S40.3: Nature, nurture and the instinct to learn
Peter Marler Section of Neurobiology, Physiology and Behaviour, University of California, Davis, California 95616, USA, fax 530 752 8391 e-mail prmarler@ucdavis.edu Marler, P. 1999. Nature, nurture and the instinct to learn. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2379-2393. Johannesburg: BirdLife South Africa.In ethological and psychological studies of the development of behaviour, issues of nature and nurture have been the subject of much dispute. The question of innateness has been especially contentious, leading many to eschew genetic contributions to development altogether in the conduct of their research, and to focus rather on nurture as a prime factor in shaping behavioural ontogeny. Several indications suggest that the tide is now turning, preparing the way for a more balanced view, acknowledging the importance of both genetic and environmental contributions in the ontogenetic equation. Signs of the trend to reinstate nature’s contributions include increasingly frequent invocation of instincts to learn. Obstacles that still need to be dispelled include the misconception that genetic control is synonymous with behavioural stereotypy, and the belief that each genome encodes instructions for one and only one mature phenotype. The phenomenon of phenotypic plasticity as studied in depth by geneticists has important implications for students of behaviour. Some examples are reviewed of the many cases in which a given genotype gives rise to environmentally triggered alternative phenotypes, both morphological and behavioural, by engaging distinct programs for development to which genetic factors make major contributions. The continued use of such terms as learned and innate as labels for classifying adult behaviours may prove to be an obstacle to progress, a point that is illustrated from studies of song development in birds. The next wave of progress in understanding mechanisms of learning may come from applying the methods and concepts of molecular and developmental genetics.
INTRODUCTION
Although I have devoted most of my professional life to behavioural experiments on mechanisms of animal learning, I have become increasingly convinced that the next wave of progress in understanding learning will come from genetics. Specifically, I believe that the mechanisms underlying natural processes of learning in animals will remain intractable to scientific analysis until we apply the methods and concepts of developmental genetics. This will be a novel and challenging enterprise, and I do not pretend to have any special insights into the approaches that should be taken or the methods that should be employed. Those are decisions for the next generation to make. However ethologists and psychologists can make a useful contribution by developing animal models of learning systems that may be amenable to genetic analysis, while at the same time pointing out some of the potential pitfalls and conceptual confusions that can arise in the study of behavioural development.
Here my aims are three. First, I will argue that concepts of innateness are making an encouraging comeback in studies of development, countering past opposition to their use that I believe has seriously hindered progress in understanding the principles of behavioural development. Secondly I will attempt to dispel the beliefs held by many that genetic controls necessarily imply complete behavioural and morphological stereotypy, and that one genome encodes developmental instructions for only one mature, behavioural phenotype. And thirdly I will use data on song development in birds to reinforce the objections that others have raised to the classification of mature behavioural phenotypes as innate or learned. My overall aim is to grapple once more with the thorny, and still basically unsolved, problem of nature and nurture, by helping to prepare the way for a major research effort, hopefully not too far in the future, applying the methods and concepts of developmental genetics as a source of new insights into the mechanisms that underlie and make possible animal learning.
NATURE, NURTURE, AND LEARNING
When it comes to the development of behaviour there are about as many different points of view on the role of nature and nurture as there are people talking about it. Whether these views are expressed in scientific publications, in conversation, the popular press, or letters to the editor, the range of opinions is extraordinary, especially on the nature side of the equation (Johnston 1988 and commentators on his paper). As far as the importance of nurture is concerned, we all seem to have roughly similar ideas, focusing on the cumulative consequences of the multitudes of experiences and interactions that organisms have with their environments, experiences that begin in the embryo, are immensely enriched at birth and continue into maturity, albeit somewhat attenuated, until death.
It is the nature side of the equation that gives us so much trouble. Opinions about the importance of concepts of innateness in understanding behavioural development cover an enormous range. Most extreme is the widespread view that to invoke nature in discussions of behavioural development is to imply a fatalistic commitment to total unequivocal predestination, with the inevitable emergence of a single, stereotyped behavioural phenotype. This is the issue I want to discuss first, the relationship between concepts of innateness and behavioural stereotypy.
INNATENESS, STEREOTYPY, AND PHENOTYPIC PLASTICITY
A surprising number of animal behaviourists seem to believe that notions of innateness, by definition, allow absolutely no option for adaptive developmental plasticity. Given the obvious fact that behaviour is developmentally variable, they go on to conclude that concepts of innateness are either illogical or in the extreme, especially in the human case, abhorrent. The critics of behavioural genetics seem to assume that all proponents of innateness postulate a virtual zygotic homunculus, guiding every one of the multitude of steps in the marathon journey to the mature phenotype. Another corollary of the belief that ‘innate’ is synonymous with ‘stereotyped’ seems to be the conviction that, if behaviour was indeed innate, there would, by definition, be only one mature phenotype for each genotype, controlled as deterministically with behaviour as is felt to be the case with morphology.
