of forest patches and the regional abundance of the species. With this in mind, Opdam (1991) reviewed these studies and noted that in most cases, area of forest patch explained a higher amount of the variation in bird occupancy patterns than did isolation. In general, results within a region tended to be species-specific and studies which combined all species together may have missed significant differences in species responses (e.g. compare Helliwell 1976 with McCollin 1993). Opdam et al. (1984 and 1985) found that variables measuring isolation significantly improved species-area regressions for several mature forest species. In a more extensive study, van Dorp & Opdam (1987) found that forest patch occupancy of six of 32 species was negatively affected by increasing interpatch distance and positively affected by amount of forest in the vicinity of the forest patch. More detailed studies on the sedentary European Nuthatch Sitta europaea and the Spotted Woodpecker Dendrocopos medius found that patch occupancy or colonisation rates were correlated with distance to other occupied patches or large patches of suitable forest (Petterson 1985; Verboom et al. 1991). Several studies focusing primarily on passerines in the eastern United States reported that 'interior' forest species, 'area-sensitive' and/or neotropical migrants were less abundant or were absent in isolated forest patches (e.g. Whitcomb et al. 1981; Lynch & Whigham 1984; Askins & Philbrick 1987; Askins et al. 1987; Robbins et al. 1989). In studies involving non-passerines, Spruce Grouse Dendragapus canadensis were less likely to occupy forest patches that were smaller and further away from occupied patches (Fritz 1979) and Red-cockaded Woodpeckers Picoides borealis were less likely to colonise isolated clusters of suitable nest trees (Thomlinson 1995).
A major difficulty with interpreting these inferential studies is separating the effects of isolation and correlated factors such as patch size and regional forest cover which in turn influence regional abundance of a species (e.g. van Dorp & Opdam 1987; Bellamy et al. 1996; Table 1). Isolated patches are often in areas with lower forest cover and these patches may have increased edge effects that could reduce local forest suitability (Dunning et al. 1995). Andrén (1994) and Fahrig (1997) found that regional forest cover may be more important than fragmentation per se in explaining species distribution across landscapes. Villard et al. (1995) attempted to separate the effects of area, isolation, vegetation structure of the fragments, breeding philopatry and a combination of these factors on the incidence of four species (Wood Thrush Hylocichla mustelina, Black-and-white Warbler Mniotilta varia, Ovenbird Seiurus aurocapilla and Scarlet Tanager Piranga olivacea) in forest patches in an agricultural area in Ontario. Isolation alone appeared to be an important predictor of extinction and recolonisation of a patch only for the Black-and-white Warbler, but including isolation in combination models improved the fit for three of the four species.
Occupancy of connected patchesAnother inferential approach has been to compare occupancy rates of fragments that are joined by corridors to those that are completely isolated. Beier & Noss (unpublished manuscript) have thoroughly reviewed studies of this type and note that the interpretation of these studies is unclear due to the confounding effects of corridor presence and overall forest cover, patch size, distance to source populations, distance away from urban centres etc. In addition, many of these studies are unreplicated ( e.g. MacClintock et al. 1977; see Nicholls & Margules (1991) for a discussion on experimental designs involving corridors). Two studies have attempted to control for some of these problems. Dunning et al. (1995) chose study sites which differed mainly in the presence or absence of corridors: they found that corridor presence facilitated colonisation of clearcuts by Bachman's Sparrow Aimophila aestivalis. Schmiegelow et al. (1997) experimentally set up isolated and connected reserves that were matched for forest type and found that reserves connected by corridors did not have higher abundance of neotropical migrants and resident songbird species than isolated reserves two years after the forest was fragmented.
In Table 1 we outline the strengths and weaknesses of the occupancy approach to inferring movement of birds. A more direct measure, although still inferential, is to document dispersal patterns using individually marked birds, which we cover in the next section.
Movement through fragmented landscapes by known individuals
A second approach to assessing dispersal abilities of birds through fragmented landscapes has been to relocate individually-marked birds or to follow birds using radio-telemetry. Dispersal studies that did not explicitly address the influence of fragmentation are not reviewed here. One approach is to colour-ring birds and then search the surrounding landscape to determine where they settled. Dispersal distances can then be compared among landscape types, the usual prediction being that if openings are barriers, then dispersal distances will be shorter in highly fragmented landscapes. Matthysen et al. (1995) used this approach for juvenile European Nuthatches. Contrary to prediction, they found that in their highly fragmented sites, dispersal distances were longer in comparison to sites in other studies which were less fragmented. They argued that the scale of dispersal necessarily expanded as forest patches were further apart. Nevertheless, once settled, birds in isolated forest patches were more sedentary, even though many of these birds may have settled in territories of poor suitability when compared to birds dispersing in large tracts of forest (see Matthysen & Currie 1996). Saunders & de Rebeira (1991) documented more movement of ringed birds between patches connected by corridors than those between isolated patches. Haas (1995), who colour-ringed adults and monitored breeding dispersal, also found more movement between sites that were connected by a wooded corridor than between isolated sites.
