S40.4: Adaptive specialisations of memory in birds
David F. Sherry
Department of Psychology, University of Western Ontario, London Ontario, Canada N6A 5C2, fax 519 661 3961, e-mail sherry@julian.uwo.ca
Sherry, D.F. 1999. Adaptive specialisations of memory in birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2394-2416. Johannesburg: BirdLife South Africa.Memory is a biological process like any other, subject to natural selection and exhibiting adaptive variation in its structure and organisation. Specialisations of memory have been described in birds, that are clearly related to behavioural and ecological specialisations in different species (Olson 1991; Olson et al. 1995). Memory in birds, and other animals, can also show specialisation within the same individual. That is, memory is not a unitary process but consists of multiple systems with different functions and different properties. This paper describes some of the available evidence for multiple memory systems in birds, in particular the evidence provided by lesion studies of the avian hippocampus. A possible adaptive explanation for the existence of multiple memory systems is discussed. Recent research on sex differences in the avian hippocampus further illustrates specialisations of memory that can result from selection for particular memory functions. The paper concludes with comments on whether avian memory systems can be considered innate.
MULTIPLE MEMORY SYSTEMS
One of the clearest demonstrations of the existence of multiple memory systems is the dissociation that can occur between different kinds of memory as a result of experimental disruption of brain function. If damage to part of the brain disrupts performance on one kind of memory task but leaves performance on another intact, this shows that these forms of memory can operate independently, and can be dissociated. The challenge for researchers is to design memory tasks so that changes in behaviour following brain lesions are informative. A large body of experimental work conducted with mammals - mostly primates and rodents - has identified memory systems that can be dissociated by lesioning or removing parts of the brain. Case studies of people who have become amnesiac following neurological damage have likewise identified dissociable memory systems in humans. In birds, multiple memory systems have also been described, and lesion studies of the avian hippocampal formation have succeeded in characterising one of these systems. This research shows that the avian hippocampal formation serves a central role in spatial memory, as assessed in a variety of behavioural tasks. Impairment of hippocampal function in birds, in contrast to the prevailing picture in research on the mammalian hippocampus, gives little indication that the avian hippocampus plays any role in memory for non-spatial information.
A question that is rarely asked in clinical work with people or in laboratory studies of rodents and primates is, why should memory be organised into multiple systems? Because research on memory in birds is often one component of a broader effort to understand the cause, function, and evolution of bird behaviour, studies of birds have provided a number of functional and evolutionary hypotheses to account for such features of cognition as the organisation of memory into multiple systems. One evolutionary hypothesis for the existence of multiple memory systems is that memory consists of specialised systems serving specific circumscribed functions. Not only are these functions different, but they are also functionally incompatible. That is, multiple memory systems may have evolved because the functional demands of memory are such that a single system of broad domain could not, in practice, simultaneously serve multiple competing functions.
MEMORY AND THE AVIAN HIPPOCAMPUS
Food-storing birdsFood-storing birds, like black-capped chickadees Parus atricapillus, depend on memory to retrieve scattered caches of food. These birds create thousands of food caches, mainly in fall and winter, and can remember the locations of individual caches for at least several weeks [for reviews see Shettleworth (1995), Sherry & Duff (1996)]. Chickadees with surgical lesions of the hippocampus continue to store food and to search for their caches but their success at finding caches falls to the chance level (Sherry & Vaccarino 1989). Analysis of what is disrupted in cache retrieval shows a dissociation between memory for spatial locations and memory of other kinds (Sherry & Vaccarino 1989; Hampton & Shettleworth 1996).
The first indication that lesions to the hippocampus disrupt the retrieval of caches was work by Krushinskaya on the Eurasian nutcracker Nucifraga caryocatactes (Krushinskaya 1966). Nutcrackers with lesions to the hippocampal region made as many caches as control birds, and later searched for their caches, but in general failed to find them. Control birds with lesions of the anterior hyperstriatum accessorium, lesions of the neostriatum caudale, or no lesions at all, successfully recovered 70 - 90% of their caches.
In black-capped chickadees, lesions of the hippocampus have comparable effects (Sherry & Vaccarino 1989). Chickadees were allowed to cache and recover seeds in an indoor aviary containing tree branches drilled with small holes to serve as cache sites. Five trials, that each consisted of caching seeds and searching for them 3 h later, were performed to obtain baseline data on the accuracy of chickadees’ memory for cache sites. Birds were then assigned to three groups and received either lesions of the hippocampal region, lesions of the hyperstriatum accessorium, or served as unoperated controls. Birds were allowed to recover from surgery and then were given five more caching and recovery trials. Chickadees with hippocampal lesions, like nutcrackers, cached seeds and later searched for them, but failed to search at the correct sites. Chickadees with hippocampal lesions established as many caches and made as many search attempts as birds in the other groups. Their search attempts were simply at the wrong locations.
How does destruction of the hippocampus affect search for cache sites in these birds, and is the primary effect a disruption of memory? Subsequent research has examined the general question of the function of the avian hippocampus by training birds, some of them food-storing birds, to perform tasks with different memory requirements and then observing the effects of hippocampal lesions. Properly designed, these tasks can help determine whether or not the effects of hippocampal damage are specific to memory and more importantly, what kind of memory is compromised by hippocampal damage.
In the experiment on cache recovery by black-capped chickadees described above, behaviour was normal in many respects. Lesioned birds continued to make caches, and observations showed that they ate and flew normally and searched for caches at the normal rate. It is thus unlikely that a general impairment in motivation, perception or motor performance was the cause of their failure to find caches, though more subtle effects on behaviour are still possible. To more directly address the effect of hippocampal damage on memory, chickadees were trained on two non-caching tasks, one that explicitly involved memory for dispersed spatial locations and one that did not. In the 'Place' task, birds had to learn which six locations, out of a possible 72, were baited with food. The experiment was carried out in the same indoor aviary as the previous experiment on cache retrieval, and the apparatus consisted of the same set of six tree branches, each drilled with 12 small holes that could hold food or be used as cache sites. In the Place task, the same six holes always held a fragment of sunflower seed and birds rapidly reached the criterion level of performance of correctly locating 3 or more food sites in their first six choices for 5 consecutive trials. This task tested memory for spatial locations. It differs somewhat from the cache recovery problem in that it requires memory for the same small set of familiar locations on all trials, rather than for a larger number of changing cache sites, but the results described below show that this does not affect interpretation of the effects of hippocampal lesions.