But of course morphology, like behaviour, is also developmentally variable, although perhaps to a lesser degree. The same genotype can yield many phenotypes, and usually does. Phenotypic plasticity is a fundamental fact of developmental biology. It has long been a basic tenet of geneticists that a given genotype encodes instructions for the development, not just of one phenotype, but many. It is true that, in the past, some have tended to treat the range of alternative phenotypes as ‘noise,’ distributed around a ‘reaction norm,’ but the situation is now changing. A majority now take the view that many environmentally-dependent alternative phenotypes are adaptive, and make significant contributions to fitness (Schlichting & Pigliucci 1998; Scheiner 1993).
You only have to look around you to see endless illustrations of phenotypic plasticity as a basic attribute of living things. Examples are readily available from invertebrates and even plants. Caste determination in social insects is a classic case. A female ant possesses developmental instructions to acquire the morphology and behaviour of either a worker or a queen. Her fate is determined by her environment, which triggers one program or the other, depending on such things as the presence of a queen, egg size, nutrition, and temperature (Holldobler & Wilson 1990). The patterning of insect life cycles is subject to many adaptive, environmentally-induced variations that result from the triggering effects of cyclic and acyclic environmental stimuli (Tauber et al. 1986).
In many lepidoptera, seasonally-distinct morphs, some originally thought to be distinct species, display apparently adaptive differences in morphology and behaviour (Shapiro 1976; Nijhout 1991). In some the triggering cues, often temperature in tropical species and photoperiod in temperate species, have been carefully studied, with genetically-determined variations in photoperiod specifications from one local population to another. Larvae of the Geometrid moth, Nemeria arizonaria feed on oak leaves and can develop either as catkin mimics or twig mimics. They differ radically in both appearance and behaviour (Greene 1989). Experimentally-displaced larvae will actively relocate on catkins or twigs, depending on the morph. In this case the cues that initiate one pattern of gene activation or the other are provided, not by temperature or photoperiod but by diet, especially seasonally changing concentration of tannins.
There are well-known cases of environmentally dependent sex determination (Bull 1983), and many examples of intraspecific variation in patterns of social organisation in fish, amphibians, birds and mammals appear to be cued by such experiential factors as population pressure, food availability and predation (Lott 1991).
Phenotypic plasticity seems to be especially important in plants, perhaps because of their predominantly sessile life style, requiring them to deal with a wide range of ambient conditions (Turesson 1922; Schlichting 1986; Bradshaw 1965). The same amphibious buttercup will grow different leaves depending on whether it is in or out of water (Cook & Johnson 1968). Variations in soil chemistry trigger growth programs that make larger or smaller plants, more leaves or more seed, with the same constituents eliciting different responses in different plant species (Ginzo & Lovell 1973). Move a plant from sun to shade and another growth program is activated; it becomes etiolated, leans towards the light, and grows differently-shaped leaves (Lee & Cavers 1981). Transplant it to a mountain top and the same genotype will yield a phenotype different from that at sea level (Clausen et al. 1940). A cloned plant will grow differently alone and in a crowd.
In vertebrates, the shape of bones varies dramatically in response to the stresses and strains to which they are exposed during growth and usage. West-Eberhard (1989) recounts the interesting case of a mutant goat with no forelegs that learned to walk on its hind feet. Study of its skeleton after it died revealed all kinds of use-dependent changes including enlarged hind legs, a curved spine, and modified muscle insertions. In birds, the wear and tear of usage makes necessary contributions to the interactions with growth processes to create the mature phenotype of beaks, feet and feathers of birds, as every aviculturalist who has dealt with abnormal growth of beaks and claws in captivity knows all too well. There are many demonstrations of diet-induced changes in the size of caeca and intestines of birds, as well as more subtle adjustments in the balance between different digestive enzymes, and the length of intestinal villi (Klasing 1998). And everything that is true of morphology is even more true of behaviour (West-Eberhard 1989).
Variation in the timing and sequencing of avian life cycle stages, within limits imposed by strong, endogenous circannual rhythms, is triggered by some kinds of experience, such as stress (Silverin 1998), and food shortage, which prove to be more potent than others, such as social factors (Gwinner, this volume; Wingfield, this volume). Specific visual stimuli such as patterns of polarisation of the sky at sunset serve to recalibrate the navigational coordinates of migratory birds, interacting with underlying patterns of responsiveness to the earth’s magnetic field (Able & Able, this volume). Species-specific auditory stimuli influence the subsequent vocal behaviour of songbirds, in some cases irreversibly, in others in open-ended fashion (Marler 1997). In all of these cases of phenotypic plasticity in the behaviour of birds, developmental plasticity takes the form of cueing by particular kinds of environmental stimulation, interacting in turn with sensory and motor predispositions that are underlain by genetic programs that differ from species to species, and even from population to population.