As with species occupancy studies, there are several problems with studies of this type (Table 1). First, for studies on juvenile dispersal, resighting probabilities are extremely low (e.g. 6.8% of 220 nestlings for Matthysen et al. (1995)). Second, biases arise because study areas are often too small to encompass full dispersal distances (reviewed by Haas 1995) and mortality of birds during dispersal is not accounted for. Replication of landscapes is rare because of the time consuming nature of these studies. Finally, Temple (1991) has noted that the probability of a bird leaving its natal patch and finding a destination patch in which to breed depends only in part on the resistance of the matrix and the distance between source and destination patches. The probability of leaving a patch may also be influenced, among other things, by the degree of natal philopatry of the species and the pressure to leave the patch (e.g. high competitor density and aggression level, few mating opportunities and low food resource levels) (Bernstein et al. 1991; Temple 1991). For example, Lens & Dhondt (1994) found that juvenile Crested Tits Parus cristatus raised in small isolated fragments had lower body mass and delayed dispersal compared with those raised in larger forest patches, suggesting that the isolated patches were of lower quality. Willow Warblers Phylloscopus trochilus breeding along a highway, were more likely to disperse away from the road and dispersal distances were longer than for males that lived further from the road (close to road was low quality habitat) (Foppen & Reijnen 1994). Interspecific and interpopulation variability in the above factors suggest that comparative approaches and more detailed behavioural studies are needed.
The use of radio telemetry to monitor bird movements circumvents some of the problems inherent in the resighting technique above (Table 1). By using telemetry, mortality of birds during dispersal and in different habitat types can be determined and the distance moved and time taken to disperse quantified. For example, Miller et al. (1997) used telemetry to monitor juvenile dispersal in Spotted Owls Strix occidentalis caurina. By taking telemetry fixes daily they discovered that juveniles chose closed canopy forest over open-canopy forest and suffered higher mortality when they used clearcuts and open areas with young saplings. Owls did not choose to move in continuous forest and their dispersal distance did not vary with respect to levels of fragmentation. Nonetheless, clearcuts, agricultural areas and urban centres appeared to be barriers to dispersal. Overall, using telemetry improves the resolution of dispersal studies and still contributes greatly to the field, but the method has drawbacks: it is expensive, labour-intensive, and the detailed trajectory of birds is usually not known (Table 1).
Measuring flow and trajectories of individuals
Given the shortcomings of inferential methods above, how can we improve significantly our understanding of bird dispersal in fragmented landscapes? Greater resolution of bird dispersal in fragmented forests can be provided by direct measures of movements of individuals, such as measuring their flow through particular landscape elements, by mapping their actual trajectories, and by making direct measures of movement decisions of birds encountering contrasting landscape elements. These approaches have clear advantages over the inferential methods because they can pinpoint the exact locations of movement and allow decision rules to be quantified for future use in dispersal models at the landscape scale. This section is necessarily brief, due to the paucity of studies of this nature, but it demonstrates the potential of flow and, particularly, trajectory studies.
Flow studies
Flow studies measure the number of birds travelling through certain parts of the landscape, thus allowing a comparison of the use of different landscape features for movements. Wegner and Merriam (1979) provided an early example of a flow study by using standardised observation posts to simply count the number of birds moving among forest patches, fencerows and open fields. In their study, the flow of songbirds was greater between forest and vegetated fencerows than between other landscape elements, and this was interpreted as evidence of channelling of birds along fencerows. Johnson and Adkisson (1985), on the other hand, measured the flow of Blue Jays Cyanocitta cristata as they dispersed beech nuts within an agricultural landscape. Of 158 Blue Jays observed during their movements, 91% followed fencerows closely, while only 9% flew over adjoining fields. Both studies suggest that forested corridors facilitate movement, but provide little information on the movement rate across fields, since there is no measure of the open area sampled and detectability is unaccounted for. More recently, Machtans et al. (1996) measured flow by comparing the detection rates of dispersing forest songbirds in riparian corridors with adjacent clearcuts. They found greater bird movement in riparian strips (measured via mist-netting) than in clear cuts (measured via observation). This conclusion, though, assumes that the observers could detect birds moving in both landscape features with the same ability, and the authors conceded that this assumption may not have been valid. In fact, equal detectability within different habitat types might be impossible to attain with birds (contrary to animals physically constrained to the ground or water) and thus might be a strong limitation inherent to flow studies (Table 1). Flow studies may be most useful to measure location-specific patterns such as the
'bottleneck effect' resulting from birds moving through a large forest patch and entering a narrow forested corridor.Passive trajectory studies
Following the path or trajectory of birds as they move through fragmented landscapes will provide evidence of avoidance or utilisation of various landscape elements. Until recently, detailed measures of trajectories have not been possible. However, recent technological advances should open the way to exciting developments in bird movement analysis. We review two such advances below: differentially-corrected Global Positioning Systems (GPS; e.g. Rempel et al. 1995) and harmonic radar (Mascanzoni & Wallin 1986; Roland et al. 1996). With GPS receivers, we obtained detailed trajectories of birds in the post-fledging period by following them visually and recording their locations. Trajectories were then plotted on a high-resolution computerised map, ready for analysis (Fig 1). High-quality GPS receivers can work very well even under forested cover (D'Eon 1995) and provide locations with a 5 meter accuracy after data correction by specialised software (Hurn 1993). Of course, the ideal situation would be to place GPS receivers on the birds themselves, either coupled with a transmitter (to find individuals and download GPS data remotely using a modem) or with the possibility to recapture the bird and download the GPS data directly. GPS-transmitter packs have only begun to be used for mammals (Edenius 1997; Moen et al. 1997; Rempel & Rodgers 1997); they are still too heavy for songbirds but we should expect lighter circuitry to be available in the coming few years. In the meantime, it is possible to follow easily detectable species within ca. 10 m while holding a GPS receiver to obtain useful trajectory data, if care is taken not to disturb the bird (Fig. 1).