Another group of birds learned a different memory task. In this task six out of a possible 72 sites also contained food, but the sites changed on every trial. The presence of food was indicated by a small card placed at each of the 72 sites. The six sites containing food had a black card and the remainder had a white card (the colour relations were counter-balanced across subjects). On this 'Cue' task birds did not have to remember spatial locations - the spatial location of food changed on every trial and was not informative - but instead had to remember the association between the colour of cards and the presence of food. Birds learned this task to the same criterion as the Place task, though the Cue task was more difficult and required more trials to reach criterion (a mean of 14.0 trials for the Cue task versus 7.2 trials for the Place task).
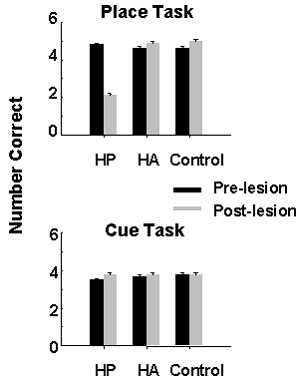
Hippocampal lesions had very different effects on performance of the two tasks: performance on the Place task decreased significantly following hippocampal lesioning, while performance on the Cue task was unaffected. Lesions of the hyperstriatum accessorium had no detectable effect on the performance of either task (Fig. 1).
This experiment shows that following hippocampal lesioning, chickadees that had learned the Place task had a deficit in their ability to remember which holes contained food, even though the six sites were highly familiar and the task had been learned more quickly than the Cue task. Interestingly, the performance of these birds remained above the chance level, though much reduced compared to their pre-lesion performance or the performance of birds in other groups. Birds performing the Cue task, which does not involve memory for spatial locations, showed no impairment in performance following lesioning, indicating that an intact hippocampus is not necessary for performance of this task. These results show a clear dissociation between the cognitive processes required for performance of the Place and Cue tasks. Hippocampal damage disrupts the Place task but not the Cue task, and an unimpaired ability to perform the Cue task does not help with performance of the Place task. Disruption of memory for spatial locations is thus the most likely explanation for the inability, described earlier, of chickadees and nutcrackers to find their caches following hippocampal lesions. Furthermore, the effects of hippocampal lesions appear similar whether the birds are searching for unique cache sites established 3 h earlier, or searching for a small set of well-learned food sites.
Birds that had learned the Place and Cue tasks showed a further effect of hippocampal lesions when performance of the birds was analysed beyond their initial six search attempts. In most trials, birds continued to search for the full 5 min duration of the trial and the behaviour of hippocampally-lesioned birds showed a striking pattern (Fig. 2). Lesioned birds made large numbers of errors that consisted of revisiting sites that they had already searched. These revisits were to both baited and unbaited sites and occurred on both the Place and Cue tasks. Birds with hyperstriatum accessorium lesions and non-lesioned birds made almost no revisiting errors (Fig. 2).
It is not surprising that revisiting errors occur on the Place task, because the basic result of the experiment was that hippocampally-lesioned birds had a specific problem remembering spatial locations. On the Cue task we expected that performance would be less affected by a purely spatial impairment because the cued sites were always on different, visually-distinct tree branches. We proposed that hippocampal lesions, in addition to any effect on spatial memory, also have a disruptive effect on working memory akin to that reported in rats following hippocampal lesions (Olton et al. 1979; Jarrard 1983). 'Working memory' is defined as memory for information relevant to the current performance of a task, in contrast to 'reference memory' which is memory for information common to all performances of a task (Honig 1978). Chickadees with hippocampal lesions appeared to have a working memory impairment that caused them to revisit sites they had already searched on the current trial, something they normally avoid doing.
The difficulty with this interpretation is that baited food sites in both the Place and Cue tasks were locations in space. If lesioned birds suffered a complete disruption of spatial memory that affected not only their ability to relocate well-learned spatial locations, but also their ability to remember any spatial locations they had visited during the current trial, then spatial deficits alone might explain the working memory errors observed. Hampton & Shettleworth (1996) tested this possibility using a touch screen to present chickadees with two types of memory problems.
A touch screen displays programmed stimuli on a computer monitor and records where on the monitor an animal makes a response by touching or pecking the screen. Spatial tasks can be designed that require the animal to view a stimulus on the screen and then indicate, after the stimulus has gone off, where the stimulus was. Non-spatial tasks can also be designed that use the touch screen to record the response of the animal. Black-capped chickadees were trained to perform a spatial location matching task and a colour matching task on a touch screen. In the spatial task, a white square appeared in one of 20 possible locations on the monitor, the bird was required to peck it 5 times, whereupon the stimulus went off. Five seconds later two squares appeared, one in the same location as the previous stimulus and one in a different location. Five pecks to the square in the original location resulted in access to food. Pecks in the other location initiated the next trial. In the colour task, a circle in one of 14 possible colours appeared on the screen, the bird was required to peck it 5 times, and then the stimulus went off. After 5 seconds two circles appeared, placed symmetrically around the previous location of the sample colour. One circle matched the original stimulus in colour while the other did not, and pecking the matching colour produced access to food while pecking the other colour initiated the next trial. Both tasks are thus working memory tasks because they required memory for the nature of a sample stimulus presented 5 s previously. Successive trials had samples in different locations, or in different colours, so accurate performance required memory for the most recently presented sample.
Once birds were performing both tasks to criterion they received hippocampal lesions, and after recovery, were tested again on the touch screen. There was a significant decrease in performance of the spatial location matching task but not the colour matching task (Fig. 3). That is, in two tasks both requiring working memory, a deficit was observed when the stimulus to be remembered was a spatial location, but not when the stimulus to be remembered was a colour. This result shows that working memory in general is not impaired following hippocampal lesioning in chickadees: the deficits that do occur are restricted to memory for spatial information. Dark-eyed juncos Junco hyemalis were also observed in this study, and the results obtained with these birds were very similar to those obtained with chickadees (Fig. 3). It seems likely that spatial memory is selectively disrupted by hippocampal lesions in both chickadees and juncos and can be dissociated from memory for colours and from working memory (Hampton & Shettleworth 1996)
Pigeons
Research with domestic pigeons Columba livia has further specified the memory deficit that follows hippocampal lesions in birds. [See Bingman et al. (1995) for review of research on the effects of hippocampal lesions on navigation by homing pigeons.] In the laboratory, pigeons with hippocampal lesions showed no deficit in a colour matching task similar to that described above, across a range of delays between presentation of the sample and the test stimuli (Good & Macphail 1994). This result indicates that working memory for colours is intact in pigeons with hippocampal lesions. A similar result was obtained using a successive delayed non-matching procedure with trial unique stimuli. For this task, subjects viewed sequences of photographs of scenes and objects. Each sequence consisted of four photographs, each presented twice. The first time a photograph was presented, pecking a key produced a food reward. The second time the photograph appeared no food reward was available and birds had to refrain from pecking the key. The second repetition of a photograph sometimes followed immediately after the first, sometimes after the intervening presentation of another photograph. Once each photograph had been presented twice, a similar sequence of four new photographs was presented. The use of photographs as stimuli makes it possible to present large numbers of unique stimuli in experimental sessions. This is a 'non-matching' task because only responses to stimuli that do not match anything seen previously are correct. The results showed that hippocampal lesions had no effect on performance of this task, over retention intervals ranging from 10 - 30 s between the presentation of successive photographs (Good & Macphail 1994; Fig. 4).