The notion of instincts to learn fits comfortably with the phenomenon of phenotypic plasticity, once we begin thinking of the behavioural and psychological consequences of learning as attributes of the phenotype to which genotype/environment interactions give rise in development. The diversity and lability of such phenotypes is surely greater than with any other biological attribute, with the possible exception of the immune system. Environmental contributions to the developmental equation are greater and more complex than with other kinds of traits, but genetic factors continue to play a crucial role, not only in cued learning but also in programming the procedural rules that underlie perception, memorisation, and the establishment of functional relationships between experience, mentation, and planning for action. Dramatic progress has been made in disentangling the complex networks of events at the cellular, synaptic and biochemical level underlying habituation, sensitisation and classical conditioning, and the role of changing patterns of gene activation is becoming clear, with parallels emerging between mechanisms of adult learning and more general principles underlying cellular and organismal development (Kandel 1983; Kandel & O’Dell 1992; Silva et al. 1998; West-Eberhard 1998).
Phenotypic plasticity is everywhere, and in many cases environmentally triggered variation is known to be adaptive. It is simply incorrect to assume that genetic controls automatically imply behavioural stereotypy. Even with highly stereotyped behavioural phenotypes, it may well be that the ontogenetic trajectories leading up to maturity are actually not one, but many, as the growing embryo corrects for the developmental perturbations to which it is probably subject (Waddington 1957).
Note that none of these environmentally-induced changes is erratic or random. In every case the change appears organised and apparently adaptive, in some cases with proven quantified consequences for fitness (Schlichting & Pigliucci 1998). Clearly the environmental stimuli responsible for such changes achieve their effect, not by simple physical impact, as when a leaf is scorched by the sun, but as cues that match a preordained predisposition, and trigger preordained responses, in which genetic pre-programming plays a major role. Thus, the pervasiveness of adaptive phenotypic plasticity, although environmentally dependent in important ways, has no place in an anti-innateness argument. On the contrary, it is yet another tribute to the subtlety of genetic contributions to patterns of reactivity to environmental stimuli, contributing in turn to the ontogenetic unfolding of developmental programs.
However, there are still some who believe that you can safely downplay or even ignore the nature side of the equation in behavioural development. Until recently, especially in the social sciences, the term innate has been suspect and its users have occasionally been the targets of vigorous but sometimes uninformed criticism, often discouraging students of behaviour from pursuing studies of innateness and its developmental implications. As a consequence, despite their centrality in Darwinian thinking, concepts of instinct and innateness and genetic contributions to behavioural development in general have had a checkered history, but there are signs that the situation is beginning to change.
INSTINCTS TO LEARN: FOUR PRO-INNATENESS CASE HISTORIES
As scientists continue to grapple with the nature/nurture problem from a developmental point of view, some are beginning to reinstate concepts of instinct and innateness. I mention here just four among many examples, each concerned with some aspect of behavioural development and learning. In each of them a replay of the entire nature/nurture debate is very close to the surface, in language studies (e.g. Pinker 1994), in computer modelling of complex behaviour (Elman et al. 1997), in song learning in birds, as I will mention later (Gould & Marler 1987; Marler 1997), and lastly, in the historically interesting case of food aversion learning, in which organisms come to recognise and avoid food that makes them ill (e.g. Palmerino, Rusiniak & Garcia 1980).
With computer modelling, it is my impression as an outsider that when some of the biologically-minded engineers and artificial intelligence theorists who, after their heady successes in dealing with games of chess and tic-tac-toe, undertook the even more daunting task of designing machines that behave and learn, they were somewhat surprised by the many unforeseen facts and procedural rules that have to be provided by the machine builder beforehand for things to work (Dreyfus & Dreyfus 1988). They often found themselves broaching behavioural and psychological issues that were still not fully understood. It seems that complex instructions are needed for machines to accomplish even the most simple behavioural tasks. By analogy, organisms that behave and learn must surely need to have such rules and facts provided beforehand. It follows that contributions from nature to the ontogenetic equation are critical.
With birdsong and with language studies, it is the thrust of empirical research that has brought nature and nurture into such sharp relief (Gould & Marler 1987; Pinker 1994; Marler 1997). Detailed analyses of particular organism/environment interactions, whether of a young bird or a growing infant, have established the dominant role in development and learning of innate predispositions. These predispositions are sometimes general and shared by many taxa and sometimes so specialised as to be unique to a species, a population or an individual, as with language. They have evolved as specific adaptations, designed to guide particular developmental processes, processes that we often include under the general umbrella of ‘learned’ behaviour, although this is a label that in itself may be of questionable value, as I will discuss later.