Harmonic radar (Mascanzoni & Wallin 1986; Roland et al. 1996) also holds promise for the detailed study of bird movement. This technology involves tags that absorb a radar signal from a transmitter, which is re-radiated at a different wavelength, which can be picked up by a Yagi antenna (commonly used for radio-tracking). Tags, which consist of wire and a diode with no internal power supply, are small, light (< 5 mg), and inexpensive. Also, conventional radio-tracking receivers can be modified easily and at a small cost to send and receive signals. In their study, Roland et al. (1996) were able to track butterflies and moths at up to 50 m from the receiver, over periods of up to 48 h. The main shortcoming of harmonic radar is its short range (approximately 100 m), which limits the kinds of studies that can rely on this method. No studies of birds have yet taken advantage of harmonic radar, although it has obvious relevance to small-scale movement analysis.
Despite technological and conceptual advances, obtaining detailed movement data in a landscape context will remain labour-intensive. Furthermore, more sophisticated radio-telemetry will not eliminate all its shortcomings. But more importantly, passive monitoring of birds moving over large areas will not control for a variety of ways in which habitat type and movement may be confounded (Table 1). For example, birds found moving along forest edges (e.g. Fig. 1) may be selecting the edge as prime foraging habitat or they may 'bounce' on an impermeable boundary. Also, however detailed, passive trajectory studies will require assumptions about the motivation of movement (e.g. dispersal or foraging). Resolving the effects of habitat elements and motivation on movement behaviour thus requires a much finer-scale understanding of individual decision-making.
To understand motivations behind bird movements, we will first need to characterise movement patterns. For this purpose, many analytical procedures already exist. Simple descriptive statistics such as step length, turning angle, and directionality indices (reviewed by Bell 1991; see also Claussen et al. 1997), have often been used to describe the searching paths of ground foraging birds (e.g. Smith 1974; Zach & Falls 1976). Methods used to compare movement patterns among species or under different environmental conditions, include the modelling of the observed movement patterns as passive diffusion (e.g. Kareiva 1983; Turchin 1991) or correlated random walk (e.g. Kareiva & Shigesada 1983; Bovet & Benhamou 1988; Crist et al. 1992) models whose input parameter values are then compared across treatments. Yet another method used to compare movement patterns, whose results are often difficult to interpret however, is fractal analysis. This method calculates the fractal dimension of movement paths in order to describe and compare movement patterns across spatial scales (e.g. Johnson et al. 1992; Wiens et al. 1995). Fractal analysis is considered to be a way of extrapolating small scale movement patterns to larger scales, and ultimately infer their influence on population processes at the landscape level (Wiens et al. 1995; but see Turchin 1996). These advanced methods have been mostly used with insects and are yet to be used with birds.
Fine-scale behavioural observations coupled with movement analysis may help separate barrier effects from confounding habitat effects. However, an important limitation of 'passive' trajectory studies, even sophisticated ones, will remain: there is no control over the motivation that causes birds to move among forest patches. Because of this limitation, a comparison of movement patterns of birds moving through fragmented or connected landscapes would test the null hypothesis that movement patterns are the same in both types of landscapes, but would not allow inferences on factors like travel costs (Table 1).
Experimental trajectory studies
To gain more insight from trajectory studies, we need to increase our control over the motivation of birds under study. One way of creating this motivation is through the use of experiments with tape-recorded songs and calls that elicit a response from one or several target species. For example, Sieving et al. (1996) and Rail et al. (1997) used species-specific playbacks of territorial songs to measure the distance that male songbirds could be lured into or across gaps in forest cover. Using the mobbing call of Black-capped Chickadees Poecile atricapillus or other widespread species as a stimulus can attract several forest species simultaneously and in multiple seasons (Hurd 1996; Desrochers & Hannon 1997; St. Clair et al. unpublished manuscript). With mobbing calls, we have previously quantified bird preferences for travelling under forest cover in both relative and absolute terms (Fig. 2). Bird species varied in their reluctance to travel outside of the forest, but overall, birds were twice as likely to travel through 50 m of forest than through 50 m of open area to reach a recording of mobbing calls. When given a choice of travelling through forest or across a gap, the majority of respondents preferred to take a detour to remain in the woodland, even if it was three times longer than the shortcut in the open. However, species differed greatly in their response to gaps (see below).
Of course, playback techniques can only provide trajectories with known destinations (as opposed to passive movement) over very short-distances. To measure how local decisions can delay or facilitate movement across larger areas, bird trajectories with known destinations can be obtained through the temporary translocation of territorial individuals (as opposed to permanent translocation as in Bendel & Therres 1994). Upon release, relocated individuals may then be followed with a Global Positioning System (GPS) receiver or accurate, continuous radio-tracking to log the routes they choose in the landscape on their way back to their territory. When following the birds is not possible, an indirect approach, such as measuring the time between release and return to a territory, may indicate the relative permeability of different landscapes. In birds, translocation studies have primarily been attempted for the study of homing mechanisms (Able 1995), and linking translocation techniques with the study of dispersal in fragmented landscapes has only begun recently. For example a preliminary translocation study conducted by Bélisle in 1997 and 1998 suggests that forest fragmentation does reduce the ability of birds to promptly return to their territory (Table 2). In this study, territorial males were attracted by conspecific song playbacks, captured and translocated to sites designed to force them to travel either through fragmented, agricultural landscapes or through predominantly forested landscapes. Translocation distances generally ranged from 1.5 to 2.0 Km, which we considered big enough to provide information at the landscape scale and small enough to limit confounding effects of navigation ability.