Finally, Good & Macphail (1994) carried out a spatial matching to sample task in which birds were required to respond by pecking a key in the same location as the sample that they had previously viewed, and found a significant impairment in pigeons with hippocampal lesions across a range of delays from 2 - 10 s (Fig. 5).
These results with pigeons, like those with black-capped chickadees and dark-eyed juncos, provide evidence of a dissociation between spatial memory and memory of other kinds following hippocampal lesions. The tasks used were designed to be as similar as possible in most respects, but to differ in their requirement for spatial information processing. Comparable experiments with pigeons have been carried out by Colombo et al. (1997a; 1997b). They, too, contrasted the performance of a spatial task with performance of non-spatial tasks by birds with hippocampal lesions. The non-spatial tasks were delayed matching to sample and concurrent discrimination. The delayed matching to sample results are similar to those reported for pigeons by Good & Macphail (1996), that is, there was no evidence that birds with hippocampal lesions performed differently from intact controls on this non-spatial task.
For the concurrent discrimination task, birds viewed six pairs of photographs of everyday objects. One member of each pair was designated as positive and pecking that slide produced a food reward. Pecking the other, negative, member of the pair turned off all stimuli and initiated the next trial. The designation of one member of the pair as positive was made arbitrarily by the experimenter and the pigeon had to determine over successive trials which member of each pair was positive and which was negative. The task thus involves discrimination - distinguishing one member of the pair from another - and was concurrent because the discrimination was being made for six pairs at the same time. After mastering the first six pairs, birds were trained on six more pairs, and then on a further six pairs.
Hippocampal lesions had no effect on the rate at which pigeons achieved a criterion level of performance of 88% correct for two successive experimental sessions (Fig. 6). The effects of hippocampal lesions on performance of the spatial task were quite different. The apparatus for this task consisted of eight cups, each containing 2 dried peas, placed on a 1 X 2 m table. The task was to collect food from each cup, and birds could increase their rate of collecting food by avoiding revisits to previously visited cups. Pigeons with hippocampal lesions eventually learned the task to a criterion of one session with no more than one revisiting error, but took a mean of 35 training sessions to do so, compared to a mean of 7 for control birds. Thus, on a search task with a spatial component, hippocampal damage impaired performance (Fig. 7).
The results of these studies with food-storing birds and pigeons show a clear dissociation between memory for spatial locations and memory of other kinds. Hippocampal lesions had no effect on memory for a simple association between food and a cue, for colours presented on a touch screen, for colours on two different key peck tasks, for photographs in a delayed successive non-matching task, or for photographs in a concurrent discrimination task. In contrast, hippocampal lesions disrupted memory for cache sites, for places in an aviary, for locations on a touch screen, for spatial locations in a key peck task, and for search among spatially-dispersed feeders.
THE FUNCTION AND EVOLUTION OF MULTIPLE MEMORY SYSTEMS
Dissociations of the kind described above, between spatial memory and memory of other kinds, have been a key to understanding the organisation of memory in animals and humans. Damage to the medial temporal lobe, in particular to the hippocampal region, produces a profound amnesia in humans, but the effect is restricted to only some types of memory. Conscious recollection of recently occurring events, sometimes called 'declarative memory', is severely disrupted in these people, while memory for events more remote in time, intellectual functioning, cognitive and motor skills, classical conditioning, and some other memory processes are not disrupted (Squire & Zola 1997). Research with neuropsychological patients, as well as dissociations in memory that can be observed in laboratory tasks with normal participants, have led to the view that memory consists of multiple systems serving distinct functions (Schacter 1996).
From an evolutionary point of view, the important questions is, why should memory be organised in this way, rather than as a single integrated memory system of broad domain? One proposal is that multiple memory systems evolve when there are different functionally incompatible demands on memory (Sherry & Schacter 1987). Memory cannot, for example, both preserve the details of unique events and ignore the details of unique events in order to extract the general patterns from experience. Preserving unique detail and ignoring unique detail are logically and functionally incompatible. We suggested that multiple systems evolve in the presence of such incompatible and competing functions.
Instances of multiple systems occur in other cognitive domains. Vision is particularly rich in examples. The compound eyes and ocelli of flying insects both play important roles in the control of flight. These anatomically separate visual systems serve different functions (Chapman 1982). The ocelli have poor spatial resolution because the focal plane of the ocellus lies behind the retina, producing an image that is out of focus. The ocelli are very sensitive to changes in level of illumination, however, even at low light levels, and convey signals rapidly to the nervous system using large neurons and a small number of integrative steps in the neural path (Wilson 1978). Compound eyes, on the other hand, are capable of finer spatial resolution and pattern perception but respond more slowly than ocelli and are less sensitive to changes in average illumination (Taylor 1981). The rapid response of the ocelli and their sensitivity to change in light level makes them particularly important in the control of roll and pitch during flight, both of which can cause the visual field of an ocellus to dip below the horizon and trigger a corrective flight response. The greater spatial resolution of the compound eyes, achieved at the cost of a slower response, contributes to the control of flight by detecting pattern and shape in the visual world. Rapid averaging of light intensity across the visual field - as performed by the ocelli - and detecting pattern in the visual field - as performed by the compound eyes - are functionally incompatible. They cannot both be carried out simultaneously. The problem has been solved in flying insects with two visual systems, each serving a different function. In humans and other primates, separate visual systems have likewise been identified for object identification versus object location and for perception versus motor control (Goodale & Milner 1992).
Like vision, memory also consists of multiple systems serving separate incompatible functions. This can be illustrated by comparing memory in two contexts that have been well described in birds, memory for cache sites in food-storing birds and memory for song in oscines. Song learning has a number of distinctive features including sensitive periods for learning and selectivity in which songs are acquired and retained from among the range of songs heard. Memory for cache sites shows no indication of sensitive periods and no known selectivity in the kinds of cache sites that can be remembered. Indeed, the properties of a sensitive period and selectivity, which make functional sense in the case of song learning, seem nonsensical in the case of memory for cache sites. The role of song in seasonal breeding and its acquisition by juveniles after hearing conspecific song provide functional reasons for sensitive periods and selectivity in learning. The functional requirements of food storing are very different and while these place a premium on large-capacity long-duration spatial memory, sensitive periods or the kind of selectivity found in song learning would seem only to reduce the effectiveness of memory for cache sites. Different memory systems, rather than a single unified system, occur in these different behavioural contexts because the functional requirements of memory in each case are mutually incompatible.