Finally, consider the case of aversive food conditioning. Back in the period when general learning theories held sway in psychology, John Garcia and his colleagues came forth with the heretical suggestion that all learning is not general, with evidence that a special kind of learning process comes into play when a rat - or a person for that matter - has an experience that makes it ill. Usually, with negative conditioning, as with a painful or frightening experience, it is something in the very recent past that becomes the conditioned stimulus. With illness as the negative experience, however, animals and probably people, cast much further back in time than is usual, to old memories about unusual events that might have caused the illness. There is an especially strong inclination, at least in mammals, to avoid the taste and smell of those novel foods that were eaten hours earlier (Palmerino, Rusiniak & Garcia 1980).
Nowadays it sounds perfectly natural and adaptive for a white rat to focus this kind of food aversion learning on taste and smell, rather than, say, on sounds or appearance, which condition less readily. But when Garcia came to publish his data, editorial reviewers were incredulous (Garcia 1981). According to theory then current, any stimulus, seen, heard, or smelled, should be equally conditionable. Also, Garcia’s time intervals between conditioned and unconditioned stimuli were much too long to be credible. Eventually he got his papers published, starting what became a mini-revolution in thinking about the adaptiveness of learning. His work added a strong emphasis to the nature side of the nature/nurture equation. Many other cases have been described since, with organisms adaptively tuned to learn much more readily from some experiences than from others, with the nature of their sensitivities varying with the task and the species. But it is still an uphill battle to overcome the widespread illusion that learning systems are open-ended and unconstrained, aside from limitations on perception and actions that can be performed.
Innate Learning Predispositions
It is my impression that, today, students of behavioural development, whether they are psychologists, psycholinguists, computer modellers, or biologists, are more and more inclined to take it for granted that their subjects are necessarily guided by innate learning predispositions. These predispositions have evolved because of the advantages that accrue to those who are especially prepared to focus the potential advantages of developmental flexibility in behavioural domains that are important for the particular organism at some stage of life. There are obvious fitness consequences to possessing some innate foreknowledge about how best to deal promptly and incisively with issues that require learning for solution, especially those classes of problems that loom large in the biological history of the species, like learning to communicate, or, in the case of learned food aversions, diagnosing the cause of illness. The message emerging from this work and much else like it is that you cannot design an organism that learns quickly and efficiently without including in the plans major elaborate genetic instructions that facilitate the emergence of certain kinds of environment-contingent variation in behaviour. As I see it, the greater the emphasis on the importance of experience in development, the greater is the burden on the genome to ensure that mechanisms are in place, at the phase of the life cycle when they are needed, to optimise the chances of generating adaptive behavioural alternatives. The encoding of instructions for multiple, environmentally-triggered developmental strategies is one important means of providing for adaptive phenotypic behavioural plasticity.
Even if we can reach agreement that genomic contributions are critical for understanding any developmental process, including behaviour, it is by no means clear what to do next. The New York Times reporter on duty at the last Neuroscience Meeting obviously felt that it is helpful to try to divide up the relative contributions of nature and of nurture to each behaviour (Blakeslee 1997). Her article was headed ‘Cups of genes and dash of experience: recipe for a brain.’ We surely have to acknowledge that, in some degree, the genome is involved in every developmental event, obscuring efforts to partition the relative contributions to each phenotype. It will be more profitable to begin thinking about the ontogeny of behaviour as a developmental geneticist might approach the problem, striving for new theoretical approaches that will be helpful in disentangling the complex networks of genome/environment interactions involved in the journey from the genes to a complex trait, such as behaviour. This is surely one of the great scientific challenges now confronting students of animal behaviour.
Are the Terms ‘Innate’ and ‘Learned’ Still Useful?
As we begin preparing for the next generation of developmental theorising, it may be timely to clear the decks by submitting some of our existing terminology and usage to critical re-examination. I have in mind especially the tendency that, as Johnston (1987) has shown, many of us indulge in to some degree, to categorise behaviours in a two-part, either-or, dichotomous classification. Is a given behaviour instinctive or is it acquired? Is it innate or is it learned? The literature is full of pairs of terms of this kind. It is true that for quite a long time some social scientists studying behaviour have tended to avoid the term ‘innate’ altogether. But in doing so, they have tended to fall back on an alternative dichotomy that is really no different. They often define behaviours as either ‘learned’ or ‘unlearned’ evidently believing, illogically in my opinion, that this distinction is more robust and objective than the distinction between innate and acquired (cf Bateson 1984). My work on song learning in birds has convinced me that dichotomous terms like these, whether applied to entire behaviours or to limited components of behaviour assembled during ontogeny by ‘intercalation’ (Lorenz 1965), are not only logically flawed, but may be harmful in a deeper sense, by encouraging a mindset in dealing with the nature/nurture problem that is counterproductive, as Johnston (1988) has argued. Let me describe how I have come to this position.
We speak of some birdsongs as innate, and others as learned. An example of the first is provided by a flycatcher, the Eastern Phoebe (Kroodsma & Konishi 1991). Fig. 1 shows examples of the two song types of this species, first heard in nature, then as developed by a bird raised in social isolation. Under both conditions, the songs of this bird always develop in the same way, and we are inclined to speak of the song as innate.