In both flow and trajectory studies, a variety of ecological, behavioural and social components of bird movement need to be addressed. Although we found no evidence that variation in weather variables influenced the tendency of birds to cross gaps in forest cover (St. Clair et al. unpublished manuscript), severe weather and experimental stimuli themselves may influence the responses of foraging birds to predation risk and it is likely that this effect extends to movement decisions. Similarly, conspecific attraction, measured by the number of individuals detected during trials, had no effect on movement decisions in our study, but clearly influences the behaviour of animals in other contexts (e.g. Beauchamp et al. 1997). Beyond these factors, we need to determine the nature of movements under study: territorial residents may have decision rules very different from those of dispersing birds. Also, needed are better and species-specific measures of perceptual range (i.e. the distance at which an individual can detect a given landscape element; Zollner & Lima 1997), in order to improve models of landscape exploration.
SCALING UP BEHAVIOUR
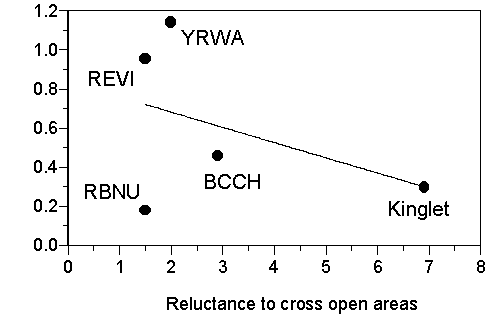
Will new behavioural tools help us 'scale-up' to explain bird occupancy patterns in the landscape? It is premature to fully answer this question here. In the short term, the success of new empirical work on short-term, small-scale trajectories and associated decision rules by dispersing birds will be measured first by its ability to generate testable predictions about landscape occupancy patterns. From our own work, certain predictions can already be made. For example, data on the reluctance of birds to cross gaps (Fig. 2; Desrochers & Hannon 1997) can be used to predict that the species most reluctant to come into the open will respond more negatively to forest patch isolation than others, at least in similar landscapes. We can provide a first, crude assessment of this prediction with data from two of our studies (Fig. 3). Despite the very small sample, Fig. 3 suggests that species reluctant to come into the open may respond more negatively to experimental isolation of their habitat. We are currently obtaining information both on gap avoidance and response to isolation with more species, so the relationship in Fig. 3 (or lack thereof) remains to be confirmed.
Beyond interspecific patterns, we may also use behavioural data to predict threshold responses to landscape elements. For example, we found that Black-capped Chickadees were consistently unwilling to cross gaps of 50 m when alternative routes existed (Desrochers & Hannon 1997; St. Clair et al. unpublished manuscript). Based on this observation, we can hypothesise that these birds might be less likely to occur in forest patches separated by 50 m or more from a neighbouring patch, . Indeed, after controlling for patch size as a covariate, the winter abundance of chickadees was lower in forest patches whose isolation exceeded this threshold (Table 3; F1,74 = 3.2, one-tailed P = 0.04). Besides the simple predictions above, detailed movement data may prove important to improve spatially-explicit models which too often are based on simplistic assumptions about the decisions made by dispersing birds. It should be possible to incorporate such empirically-based behavioural rules without compromising model tractability (Turchin 1991) to generate highly specific predictions not only about bird movement in landscapes, but also in a variety of population models.
CONCLUSION
Our brief review highlights the current emphasis on patterns of distribution rather than underlying processes, which is diagnostic of a field of research still in its infancy. Despite many problems with confounding effects and lack of replication, ornithologists have obtained convincing and geographically robust patterns showing the decreased value of fragmented forests to a significant number of bird species. We know, not just from simulations but also from observation, that isolation of forest patches per se sometimes reduces the capacity of birds to colonise, even though many other factors also influence patch occupancy patterns. However, the taxonomic variety of responses to forest fragmentation is bewildering. It would thus appear futile to search for a species-independent theory of fragmentation, even with forest specialists only. A more realistic challenge will be to take advantage of differences among species to group or rank species-specific responses to fragmentation of forests (or other habitats), and thus create testable hypotheses as illustrated in Fig. 3. Opdam et al. (1995) advocate a top-down approach to evaluating and predicting fragmentation effects. However, we feel that a bottom-up understanding of behaviour and decision-making is an important way to enhance our ability to predict species responses to landscape change. This ability will be the longer-term measure of the success of the behavioural approach to dispersal. So far, the explanatory power of our theory of forest fragmentation has been low, despite the statistical significance of many models. Even though the stochasticity of the dispersal process will remain a major impediment, it should not deter ornithologists from finding creative ways to measure the actual movements of birds, or from re-visiting their own behavioural data from a new perspective.
ACKNOWLEDGEMENTS
We thank Steve Brisson, Isabelle Chouinard, Jean-Michel Roberge, and Stéphane Roy for assistance with the field work. Thomas C. Grubb, Jr. and Jeff Walters provided useful comments on an early version of this text. Work by the authors was supported by the Natural Sciences and Engineering Research Council of Canada through operating grants to A.D. and S.J.H., and a research grant by the FCAR (Québec) to A.D. M.B. was funded by a N.S.E.R.C. scholarship and a P.Q.S.P.B. grant (Québec). C.C.S. was funded by Killam and C.R.B.F. post-doctoral fellowships.