SEX DIFFERENCES IN MEMORY
In addition to multiple memory systems co-existing within the same nervous system, differences in memory between individuals can also provide some insight into the adaptive specialisation of memory. Sex differences in memory, and in spatial memory in particular, are well-known in animals and humans (Williams et al. 1990; Voyer et al. 1995; Sherry & Hampson 1997). There is debate about the developmental origins of such sex differences, particularly for humans, but the differences themselves are consistently observed. In birds there are a variety of ecological contexts in which sex differences in memory might be expected to evolve. Among the clearest are the sex differences in behaviour that occur in brood-parasitic cowbirds. Female brown-headed cowbirds Molothrus ater search for potential host nests unassisted by males. Research on the brood parasitic cowbirds has not compared memory between the sexes directly, but has instead examined the hippocampus itself, in a search for sex differences that might be meaningfully correlated with sex differences in behaviour.
Brown-headed cowbirds are obligate brood parasites. They exploit over 200 species of host, primarily forest- and forest edge-nesting passerines. Females produce about 40 eggs in a breeding season and search assiduously for host nests in which to lay their eggs. Females have been observed sitting in the canopy and watching nest building activity by potential hosts, walking silently on the forest floor scanning the canopy, and flying noisily into understorey shrubs, presumably to flush incubating hosts (Norman & Robertson 1975). Females lay at or before dawn and thus have little time to search for a host nest immediately prior to laying. Instead, females probably lay in a nest that they have located on a previous day. Female cowbirds search for host nests during the morning, following laying, then join males to feed in grain fields and livestock yards some distance from the forest and edge habitats where potential host nests are most likely to be found. Females are somewhat selective about the host nests in which they will lay (King 1979). Females prefer a completed nest with at least one but not more than two host eggs. This preference makes some adaptive sense. Laying in a nest not yet completed may cause the host to build the cowbird egg into the nest or to abandon the nest entirely. Laying in a nest with a completed clutch may result in the host’s young hatching and fledging before the cowbird nestling does. There is no indication in brown-headed cowbirds that males assist females in search for host nests.
It is likely that females hold in memory the locations of some number of potential host nests, at the appropriate stage of nest completion, for periods of at least several days in order to locate nests quickly when laying. Female cowbirds thus experience demands on spatial memory for host nests that they do not share with males. Males are not without spatial memory: they search for food, undergo migratory movements, perhaps hold in memory detailed representations of their local habitat. Females experience these demands on memory, too, but have, in addition, a further requirement to locate and remember the locations of potential host nests. It seemed likely that this sex difference in spatial behaviour might have associated with it a sex difference in the part of the brain known to play a role in spatial memory, the hippocampus.
We compared the relative size of the hippocampus in male and female brown-headed cowbirds and two other closely related icterids, red-winged blackbirds and common grackles (Sherry et al. 1993). Red-winged blackbirds and common grackles are in the same icterid tribe as brown-headed cowbirds, the Agelaiini, and share many aspects of diet, migration, and morphology with cowbirds. They differ, however, in breeding behaviour. Red-winged blackbirds breed polygynously in dense colonies while common grackles breed in loose colonial aggregations.
We determined the size of the hippocampus, relative to the size of the forebrain, in males and females of all three species and found a significant sex difference, in favour of females, in cowbirds. There was no sex difference in the size of the hippocampus in red-winged blackbirds, and although the sample size was quite small, no indication of a sex difference in common grackles either (Fig. 8).
Thus in the species in which a sex difference in spatial behaviour is known to occur, and a sex difference in spatial memory may occur, a sex difference in the predicted direction is found in the relative size of the hippocampus. One unusual feature of this sex difference is that it favours females rather than males, in contrast to nearly all previously described sex differences in spatial memory, spatial ability, and the hippocampus (Jacobs et al. 1990; Williams et al. 1990; Gaulin 1992; Sherry et al. 1992; Kimura & Hampson 1993; Gaulin 1995; Hampson 1995; Voyer et al. 1995; Galea et al. 1996).
There is variation in the breeding systems of cowbirds in the genus Molothrus. The bay-winged cowbird M. badius, for example, is not a brood parasite but breeds monogamously, incubates its own eggs and raises its own young (Fraga 1991). The screaming cowbird M. rufoaxillaris is a brood parasite that specializes on one host, the non-parasitic bay-winged cowbird. In contrast to brown-headed cowbirds, male and female screaming cowbirds search together for bay-winged cowbird nests (Mason 1987), which might be expected to eliminate any sex-specific selection on the hippocampus for spatial memory. Then there are others, like the generalist shiny cowbird (M. bonariensis) that are more similar to the brown-headed cowbird. Female shiny cowbirds parasitise over 150 species of host, and search for host nests without male assistance (Mason 1987).
Males and females of all three species were collected in Argentina during the breeding season by Reboreda et al. (1996). Their predictions were that the sex difference in search for host nests in the generalist shiny cowbird would select for a sex difference in the hippocampus similar to that found in brown-headed cowbirds, while search for host nests by both sexes of screaming cowbirds would produce no sex difference. The absence of search for host nests in the bay-winged cowbird would, likewise, result in no sex difference in the hippocampus. These predictions were confirmed, as shown in Figure 9.
The hippocampus was larger, relative to the size of the forebrain, in the two brood parasites compared to the non-parasite, suggesting that brood parasitism does, on average, result in increased selection for spatial ability. There was a significant sex difference, in favour of females, only in the shiny cowbird, however, and none in either of the other species. These results are particularly persuasive evidence that selection acting on very similar genetic and neural backgrounds can produce sex differences in that region of the brain known to be specifically involved in spatial memory in birds.