Now compare an example of a so-called ‘learned’ song. Fig. 2 shows sound spectrograms of Song Sparrow song, as recorded in nature, and as developed by a male raised out of hearing of its own kind. Here the picture is very different (Mulligan 1966; Kroodsma 1977; Marler & Sherman 1985). We know that song structure is highly degraded and variable if the bird is unable to hear its own voice, as Konishi (1965) discovered in White-crowned Sparrows many years ago, but the song of a social isolate with its hearing intact is much more structured. Nevertheless, it is always abnormal, with a simpler structure than usual and a slower tempo (Fig. 2). Playing tape recordings of natural song during the young male’s sensitive period suffices to restore singing behaviour that is fully normal. Given these facts, we hardly hesitate to classify Song Sparrow songs as learned. I want to re-examine this habit that we so easily fall into of submitting behaviours to this kind of dichotomous classification. Like many others (Johnston 1987) I am increasingly convinced that there are as many problems with labelling behaviour as ‘learned’ as with labelling it ‘innate.’
Is Song Sparrow Song Learned or Not?
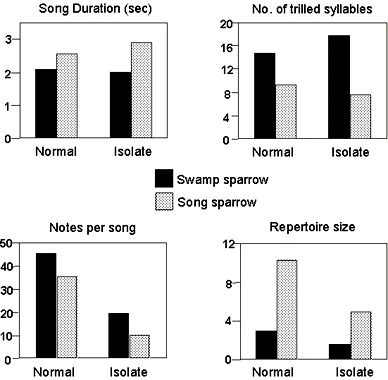
Let us re-examine the conclusion that Song Sparrow song is learned. If we look more closely at isolate Song Sparrow song, it is clearly not structureless. While you might be justified in concluding that the amorphous song of a deaf bird is completely lacking structure (Nottebohm 1966, 1968; but see Marler & Sherman 1983; Marler in press), isolate song still has a number of Song Sparrow-like features (Marler & Sherman 1985). There are several parts to the song, and we can sometimes see an intriguing tendency to accelerate the very first trill in the song, something that happens frequently in natural Song Sparrow songs (Fig. 2). Song duration and the number of trilled syllables per song, for example, are very similar in normal and isolate songs (Fig. 3, top). But other song features are changed (Fig. 3, bottom). How should we interpret these aspects of the song that are abnormal in isolation? Do the abnormalities recur in other songbirds with learned songs? Perhaps they represent something like a general default alternative that many songbirds would fall back on when they are raised without access to adult mentors? What do the unchanged song features represent? Are they very conservative traits, widely shared by different songbirds, or are some of them specific to Song Sparrows, so firmly and deeply rooted in the ontogenetic programming of this species that they resist developmental change?
Cross-Species Similarities
We achieve a new perspective by considering a second songbird with very different songs (Marler & Sherman 1985). What happens if we take a male Swamp Sparrow and raise it under the same conditions of social isolation as our Song Sparrow subjects? In both species the isolate songs are simpler than normal (Fig. 2). The impression that they are similarly affected by social isolation is reinforced by the fact that, if you study them quantitatively, you see that their abnormalities have a rather similar flavour.
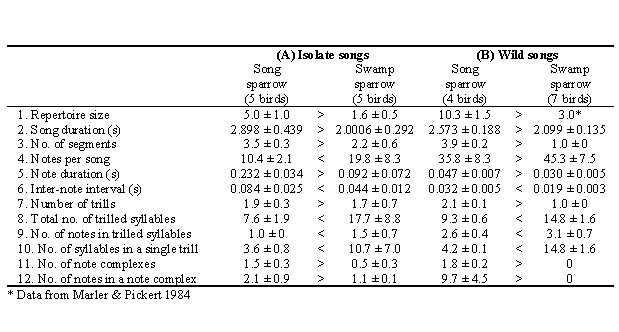
Table 1 shows data on some ten birds taken as eggs in the wild, hatched under Canaries, and then raised by hand and placed in individual isolation. It is clear that the songs of all ten were abnormal. Looking at the Song Sparrow data first, you see that individual song type repertoires were smaller in isolates, and there were fewer notes per song (Fig. 3, bottom). In addition, isolates had fewer notes per syllable, and the duration of notes and pauses between notes were increased so that the tempo of the whole song was slower. If you now compare the Swamp Sparrow data you see very similar general trends, in one measure after another. So in both species, song develops abnormally in isolation, but they develop normal song if, under the same conditions, we simply play tape recordings of song to them. Given these findings, it seems completely appropriate to describe these songs as ‘learned,’ as I and others have done many times in the past.