REFERENCES
Able, K.P. 1995. Orientation and navigation: a perspective on fifty years of research. Condor 97: 592-604.
Addicott, J.F., Aho, J.M., Antolin, M.F., Padilla, D.K., Richardson, J.S. & Soluk, D.A. 1987. Ecological neighborhoods: scaling environmental patterns. Oikos 49: 340-346.
Allen, T.F.H. & Starr, T.B. 1982. Hierarchy: Perspectives for ecological complexity. Chicago, Illinois, U.S.A.; University of Chicago Press: 310pp.
Andrén, H. 1994. Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71: 355-366.
Askins, R.A. 1995. Hostile landscapes and the decline of migratory songbirds. Science 267: 1956-1957.
Askins, R.A. & Philbrick, M.J. 1987. Effects of changes in regional forest abundance on the decline and recovery of a forest bird community. Wilson Bulletin 99: 7-21.
Askins, R.A., Philbrick, M.J. & Sugeno, D.S. 1987. Relationship between the regional abundance of forest and the composition of forest bird communities. Biological Conservation 39: 129-152.
Badyaev, A.V., Martin, T.E. & Etges, W.J. 1996. Habitat sampling and habitat selection by female Wild Turkeys: ecological correlates and reproductive consequences. Auk 113: 636-646.
Beauchamp, G., Bélisle, M. & Giraldeau, L.-A. 1997. Influence of conspecific attraction on the spatial distribution of learning foragers in a patchy habitat. Journal of Animal Ecology 66: 671-682.
Bell, W.J. 1991. Searching behaviour. The behavioural ecology of finding resources. London; Chapman & Hall: 358pp.
Bellamy, P.E., Hinsley, S.A. & Newton, I. 1996. Local extinctions and recolonisations of passerine bird populations in small woods. Oecologia 108: 64-71.
Bendel, P.R. & Therres, G.D. 1994. Movements, site fidelity and survival of Delmarva fox squirrels following translocation. American Midland Naturalist 132: 227-233.
Bender, D.J., Contreras, T.A. & Fahrig, L. 1998. Habitat loss and population decline: a meta-analysis of the patch size effect. Ecology 79: 517-533.
Bernstein, C., Krebs, J.R. & Kacelnik, A. 1991. Distribution of birds amongst habitats: theory and relevance to conservation. In: Perrins, C.M., Lebreton, J.-D. & Hirons, G.J.M. (eds) Bird population studies: relevance to conservation and management; Oxford, U.K.; Oxford University Press: 317-345.
Bovet, P. & Benhamou, S. 1988. Spatial Analysis of Animals' Movements Using a Correlated Random Walk Model. Journal of theoretical Biology 131: 419-433.
Brewer, R. & Harrison, K.G. 1975. The time of habitat selection by birds. Ibis 117: 521-522.
Burke, D.M. & Nol, E. 1998. Influence of food abundance, nest-site habitat, and forest fragmentation on breeding ovenbirds. Auk 115: 96-104.
Claussen, D.L., Finkler, M.S. & Smith, M.M. 1997. Thread trailing of turtles: methods for evaluating spatial movements and pathway structure. Canadian Journal of Zoology 75: 2120 - 2128.
Clemmons, J.R. & Buchholz, R. (eds) 1997. Behavioral approaches to conservation in the wild. Cambridge, U.K.; Cambridge University Press: 400pp.
Crist, T.O., Guertin, D.S., Wiens, J.A. & Milne, B.T. 1992. Animal movement in heterogeneous landscapes: an experiment with Eloedes beetles in shortgrass prairie. Functional Ecology 6: 536-544.
D'Eon, S.P. 1995. Accuracy and signal reception of a hand-held global positioning system (GPS) receiver. Forestry Chronicle 71: 192-196.
Desrochers, A. & Hannon, S.J. 1997. Gap crossing decisions by dispersing forest songbirds. Conservation Biology 11: 1204-1210.
Dunning, J.B., Jr. , Borgella, R., Jr. , Clements, K. & Meffe, G.K. 1995. Patch isolation, corridor effects, and colonization by a resident sparrow in a managed pine woodland. Conservation Biology 9: 542-550.
Edenius, L. 1997. Field test of a GPS location system for moose Alces alces under Scandinavian boreal conditions. Wildlife Biology 3: 39-43.
Fahrig, L. 1997. Relative effects of habitat loss and fragmentation on population extinction. Journal of Wildlife Management 61: 603-610.
Foppen, R. & Reijnen, R. 1994. The effects of car traffic on breeding bird populations in woodland. II. breeding dispersal of male willow warblers (Phylloscopus trochilus) in relation to the proximity of a highway. Journal of Applied Ecology 31: 95-101.
Fritz, P.S. 1979. Consequences of insular population structure: distribution and extinction of Spruce Grouse populations. Oecologia 42: 57-65.
Gutzwiller, K.J. & Anderson, S.H. 1992. Interception of moving organisms: influences of patch shape, size, and orientation on community structure. Landscape Ecology 6: 293-303.
Haas, C.A. 1995. Dispersal and use of corridors by birds in wooded patches on an agricultural landscape. Conservation Biology 9: 845-854.
Helliwell, D.R. 1976. The effects of size and isolation on the conservation value of wooded sites in Britain. Journal of Biogeography 3: 407-416.