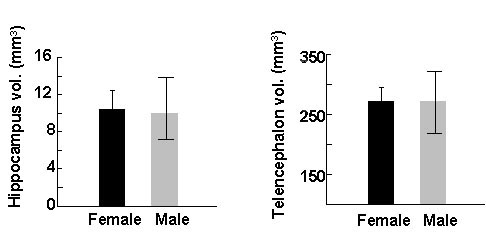
It might be argued that such correlations between sex differences in behaviour and sex differences in the brain are not a particularly surprising outcome. Sex differences of one kind or another in behaviour and the brain are not uncommon and producing a post hoc evolutionary explanation for their correlation may be relatively easy. To test this account of sex differences in the hippocampus, Karin Petersen and I looked for a species of bird in which there is no ecological or behavioural reason to predict a sex difference in spatial ability or spatial memory, a search which brought us back to food-storing birds (Petersen & Sherry 1996). Male and female black-capped chickadees both store and retrieve food, have the same diet, defend the breeding and winter flock territories, feed nestlings and fledglings, and are obviously found in the same habitat. They differ in some aspects of behaviour such as dominance, and of course differ in reproductive physiology. Nevertheless, we were unable to identify any ecological or behavioural difference between the sexes in black-capped chickadees that would predict a sex difference in spatial memory or the hippocampus. Observations of birds in captivity confirmed that there was no sex difference in food storing or in the accuracy of memory for cache sites. No sex difference in the relative size of the hippocampus was found in these birds either (Fig. 10), indicating that sex differences in the hippocampus do not occur as a matter of course and are not found in the absence of behavioural or ecological differences between the sexes in spatial behaviour.
Sex differences in the avian hippocampus, like the organisation of memory into multiple systems, can be understood as the evolutionary outcome of natural selection acting on memory. Differences in behaviour between the sexes expose them to different selective regimes with different functional demands on memory (Jacobs & Spencer 1994; Gaulin 1995; Sherry & Hampson 1997). The effects of sex-specific selection on memory can be seen in sex differences like those described in the hippocampus of brood-parasitic cowbirds.
INNATE PROPERTIES OF MEMORY IN BIRDS
Memory systems are clearly something different from the content or representations held in memory. The content of memory is the result of individual experience. In birds, memory for songs, stellar orientation cues, mates, offspring, predators, territory boundaries, cache sites, and many other things depends on experience and varies among individuals. Memory systems, on the other hand, are the cognitive processes by which information is acquired, retained, and made available to guide behaviour. In the foregoing discussion we have seen how these systems can be dissociated by neural intervention and how they can differ between the sexes. We have also seen that memory systems have specialised restricted functions, at least in the case of the spatial memory system of the avian hippocampus. Memory systems permit birds to obtain information from the environment, and seem unlikely to result from experience in the same way that the information held in memory does. Are memory systems, therefore, innate?
The answer to this question depends on exactly what is meant by 'innate'. A reasonable working definition of an innate trait is one that is highly probable given normal developmental experience. Food-storing chickadees and tits have a much larger hippocampus than passerines that do not store food (Krebs et al. 1989; Sherry et al. 1989). This large hippocampus is, in part, a consequence of food-storing experience (Clayton & Krebs 1994; Clayton 1995). But food-storing experience is part of normal development and the resulting increase in hippocampal size is thus a highly probable outcome in these birds. Innate traits, by this definition, may be experience-dependent and plastic, although the final outcome is nevertheless highly probable, and exhibits little variation among individuals.
Recent work on connectionist models of human cognition suggest that such outcomes, though highly probable and shared with few exceptions by all members of a species, can develop despite very loose specifications of the initial structure of the nervous system (Elman et al. 1996). That is, innate behaviour or innate properties of memory and cognition can occur, at least in theory, without there being very much that is innate in the sense of being hard-wired or specified beforehand in the nervous system.
Connectionist models are attempts to simulate cognitive processes using inter-connected networks of elements with simple neuron-like properties. The elements, or nodes, of the network can vary in their state of activation, and are connected to each other (hence 'connectionist') in a way that is loosely analogous to synaptic connections among neurons. The influence one node in the network has on other nodes can be modified, along with its state, in the course of the simulation. Networks of this kind can be implemented in a computer program and exposed to input of various kinds. The behaviour of the network can be examined for its dynamic responses to input and its final steady state.
From the connectionist perspective adopted by Elman et al. (1996), 'innate' describes consistent re-occurring patterns in a simple network. Information is not explicitly represented in the network initially, and the architecture of the network is usually specified in a very generalised way. Nevertheless, outcomes are observed in models of imprinting in birds (O'Reilly & Johnson 1994) and language learning in children (Plunkett & Marchman 1991), for example, that satisfy our working definition of innate: highly probable given normal developmental experience. It is this kind of result that leads Elman et al. (1996) to conclude that normal patterns of experience and learning, especially normal patterns in the sequential timing of experience, rather than innate information or innate cognitive architecture, may be the key to observed innateness in animal and human cognition. Although connectionist models are models, not nervous systems, and the changes they undergo are simulations, not development, they can be a useful source of hypotheses about cognitive development.
This is some way from the idea of innate as understood by Konrad Lorenz and attacked by Daniel Lehrman in their influential debate over the importance of nature and nurture in animal behaviour (Lorenz 1965; Lehrman 1970). Lorenz equated innate with genetically specified, evolutionarily adaptive, hard-wired in the nervous system, and resistant to experience. Lehrman argued that few aspects of behaviour, if any, are truly impervious to the effects of experience. Interestingly, most of the research results used by Lorenz and Lehrman to support their positions came from the study of bird behaviour. It is clear that Lorenz felt something important had been lost in the theoretical rapprochement that occurred between those he calls the 'English speaking ethologists' and the 'American behaviourists' on the subject of innateness. He refers often to 'what we formerly called innate', attempting to refine an elusive biological concept that he felt was not adequately captured by the idea that all behaviour is the result of an interaction between genotype and environment. The hypothesis derived from connectionist models, that innate behaviour and cognitive organisation are the outcome of normal developmental experience, rather than the antithesis of developmental experience, may be a step toward coming to grips with 'what we formerly called innate'.
Whether memory systems in birds require appropriate experience to develop normally, and exactly how experience might affect the development of memory systems, are questions that await further research. It may seem unlikely that the specialised functions of the hippocampal memory system, and its apparent independence from memory of other kinds, is itself the result of experience, but there is little research that directly addresses this question. The data that are available provide evidence for a good deal of plasticity, but something well short of completely open-ended plasticity (Clayton & Krebs 1994).
CONCLUSIONSRecent research on spatial memory and the hippocampus in food-storing birds and domestic pigeons shows that spatial memory can be dissociated from memory of other kinds by hippocampal damage. This provides good evidence that memory is not a unitary process, but is organised into multiple systems that, at least in this case, can function independently of each other. In contrast to results observed in humans, non-human primates, and rodents, there is presently little evidence that the hippocampus of birds plays a role in memory of any kind except spatial memory. Whether this is an accurate assessment of the role of the avian hippocampus in memory can only be established by future research directed specifically at examining non-spatial memory functions of the hippocampus. Sex differences in hippocampal size in brood-parasitic cowbirds reveal the effect of differential selection for spatial memory on the two sexes. Such sex differences are absent in cowbirds and other species in which there is no sex-specific selection for spatial memory. Whether adaptively specialised memory systems are innate or not depends on exactly how the term ' innate' is interpreted. It is clear that the avian brain possesses specialised cognitive and neural architecture to handle functional problems like spatial memory. The extent to which adaptively specialised memory systems are structured by input in the course of normal development is a problem that can only be answered by future research.