Cross-Species Differences
There is another side to this coin. It is obvious from Fig. 2 that, despite the abnormalities, isolate Swamp and isolate Song Sparrow songs are easy to tell apart. It looks as though some song features in which the two species differ persist in normal fashion in isolates, and measurements confirm this. The Song Sparrow data in Table 1 reveal a number of features that are not significantly different in normal song and in isolate song. Some of the same trends can be seen in Swamp Sparrow song as well, although syntactical features of isolate song of this species are less stable ontogenetically. If we focused our attention only on the list of features that are similar in natural and isolate song, we might conclude that sparrow songs are innate. So we have something of a paradox. Depending on the features you look at, you can view sparrow songs as either innate or as learned.
A closer look reveals something even more interesting. I have already mentioned the seemingly irresistible tendency to synonymize innateness with fixed immutability. It is clear that some song features are indeed relatively fixed and resistant to change. Can we then use potential mutability as a kind of yardstick in such judgements? Could we view developmentally stable traits as innate, and use developmental lability as a diagnostic marker for the absence of innateness, concluding instead that such traits are learned?
By comparing the two species more closely we can show this simply doesn’t work. Note first that some of the developmentally labile song features, such as repertoire size and numbers of notes per song (Fig. 3, bottom), are different in natural songs of the two species. The existence of species differences permits us to approach the nature/nurture issue in a different way by asking whether any of those same differences persist in isolation.
In every one of the 12 song features represented in Table 1, Song and Swamp Sparrows differ in the same direction, in natural song and in songs developed in isolation. Notice also that some of these same features are radically changed in isolation. Yet the species differences persist. Recall the slow tempo of isolate Sparrow songs. Note duration and inter-note intervals are both drastically lengthened in isolates of both species. Yet the averages are still shorter in Swamp Sparrows than in Song Sparrows, just as in normal song.
There are other such cases. As has been found in other songbirds, song repertoires tend to shrink in social isolates. In Song and Swamp Sparrows they are reduced to about half of normal size. In nature, Song Sparrows have individual repertoires of 10 to a dozen song types, three times larger than the 3 or so song types of a Swamp Sparrow. If we now compare isolate songs of the two species we find that despite the shrinkage, Song Sparrow repertoires are again about three times larger than those of Swamp Sparrows (Fig. 3, bottom). So here we have a song feature that is highly labile developmentally, but species differences, presumably genetically based, are still very clearly displayed. The number of notes per song presents a similar case (Fig. 3). Do we conclude then that repertoire size and note number are learned or have we shown that they are innate? Clearly neither conclusion is satisfactory. Like others (Oyama 1985; Johnston 1987) I am increasingly convinced that casual use of dichotomous terms like ‘learned’ and ‘innate’ convenient though it is for some purposes, as a way of classifying mature behavioural phenotypes, is actually a hindrance to future progress.
CONCLUSIONS
When we apply the term ‘learned’ to a behaviour, what we usually mean to imply is that there is a contrast between the developmental trajectories that different individuals follow either with or without a particular set of experiences in their personal history. But the term is often used more casually as a categorical label. We don’t of course regard the entire behaviour as learned, with all of the embryological, neural, neuromuscular and somatic events that are its necessary antecedents. Nevertheless, as future students think about how to design the next generation of studies, there is a strong temptation to assume that because a behaviour has not been labelled as ‘innate’ the relevance of such concepts as species-specific, genetically-shaped learning predispositions can safely be discounted. The literature of behavioural science is replete with cases where a preoccupation with effects of experience on behavioural development has led to neglect of the genetic side of the developmental equation. This was the cardinal sin of the behaviourism movement that held sway in the study of learning for so long.
Similarly the practice of labelling behaviours as ‘innate’ has surely lead people to neglect the role of experience in designing studies of behavioural development as many studies have shown, from Lehrman (1953) to the present. I am increasingly convinced that casual, categorical usage of the learned-innate dichotomy to classify adult behavioural phenotypes is at least partly responsible for polarising our thinking about the nature-nurture controversy. Most of the verbiage on this issue, often quite vituperative, is to be found in criticisms of users of concepts of innateness (Johnston 1988), but in fact the same criticisms pertain to casual use of the term ‘learned’ (Lehrman 1953).
One way to escape from the dilemma might be, not to classify behaviours as learned or innate, but to focus our questions about nature and nurture on differences between individuals, populations, and species, and how those differences develop. This has been the aim of much of my own research on song learning in birds. Genomic manipulations and comparative studies of patterns of gene regulation have enormous potential for new insights into the role of nature in behavioural development, with a wealth of new methods for the experimenter’s deployment (Clayton 1997). Whichever solutions turn out to be most productive, some reappraisal of the terminology that we use may be a useful step in the right direction, if we are ever going to properly understand ‘instincts to learn.’
Above all, it is crucial to set aside the misconception that the invocation of genetic contributions to behavioural development implies stereotypy, and the absence of individual and group differences. It behoves any student of behaviour who ventures into the nature-nurture thicket to attend closely to what geneticists have to tell us about the emerging topic of phenotypic plasticity, to say nothing of the multitude of revolutionary new insights that molecular and developmental geneticists have to offer, if only we can figure out how to incorporate them into studies of behavioural development.