Hurd, C.R. 1996. Interspecific attraction to the mobbing calls of Black-capped Chickadees (Parus atricapillus). Behavioral Ecology and Sociobiology 38: 287-292.
Hurn, J. 1993. Differential GPS explained. Sunnyvale, CA, U.S.A.; Trimble Navigation: 55pp.
Johnson, A.R., Milne, B.T. & Wiens, J.A. 1992. Diffusion in fractal landscapes: simulations and experimental studies of tenebrionid beetle movements. Ecology 73: 1968-1983.
Johnson, W.C. & Adkisson, C.S. 1985. Dispersal of beech nuts by Blue Jays in fragmented landscapes. American Midland Naturalist 113: 319-324.
Kareiva, P.M. 1983. Local movement in herbivorous insects: applying a passive diffusion model to mark-recapture field experiments. Oecologia 57: 322-327.
Kareiva, P.M. & Shigesada, N. 1983. Analyzing Insect Movement as a Correlated Random Walk. Oecologia 56: 234-238.
Kotliar, N.B. & Wiens, J.A. 1990. Multiple scales of patchiness and patch structure: a hierarchical framework for the study of heterogeneity. Oikos 59: 253-260.
Lens, L. & Dhondt, A.A. 1994. Effects of habitat fragmentation on the timing of Crested Tit Parus cristatus natal dispersal. Ibis 136: 147-152.
Lima, S.L. & Zollner, P.A. 1996. Towards a behavioral ecology of ecological landscapes. Trends in Ecology and Evolution 11: 131-135.
Lynch, J.F. & Whigham, D.F. 1984. Effects of forest fragmentation on breeding bird communities in Maryland, USA. Biological Conservation 28: 287-324.
MacClintock, L., Whitcomb, R.F. & Whitcomb, B.L. 1977. Evidence for the value of corridors and minimization of isolation in preservation of biotic diversity. American Birds 31: 6-23.
Machtans, C.S., Villard, M.-A. & Hannon, S.J. 1996. Use of riparian buffer strips as movement corridors by forest birds. Conservation Biology 10: 1366-1379.
Mascanzoni, D. & Wallin, H. 1986. The harmonic radar: a new method for tracing insects in the field. Ecological Entomology 11: 387-390.
Matthysen, E., Adriaensen, F. & Dhondt, A.A. 1995. Dispersal distances of nuthatches, Sitta europaea, in a highly fragmented forest habitat. Oikos 72: 375-381.
Matthysen, E. & Currie, D. 1996. Habitat fragmentation reduces disperser success in juvenile nuthatches Sitta europaea: evidence from patterns of territory establishment. Ecography 19: 67-72.
McCollin, D. 1993. Avian distribution patterns in a fragmented wooded landscape (north-humberside, UK): the role of between-patch and within-patch structure. Global Ecology and Biogeography Letters 3: 48-62.
Miller, G.S., Small, R.J. & Meslow, E.C. 1997. Habitat selection by Spotted Owls during natal dispersal in western Oregon. Journal of Wildlife Management 61: 140-150.
Moen, R., Pastor, J. & Cohen, Y. 1997. Accuracy of GPS telemetry collar locations with differential correction. Journal of Wildlife Management 61: 530-539.
Morton, M.L. 1992. Effects of sex and birth data on premigration biology, migration schedules, return rates and natal dispersal in the Mountain White-crowned Sparrow. Condor 94: 117-133.
Morton, M.L. 1997. Natal and breeding dispersal in the Mountain White-crowned Sparrow Zonotrichia Leucophrys oriantha. Ardea 85: 145-154.
Nicholls, A.O. & Margules, C.R. 1991. The design of studies to demonstrate the biological importance of corridors. In: Saunders, D.A. & Hobbs, R.J. (eds) Nature Conservation 2: The role of corridors; Norton, New South Wales, Australia; Surrey Beatty and Sons: 49-61.
Opdam, P. 1991. Metapopulation theory and habitat fragmentation: A review of holarctic breeding bird studies. Landscape Ecology 5: 93-106.
Opdam, P., Foppen, R., Reijnen, R. & Schotman, A. 1995. The landscape ecological approach in bird conservation: integrating the metapopulation concept into spatial planning. Ibis 137: S139-S146.
Opdam, P., Rijsdijk, G. & Hustings, F. 1985. Bird communities in small woods in an agricultural landscape: effects of area and isolation. Biological Conservation 34: 333-352.
Opdam, P., van Dorp, D. & ter Braak, C.J.F. 1984. The effect of isolation on the number of woodland birds in small woods in the Netherlands. Journal of Biogeography 11: 473-478.
Petterson, B. 1985. Relative importance of habitat area, isolation and quality for the occurrence of middle spotted woodpecker Dendrocopos medius (L.) in Sweden. Holarctic Ecology 8: 53-58.
Plissner, J.H. & Gowaty, P.A. 1996. Patterns of natal dispersal, turnover and dispersal costs in Eastern Bluebirds. Animal Behaviour 51: 1307-1322.
Rail, J.-F., Darveau, M., Desrochers, A. & Huot, J. 1997. Territorial responses of boreal forest birds to habitat gaps. Condor 99: 976-980.
Rempel, R.S. & Rodgers, A.R. 1997. Effects of differential correction on accuracy of a GPS animal location system. Journal of Wildlife Management 61: 525-530.
Rempel, R.S., Rodgers, A.R. & Abraham, K.F. 1995. Performance of a GPS animal location system under boreal forest canopy. Journal of Wildlife Management 59: 543-551.