ACKNOWLEDGEMENTS
I would like to thank Michael Boisvert and Peter Cain for their helpful comments on the manuscript. Preparation of this article and the author’s research were supported by the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
Bingman, V.P., Jones , T.-J., Strasser, R., Gagliardo, A. & Ioalé, P.1995. Homing pigeons, hippocampus and spatial cognition. In: Alleva, E., Fasolo, A., Lipp, H.-P., Nadel, L. & Ricceri, L. (eds) Behavioural brain research in naturalistic and semi-naturalistic settings (Proceedings of the NATO Advanced Study Institute, Acquafredda di Maratea, Italy, Sept 10-20, 1994). Dordrecht; Kluwer Academic Publishers: 207-223.
Chapman, R.F. 1982. The insects: structure and function. London; Hodder and Stoughton.
Clayton, N.S. 1995. The neuroethological development of food-storing memory: a case of use it, or lose it! Behavioural Brain Research 70: 95-102.
Clayton, N.S. & Krebs, J.R. 1994. Hippocampal growth and attrition in birds affected by experience. Proceedings of the National Academy of Science USA 91: 7410-7414.
Colombo, M., Cawley, S. & Broadbent, N. 1997a. The effects of hippocampal and area parahippocampalis lesions in pigeons: II. Concurrent discrimination and spatial memory. Quarterly Journal of Experimental Psychology 50B: 172-189.
Colombo, M., Swain, N., Harper, D. & Alsop, B. 1997b. The effects of hippocampal and area parahippocampalis lesions in pigeons: I. Delayed matching to sample. Quarterly Journal of Experimental Psychology 50B: 149-171.
Elman, J.L., Bates, E.A., Johnson, M.H., Karmiloff-Smith, A., Parisi, D. & Plunkett, K. 1996. Rethinking innateness: a connectionist perspective on development. Cambridge MA; MIT Press: 447 pp.
Fraga, R. M. 1991. The social system of a communal breeder, the bay-winged cowbird, Molothrus badius. Ethology 89: 195-210.
Galea, L.A.M., Kavaliers, M. & Ossenkopp, K.-P. 1996. Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. Journal of Experimental Biology 199: 195-200.
Gaulin, S.J.C. 1992. Evolution of sex differences in spatial ability. Yearbook of Physical Anthropology 35: 125-151.
Gaulin, S.J.C. 1995. Does evolutionary theory predict sex differences in the brain? In: Gazzaniga, M. S. (ed.) The Cognitive Neurosciences. Cambridge MA; MIT Press: 1211-1225.
Good, M. & Macphail, E.M. 1994. The avian hippocampus and short-term memory for spatial and non-spatial information. Quarterly Journal of Experimental Psychology 47B: 293-317.
Goodale, M.A. & Milner, A.D. 1992. Separate visual pathways for perception and action. Trends in Neurosciences 15: 20-25.
Hampson, E. 1995. Spatial cognition in humans: possible modulation by androgens and estrogens. Journal of Psychiatry & Neuroscience 20: 397-404.
Hampton, R.R. & Shettleworth, S.J. 1996. Hippocampal lesions impair memory for location but not color in passerine birds. Behavioral Neuroscience 110: 831-835.
Honig, W. K. 1978. Studies of working memory in the pigeon. In: Hulse, S. H., Fowler, H. & Honig, W. K. (eds) Cognitive processes in animal behavior. Hillsdale NJ; Lawrence Erlbaum: 211-248.
Jacobs, L.F., Gaulin, S.J.C., Sherry, D.F. & Hoffman, G.E. 1990. Evolution of spatial cognition: sex-specific patterns of spatial behavior predict hippocampal size. Proceedings of the National Academy of Science USA 87: 6349-6352.
Jacobs, L.F. & Spencer, W.D. 1994. Natural space-use patterns and hippocampal size in kangaroo rats. Brain Behavior and Evolution 44: 125-132.
Jarrard, L.E. 1983. Selective hippocampal lesions and behavior: effects of kainic acid lesions on performance of place and cue tasks. Behavioral Neuroscience 97: 873-889.
Kimura, D. & Hampson, E. 1993. Neural and hormonal mechanisms mediating sex differences in cognition. In: Vernon, P. A. (ed) Biological approaches to the study of human intelligence. New Jersey; Ablex: 375-397.
King, A.P. 1979. Variables affecting parasitism in the North American cowbird (Molothrus ater). Ph.D. Dissertation. Ithaca NY: Cornell University.
Krebs, J.R., Sherry, D.F., Healy, S.D., Perry, V.H. & Vaccarino, A.L. 1989. Hippocampal specialisation of food-storing birds. Proceedings of the National Academy of Science USA 86: 1388-1392.
Krushinskaya, N.L. 1966. Some complex forms of feeding behaviour of nut-cracker Nucifraga caryocatactes, after removal of old cortex. Zhurnal Evoluzionni Biochimii y Fisiloggia II: 563-568.
Lehrman, D.S. 1970. Semantic and conceptual issues in the nature-nurture problem. In: Aronson, L. E., Tobach, E., Lehrman, D. S. & Rosenblatt, J.S. (eds) Development and Evolution of Behavior. San Francisco; W.H. Freeman: 17-52.
Lorenz, K. 1965. Evolution and modification of behavior. Chicago; University of Chicago Press: 121 pp.
Mason, P. 1987. Pair formation in cowbirds: evidence found for screaming but not shiny cowbirds. Condor 89: 349-356.
Norman, R.F. & Robertson, R.J. 1975. Nest-searching behavior in the brown-headed cowbird. Auk 92: 610-611.
Olson, D.J. 1991. Species differences in spatial memory among Clark's nutcrackers, scrub jays and pigeons. Journal of Experimental Psychology: Animal Behavior Processes 17: 363-376.
Olson, D.J., Kamil, A.C., Balda, R.P. & Nims, P.J. 1995. Performance of four seed-caching corvid species in operant tests of nonspatial and spatial memory. Journal of Comparative Psychology 109: 173-181.
Olton, D.S., Becker, J.T. & Handelmann, G.E. 1979. Hippocampus, space, and memory. Behavioral and Brain Sciences 2: 313-365.
O'Reilly, R.C. & Johnson, M.H. 1994. Object recognition and sensitive periods: A computational analysis of visual imprinting. Neural Computation 6: 357-389.