REFERENCES
Bateson, P.P.G. 1984. Genes, evolution and learning. In P. Marler & H.S. Terrace (Eds.) The Biology of Learning. 75-88. Springer Verlag, Berlin.
Blakeslee, S. 1997. Cups of genes and dash of experience: recipe for a brain. New York Times, November 4.
Bradshaw, A.D. 1965. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics 13: 115-155.
Bull, J.J. 1983. Evolution of sex determining mechanisms. Menlo Park, California; Benjamin Cummings.
Clausen, J., Keck, D.D., & Hiesey, W.M. 1940. Experimental studies on the nature of plant species. 1. The effect of varied environments on Western North American plants. Carnegie Institute of Washington Publications. 520.
Clayton, D.F. 1977. Role of gene regulation in song circuit development and song learning. Journal of Neurobiology 33: 549-571.
Cook, S.A. & Johnson, M.P. 1968. Adaptations to heterogeneous environments. I Variation in heterophylly in Ranunculus flammula. Evolution 22: 496-516.
Dreyfus, H.L. & Dreyfus, S.E. 1988. Making a mind versus modeling the brain: artificial intelligence back at a branchpoint. Daedalus, Winter 1988: 15-43.
Elman, J.L., Bates, E.A., Johnson, M.H., Karmiloff-Smith, A., Parisi, D. & Plunkett, K. 1997. Rethinking innateness: a connectionist’s perspective on development. Cambridge, Massachusetts; M.I.T. Press.
Garcia, J. 1981. Tilting at the paper mills of Academe. American Psychologist 36: No. 2: 149-158.
Ginzo, H.D. & Lovell, P.H. 1973. Aspects of the comparative physiology of Ranunculus bulbosus L. and Ranunculus repens L. Annals of Botany 37: 753-764.
Gould, J.L. & Marler, P. 1987. Learning by instinct. Scientific American 255: No. 1: 74-85.
Greene, E. 1989. A diet-induced developmental polymorphism in a caterpillar. Science 243: 643-646.
Holldobler, B. & Wilson, E.O. 1990. The Ants. Cambridge, Massachusetts; Harvard University Press.
Johnston, T.D. 1987. The persistence of dichotomies in the study of behavioral development. Developmental Review 7: 149-182.
Johnston, T.D. 1988. Developmental explanation and the ontogeny of birdsong: nature/nurture redux. Behavioral Brain Science 11: 617-663.
Kandel, E.R. 1983. Neurobiology and molecular biology: the second encounter. Cold Spring Harbor Symposia on Quantitative Biology. Vol. 48. Molecular Neurobiology: pages 891-908.
Kandel, E.R. & O’Dell, T.J. 1992. Are adult learning mechanisms also used for development? Science 258: 243-245.
Klasing, K.S. 1998. Comparative Avian Nutrition. New York, CAB International.
Konishi, M. 1965. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Zeitschrift fur Tierpsychologie 22: 770-783.
Kroodsma, D.E. 1977. A re-evaluation of song development in the song sparrow. Animal Behaviour 25: 390-399.
Kroodsma, D.E. & Konishi, M. 1991. A suboscine bird (Eastern Phoebe, Sayornis phoebe) develops normal song without auditory feedback. Animal Behaviour 42: 477-487.
Lee, S.M. & Cavers, P.B. 1981. The effects of shade on growth, development and resource allocation patterns of three species of foxtail (Setaria). Canadian Journal of Botany 59:1776-1786.
Lehrman, D.S. 1953. A critique of Konrad Lorenz’s theory of instinctive behavior. Quarterly Review of Biology 28: 337-363.
Lorenz, K. 1965. Evolution and Modification of Behavior. Chicago, University of Chicago Press.
Lott, D.F. 1991. Intraspecific variation in social systems of wild vertebrates. Cambridge, New York, Cambridge University Press.
Marler, P. 1997. Three models of song learning: evidence from behavior. Journal of Neurobiology 33: 501-516.
Marler, P. In press. On innateness: are sparrows learned or innate? In M. Hauser & M. Konishi (Eds.). Behavioral and Neural Mechanisms of Communication. Cambridge, MA, MIT Press.
Marler, P. & Sherman, V. 1983. Song structure without auditory feedback: emendations of the auditory template hypothesis. Journal of Neuroscience 3: 517-531.
Marler, P. & Sherman, V. 1985. Innate differences in singing behaviour of sparrows reared in isolation from adult conspecific song. Animal Behaviour 33: 57-71.
Mulligan, J.A. 1966. Singing behavior and its development in the song sparrow, Melospiza melodia. University of California, Berkeley, Zoology Publications 81: 1-76.
Nijhout, H.F. 1991. The development and evolution of butterfly wing patterns. Washington; Smithsonian Institution Press.