Robbins, C.S., Dawson, D.K. & Dowell, B.A. 1989. Habitat area requirements of breeding forest birds of the Middle Atlantic States. Wildlife Monographs 103: 1-34.
Robinson, S.K., Thompson, F.R., Donovan, T.M., Whitehead, D.R. & Faaborg, J. 1995. Regional forest fragmentation and the nesting success of migratory birds. Science 267: 1987-1990.
Roland, J., Mckinnon, G., Backhouse, C. & Taylor, P.D. 1996. Even smaller radar tags on insects. Nature 381: 120-120.
Saunders, D.A. & de Rebeira, C.P. 1991. Values of corridors to avian populations in a fragmented landscape. In: Saunders, D.A. & Hobbs, R.J. (eds) Nature conservation 2: the role of corridors; Chipping Norton, Australia; Surrey Beatty and Sons: 221-240.
Saunders, D.A., Hobbs, R.J. & Margules, C.R. 1991. Biological consequences of ecosystem fragmentation: a review. Conservation Biology 5: 18-32.
Schippers, P., Verboom, J., Knaapen, J.P. & van Apeldoorn, R.C. 1996. Dispersal and habitat connectivity in complex heterogeneous landscapes: An analysis with a GIS-based random walk model. Ecography 19: 97-106.
Schmiegelow, F.K.A., Machtans, C.S. & Hannon, S.J. 1997. Are boreal birds resilient to forest fragmentation? An experimental study of short-term community responses. Ecology 78: 1914-1932.
Sieving, K.E., Willson, M.F. & De Santo, T.L. 1996. Habitat barriers to movement of understory birds in fragmented south-temperate rainforest. Auk 113: 944-949.
Smith, J.N.M. 1974. The food searching behaviour of two European thrushes. I. Description and analysis of search paths. Behaviour 48: 276-300.
St. Clair, C.C., Bélisle, M., Desrochers, A. & Hannon, S.J. unpublished manuscript. Winter responses of forest birds to habitat corridors and gaps. Conservation Ecology (On-line)
Temple, S.A. 1991. The role of dispersal in the maintenance of bird populations in a fragmented landscape. Acta XX Congressus Internationalis Ornithologici 4: 2298-2305.
Thomlinson, J.R. 1995. Landscape characteristics associated with active and abandoned red-cockaded woodpecker clusters in east Texas. Wilson Bulletin 107: 603-614.
Turchin, P. 1991. Translating foraging movements in heterogeneous environments into the spatial distribution of foragers. Ecology 72: 1253-1266.
Turchin, P. 1996. Fractal analyses of animal movement: a critique. Ecology 77: 2086-2090.
Tyler, J.A. & Hargrove, W.W. 1997. Predicting spatial distribution of foragers over large resource landscapes: a modeling analysis of the Ideal Free Distribution. Oikos 79: 376-386.
van Dorp, D. & Opdam, P. 1987. Effects of patch size, isolation and regional abundance on forest bird communities. Landscape Ecology 1: 59-73.
Verboom, J., Schotman, A., Opdam, P. & Metz, J.A.J. 1991. European Nuthatch metapopulations in a fragmented agricultural landscape. Oikos 61: 149-156.
Villard, M.A., Merriam, G. & Maurer, B.A. 1995. Dynamics in subdivided populations of neotropical migratory birds in a fragmented temperate forest. Ecology 76: 27-40.
Wegner, J.F. & Merriam, G. 1979. Movements of birds and small mammals between a wood and adjoining farmland habitats. Journal of Applied Ecology 16: 349-357.
Whitcomb, R., Robbins, C.S., Lynch, J.F., Whitcomb, B.L., Klimkiewicz, M.K. & Bystrak, D. 1981. Effects of forest fragmentation on avifauna of the Eastern deciduous forest. In: Burgess, R.L. & Sharpe, D.M. (eds) Forest island dynamics in man-dominated landscapes; New York, N.Y., U.S.A.; Springer-Verlag: 125-205.
Wiens, J.A. 1989. Spatial scaling in ecology. Functional Ecology 3: 385-397.
Wiens, J.A. 1992. Ecological flows across landscape boundaries: a conceptual overview. In: Hansen, A.J. & di Castri, F. (ed) Landscape boundaries: consequences for biotic diversity and ecological flows; New York, N.Y., U.S.A.; Springer-Verlag: 217-235.
Wiens, J.A., Crist, T.O., With, K.A. & Milne, B.T. 1995. Fractal patterns of insect movement in microlandscape mosaics. Ecology 76: 663-666.
Wilcove, D.S. 1985. Nest predation in forest tracts and the decline of migratory songbirds. Ecology 66: 1211-1214.
Wilcove, D.S., McLellan, C.H. & Dobson, A.P. 1986. Habitat fragmentation in the temperate zone. In: Soulé, M.E. (ed.) Conservation biology: the science of scarcity and diversity; Sunderland, Mass., U.S.A.; Sinauer Associates: 237-256.
Zach, R. & Falls, J.B. 1976. Ovenbirds (Aves: Parulidae) hunting behavior in a patchy environment: an experimental study. Canadian Journal of Zoology 54: 1863-1879.
Zollner, P.A. & Lima, S.L. 1997. Landscape-level perceptual abilities in white-footed mice: perceptual range and the detection of forested habitat. Oikos 80: 51-60.