Petersen, K. & Sherry, D.F. 1996. No sex difference occurs in hippocampus, food-storing, or memory for food caches in black-capped chickadees. Behavioral Brain Research 79: 15-22.
Plunkett, K. & Marchman, V. 1991. U-shaped learning and frequency effects in a multi-layered perceptron: implications for child language acquisition. Cognition 38: 43-102.
Reboreda, J.C., Clayton, N.S. & Kacelnik, A. 1996. Species and sex differences in hippocampus size in parasitic and non-parasitic cowbirds. Neuroreport 7: 505-508.
Schacter, D.L. 1996. Searching for memory. New York; Basic Books: 398pp.
Sherry, D.F. & Duff, S.J. 1996. Behavioral and neural bases of orientation in food-storing birds. Journal of Experimental Biology 199: 165-172.
Sherry, D.F., Forbes, M.R.L., Khurgel, M. & Ivy, G.O. 1993. Females have a larger hippocampus than males in the brood-parasitic brown-headed cowbird. Proceedings of the National Academy of Science USA 90: 7839-7843.
Sherry, D.F. & Hampson, E. 1997. Evolution and the hormonal control of sexually-dimorphic spatial abilities in humans. Trends in Cognitive Sciences 1: 50-56.
Sherry, D.F., Jacobs, L.F. & Gaulin, S.J.C. 1992. Spatial memory and adaptive specialisation of the hippocampus. Trends in Neurosciences 15: 298-303.
Sherry, D.F. & Schacter, D.L. 1987. The evolution of multiple memory systems. Psychological Review 94: 439-454.
Sherry, D.F. & Vaccarino, A.L. 1989. Hippocampus and memory for food caches in black-capped chickadees. Behavioral Neuroscience 103: 308-318.
Sherry, D.F., Vaccarino, A.L., Buckenham, K. & Herz, R.S. 1989. The hippocampal complex of food-storing birds. Brain Behaviour and Evolution 34: 308-317.
Shettleworth, S.J. 1995. Comparative studies of memory in food storing birds: from the field to the Skinner box. In: Alleva, E., Fasolo, A., Lipp, H.-P., Nadel, L. & Ricceri, L. (eds) Behavioural brain research in naturalistic and semi-naturalistic settings : possibilities and perspectives (Proceedings of the NATO Advanced Study Institute, Acquafredda di Maratea, Italy, Sept 10-20, 1994). Dordrecht; Kluwer Academic Publishers: 159-192.
Squire, L.R. & Zola, S.M. 1997. Amnesia, memory and brain systems. Philosophical Transactions of the Royal Society of London B 352: 1163-1673.
Taylor, C.P. 1981. Contribution of compound eyes and ocelli to steering of locusts in flight. I. Behavioural analysis. Journal of Experimental Biology 93: 1-18.
Voyer, D., Voyer, S. & Bryden, M.P. 1995. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychological Bulletin 117: 250-270.
Williams, C.L., Barnett, A.M. & Meck, W.H. 1990. Organisational effects of early gonadal secretions on sexual differentiation in spatial memory. Journal of Neuroscience 104: 84-97.
Wilson, M. 1978. The functional organisation of locust ocelli. Journal of Comparative Physiology A 124: 297-316.
Fig. 1. The mean number of correct choices in the first six search attempts for six groups of black-capped chickadees (n=4 per group). Birds learned either the Place task or the Cue task, and after reaching criterion performance received aspiration lesions of either the hippocampus (HP), hyperstriatum accessorium (HA) or served as unoperated controls (Control). Each bar shows mean performance for five trials (error bars equal ± 1 S.E.). The only significant effect is between performance pre- and post-lesion for birds trained on the Place task (Tukey’s HSD9 > 1.06, P < 0.01). The number correct expected by chance is 0.5 ± 0.23 (var). From Sherry & Vaccarino (1989).

Fig. 2. The cumulative number of revisits by black-capped chickadees to baited sites on the Cue task. Mean performance in five 5 min trials is shown. Filled circles show the behaviour of birds with hippocampal lesions, other symbols show the behaviour of these birds before surgery, the behaviour of control birds, and the behaviour of birds before and after aspiration of the hyperstriatum accessorium (n = 4 per group). Chickadees with hippocampal lesions made more revisiting errors than birds in any other group (Tukey’s HSD9 > 21.51, P < 0.01) and made more searches in total (HSD9 > 6.24, P < 0.05). From Sherry & Vaccarino (1989).
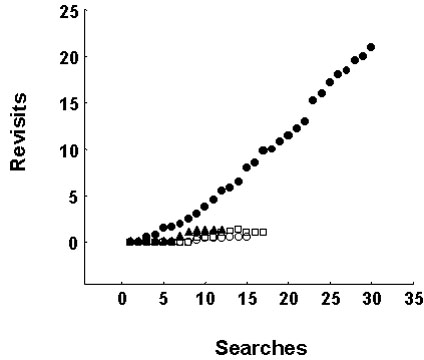
Fig. 3. The behaviour of individual dark-eyed juncos and black-capped chickadees on spatial location matching (Space) and colour matching (Colour) tasks, pre- and post-hippocampal lesions. There is no significant difference between species. Mean performance for all subjects of both species is shown (Mean). There is a significant interaction between lesioning and type of matching task (F1,6 = 28.27, P < 0.01), confirming that performance on the spatial task deteriorates following surgery, while performance on the colour task does not. From Hampton & Shettleworth (1996).
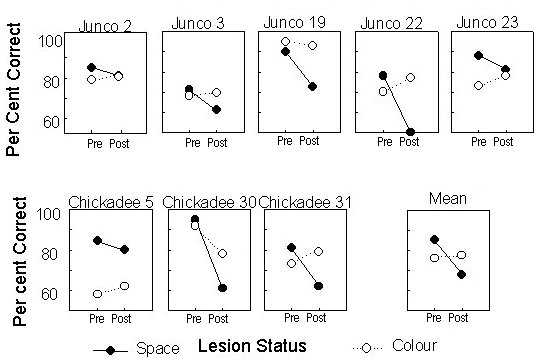
Fig. 4. Responses to the first, rewarded, presentation (S+) and the second, non-rewarded, presentation (S-) of photographs in a successive delayed non-matching task. Pigeons either had hippocampal lesions (n = 16) or were unoperated control birds (n = 15). For each delay between presentation of successive photographs (Delay) mean responses in five sessions of 48 trials per session are shown. There is a significant difference between S+ and S- trials (F1,29 = 66.67, P < 0.001), but no significant effect of lesioning, delay, or any interaction among these factors. From Good & Macphail (1994).