Nottebohm, F. 1966. The role of sensory feedback in the development of avian vocalizations. PhD Thesis, University of California, Berkeley.
Nottebohm, F. 1968. Auditory experience and song development in the chaffinch Fringilla coelebs. Ibis 110: 549-568.
Oyama, S. 1985. The Ontogeny of Information: Developmental Systems and Evolution. Cambridge, Cambridge University Press.
Palmerino, C.C., Rusiniak, K.W. & Garcia, J. 1980. The peculiar roles for odor and taste in memory for poison. Science 208: 753-755.
Pinker, S. 1994. The Language Instinct. New York; W. Morrow and Company.
Scheiner, S.M. 1993. Genetics and evolution of phenotypic plasticity. Annual Review of Ecological Systems 24: 35-68.
Schlichting, C.D. 1986. The evolution of phenotypic plasticity in plants. Annual Review of Ecology and Systematics 17: 667-693.
Schlichting, C.D. & Pigliucci, M. 1998. Phenotypic Evolution: A Reaction. Norm Perspective. Sunderland, Mass.; Sinauer Associates Inc.
Shapiro, A.M. 1976. Seasonal polyphenism. Evolutionary Biology 9: 259-333.
Silva, A.J., Kogan, J.H., Frankland, P.W. & Kida, S. 1998. CREB and memory. Annual Review of Neuroscience 21: 127-148.
Silverin, B. 1998. Behavioural and hormonal responses of the pied flycatcher to environmental stressors. Animal Behaviour 55: 1411-1420.
Tauber, M.J., Tauber, C.A. & Masaki, S. 1986. Seasonal Adaptations of Insects. New York: Oxford University Press.
Turesson, G. 1922. The genotypical response of the plant species to the habitat. Hereditas 3: 211-350.
Waddington, C.H. 1957. The Strategy of the Genes. London; Allen and Unwin.
West-Eberhard, M.J. 1989. Phenotypic plasticity and the origins of diversity. Annual Review of Ecological Systems 20: 249-278.
West-Eberhard, M.J. 1998. Evolution in the light of developmental and cell biology. Proceedings of the National Academy of Sciences, U.S.A. 95: 8417-8419.
Table 1. Mean values of twelve features measured in normal and isolate songs of Swamp and Song Sparrows. The signs between column pairs indicate which value is larger. Note that, for all measures, songs of the two species differ in the same direction in isolate songs as they do in normal songs. (Data from Marler & Sherman 1985, where details are given on how the data was gathered).

Fig. 1. Sonagrams of Type 1 and Type 2 songs of the Eastern Phoebe as they develop normally, and in isolates. (After Kroodsma & Konishi 1991).
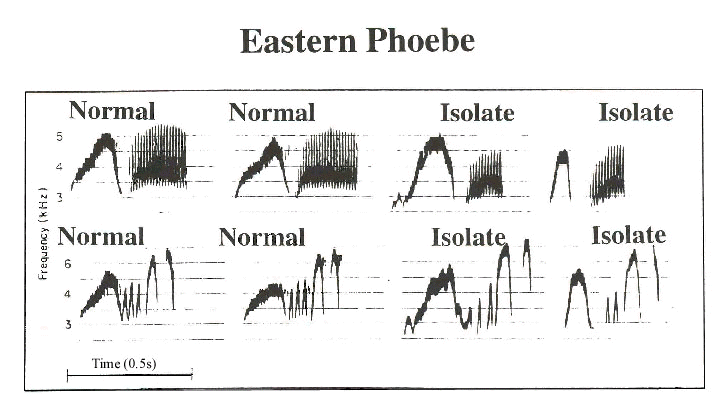
Fig. 2. Sonagrams of pairs of normal and isolate songs of Song and Swamp Sparrows. (After Marler & Sherman 1985).
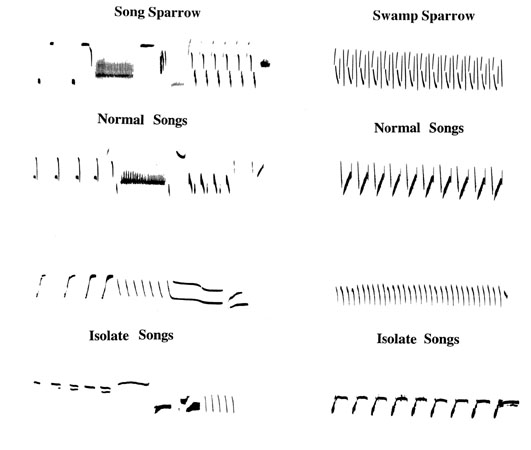
Fig. 3. Mean values of four measures of normal and isolate songs of Swamp and Song Sparrows. The top two measures, song duration and the number of trilled syllables, are similar in normal and isolate songs. At the bottom, notes per song and repertoire sizes are both reduced in isolates, but species differences persist despite the reductions. (Data from Marler & Sherman 1985 and Table 1).