Table. 1. Strengths and weaknesses of approaches to studying dispersal through fragmented landscapes.

Table. 2. Homing success (counts in parentheses) of male birds relocated ca. 2 km away from their territory through fragmented (agricultural) and connected/continuous forests near Québec city, Canada, 1997 and 1998. Species included Black-capped Chickadees Poecile atricapillus, Black-throated blue Warblers Dendroica caerulescens and Ovenbirds Seiurus aurocapillus. Treatments were alternated through time within species.

Table 3. Winter abundance of Black-capped Chickadees in forest fragments isolated from the nearest neighboring fragment by greater than or less than 50 m. Chickadee abundance in each fragment was determined by the average number of birds to respond over three census dates to a constant-volume broadcast of a chickadee mobbing calls (audible for approximately 250 m). Data were collected from aspen- (Populus tremuloides) dominated forest fragments in northern Alberta in January through March, 1997.

Fig. 1. Movement paths of late-summer dispersing songbirds in wooded parts of a golf course near Québec city, Canada, August and September 1997. Shaded and clear areas depict open (lawn) and wooded areas, respectively. Letters 's' and 'e' refer to the start and end of paths, respectively. Path I: Black-throated green Warbler Dendroica virens, length: 1680 m, positions: 156, duration: 21 min. Path II: Red-eyed Vireo Vireo olivaceus, length: 5294 m, positions: 1468, duration: 79 min. Path III: Red-eyed Vireo, length: 2439 m, positions: 564, duration: 37 min. Data collected with a GPS receiver. Positions differentially corrected (accuracy ± 5 m) and map digitized from a corrected 1:5000 aerial photo.
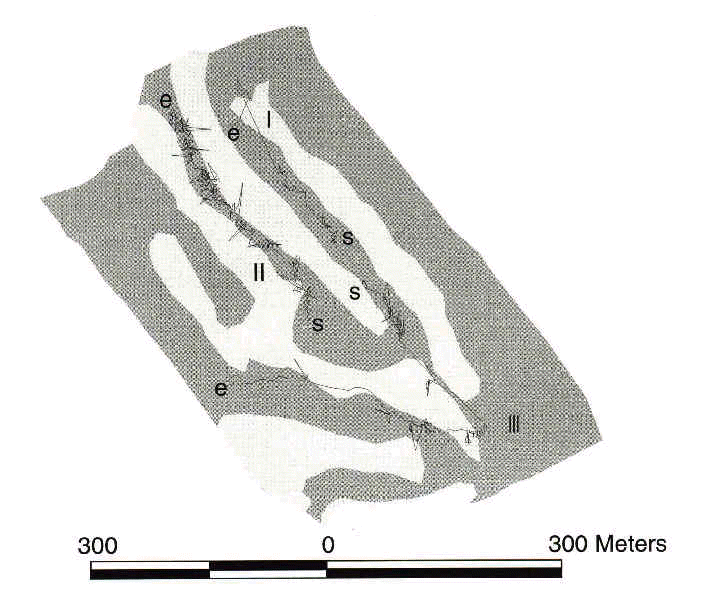
Fig. 2. Willingness of passerine birds to travel across open areas relative to forested areas. Panel A shows the probability of crossing gaps (filled circles and solid lines) or forested stands (stars and dashed line) of different widths during 5-minute periods during which a playback of Black-capped Chickadee mobbing calls was used to lure birds across gaps or forest. Panel B shows the probability that Black-capped Chickadees will take detours around open areas instead of flying directly to the playback, against the length of the detour relative to the short-cut through the open area. Each point represents >25 trials. Lines represent logistic regressions. Adapted from Desrochers & Hannon (Desrochers & Hannon 1997).
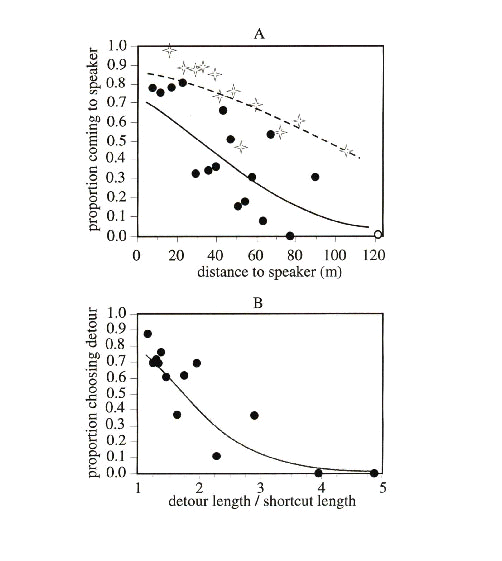
Fig. 3. Possible relationship between response to experimental isolation of forest patch and avoidance of open areas by five species of birds. Isolation responses were calculated as percent abundance change in response to two years of isolation by 200 m of clearcutting divided by percent abundance change in continuous sites (Schimegelow et al. 1997; Table 6). Reluctances to cross open areas were calculated by dividing probability of crossing a 50 m tract of forest by probability of crossing a 50 m open area during playback experiments (Desrochers & Hannon 1997). BCCH= Black-capped Chickadee, REVI= Red-eyed Vireo, RBNU=Red-breasted Nuthatch S. canadensis, YRWA=Yellow-rumped Warbler D. coronata. Kinglets are Regulus satrapa (behaviour data) and R. calendula (isolation data) and were pooled because of insufficient data. The line was calculated by linear regression.