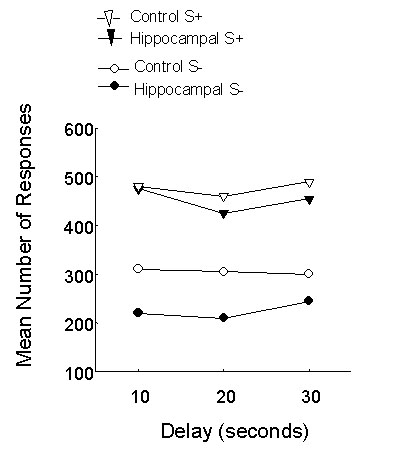
Fig. 5. Mean percentage correct responses by pigeons with hippocampal lesions (n = 14) and unoperated control birds (n = 12) in a delayed spatial matching to sample task. Mean performance is shown for five sessions of 24 trials per session at each delay between presentation of sample and test stimulus. There are significant effects of hippocampal lesioning (F1,24 = 4.52, P < 0.05), and delay (F3, 72 = 10.62, P < 0.001) but no interaction between these factors. From Good & Macphail (1994).

Fig. 6. The mean number of sessions required for pigeons with hippocampal lesions (Lesion, n = 5) and unoperated control birds (Control, n = 5) to acquire a concurrent delayed matching to sample task. 'Test' refers to three different sets of sample stimuli. There was no significant difference between lesioned and control birds for any set of sample stimuli. From Colombo et al. (1997b).
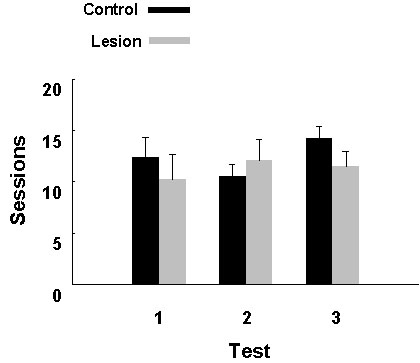
Fig. 7. The number of sessions required for unoperated control pigeons (Control, n = 5) and hippocampally lesioned pigeons (Lesion, n = 5), shown individually, to learn to feed from food cups (Shaping) and then to reach a criterion of one session with no more than one revisiting error (Acquisition). There is no effect of hippocampal lesioning on the number of sessions required to reach the shaping criterion, but a significant difference between groups in the number of sessions needed to acquire the spatial search task (t8 = 3.56, P < 0.01). From Colombo et al. (1997b).
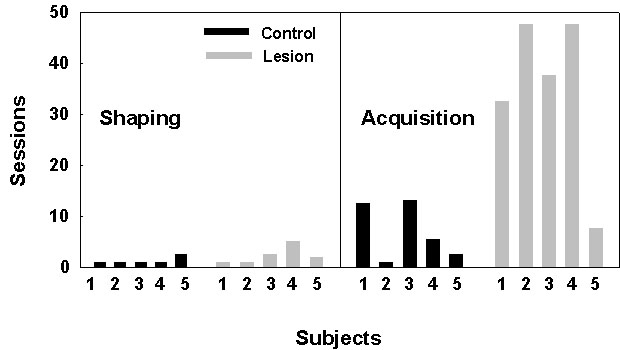
Fig. 8. Mean size of the
hippocampus in male and female brown-headed cowbirds (n = 6 ![]() , 6
, 6 ![]() ), red-winged blackbirds (n = 9
), red-winged blackbirds (n = 9
![]() , 7
, 7 ![]() ) and
common grackles (n = 2
) and
common grackles (n = 2 ![]() , 2
, 2 ![]() ). The hippocampus is larger in females than in males, after
covarying for size of the telencephalon, in cowbirds (ANCOVA F1,8 = 9.18, P
< 0.02) but not in red-winged blackbirds (ANCOVA F1,13 = 0.43, P < 0.04).
The grackle sample is too small for statistical analysis. From Sherry et al.
(1993).
). The hippocampus is larger in females than in males, after
covarying for size of the telencephalon, in cowbirds (ANCOVA F1,8 = 9.18, P
< 0.02) but not in red-winged blackbirds (ANCOVA F1,13 = 0.43, P < 0.04).
The grackle sample is too small for statistical analysis. From Sherry et al.
(1993).
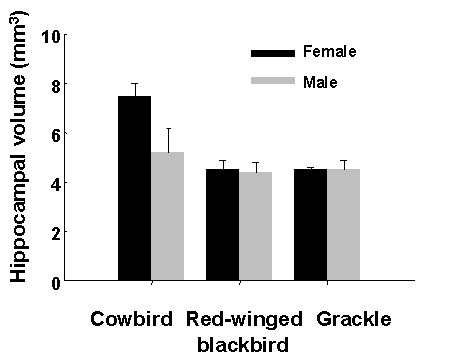
Fig. 9. Residuals of linear
regressions of hippocampal volume against telencephalon volume in shiny cowbirds (M.
bonariensis; n = 8 ![]() , 9
, 9 ![]() ) screaming cowbirds (M. rufoaxillaris; n = 6
) screaming cowbirds (M. rufoaxillaris; n = 6 ![]() , 6
, 6 ![]() ) and bay-winged cowbirds (M.
badius; n = 3
) and bay-winged cowbirds (M.
badius; n = 3 ![]() , 4
, 4 ![]() ). Positive residuals indicate a relatively large hippocampus and negative
residuals a relatively small hippocampus. There is a significant sex difference favouring
females in shiny cowbirds (t15 = 4.90, P < 0.001) but not in the other two
species. From Reboreda et al. (1996).
). Positive residuals indicate a relatively large hippocampus and negative
residuals a relatively small hippocampus. There is a significant sex difference favouring
females in shiny cowbirds (t15 = 4.90, P < 0.001) but not in the other two
species. From Reboreda et al. (1996).
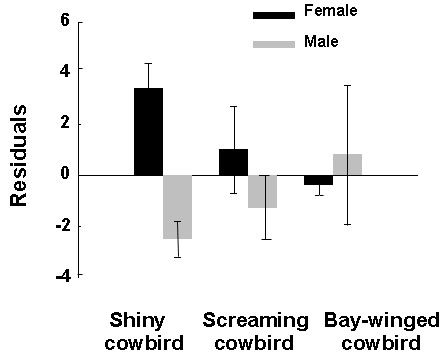
Fig. 10. Mean size of the hippocampus and telencephalon in female (n = 6) and male (n = 16) black-capped chickadees. There is no sex difference in the absolute size of the hippocampus or in the size of the hippocampus after covarying for the size of the telencephalon. From Petersen & Sherry (1996).