S39.4: The significance of parasite loads in intertidal and freshwater invertebrates for their shorebird predators
Raymond McNeil1 & Marcos Tulio Díaz2
1Université de Montréal, Québec, Canada, e-mail mcneilr@ere.umontreal.ca; 2Universidad de Oriente, Cumaná, Sucre, Venezuela
McNeil, R. & Díaz, M.T. 1999. The significance of parasite loads in intertidal and freshwater invertebrates for their shorebird predators. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2329-2344. Johannesburg: BirdLife South Africa.Many invertebrate prey of shorebirds are infested by helminths which have adopted a variety of evolutionary strategies that increase their chances of being taken in by potential hosts for transmission to the final definitive host. The major types of strategies adopted by helminths that infest invertebrates taken by shorebirds are: inducing pathology and reduced stamina, coincidence in time, coincidence in place or habitat between the infested prey and the final host, and increased conspicuousness and attractiveness. Although some shorebirds may sometimes gain indirect advantages from the helminths that infest their prey, usually they are disadvantaged. Indeed, pathogenic helminths depend upon the host for nourishment and may produce nutrient deficiencies or toxic substances with local or general action detrimental to the host, damage the host's intestinal mucosa, cause intestinal obstruction, have irritative and inflammatory actions, produce enteritis and diarrhoea, sometimes undertake complex migrations through various organs of the hosts, produce anaemia and haemorrhage, pave the way for pathogenic microbes and viruses, sensitise the hosts to other diseases, and even cause death of hosts. In addition, there appears to be a relationship between trematode infestation, absence of or delay in developing premigratory condition, and oversummering of shorebirds on their tropical wintering grounds. Indeed, although most shorebirds that oversummer on wintering grounds were born the year before, the age as such is not the causative factor. For example, adult Greater Yellowlegs Tringa melanoleuca recently arrived on the wintering grounds in northern Venezuela were more heavily infested than juveniles. By spring, first-year birds tended to contain more trematodes than adults. There was an inverse relationship between fat loads and parasite loads in birds collected prior to the spring migration. It seems likely that trematode infestation may be a significant factor responsible for oversummering in at least some shorebird species, and can interfere with their migration, and ultimately with their reproductive success.
INTRODUCTION
Many invertebrate prey of shorebirds, both in freshwater and intertidal environments, are infested by helminth endoparasites (see Swennen 1969; Helluy 1983b; Curtis 1987; Jensen & Mouritsen 1992; Bick 1994; Mouritsen et al. 1997). Consequently, members of various shorebird species are often heavily infested with helminths (Cable et al. 1960; Loftin 1960; Threlfall 1963, 1968; Schmidt & Neiland 1968; Cabot 1969a, 1969b; Dronen & Badley 1979; Tallman et al. 1985; Goater & Bush 1988; Mariaux 1989; Ching 1990; McNeil et al. 1995, 1996).
Helminths (Platyhelminthes, Nematoda and Acanthocephala) typically parasitise vertebrates, although invertebrates, particularly arthropods and mollucs, act as intermediate hosts (see Smyth 1962). The Platyhelminthes that parasitise shorebirds are restricted to two groups: the Digenean Trematoda and the Cestoda. The Digenea (flukes) are found in strong and persistent association with aquatic habitats. They have complex life-cycles, typically with a molluscan primary host in which multiplication occurs, an intermediate transport host, and a vertebrate final host (Erasmus 1972). The Cestoda (tapeworms), in the adult stage, parasite the intestines of vertebrates; they also have complex life cycles with two or more hosts (Erasmus 1972). Many NEMATODA (roundworms) are free-living in soil and water but many others have intermediate stages in arthropods or are parasites in the tissues and fluids of plants and animals (Storer & Usinger 1957). The larvae of the ACANTHOCEPHALA (spiny-headed worms) occur in arthropods, but the adults parasitize the intestines of vertebrates (Storer & Usinger 1957).
According to Bush et al. (1990), the species richness of the helminth parasite community is, on average, lower in terrestrial hosts than in aquatic ones. The mean number of helminth species reported from different classes of aquatic hosts within vertebrates increases from fishes through herptiles to birds, declining slightly in mammals. Because migratory birds, in contrast to resident forms, are exposed to more than a single environment and its parasites, we should expect migratory species to have more severe infections, to allocate relatively more resources to anti-parasite defence, and to invest more in immune defence than closely related resident species (Møller & Erritzøe 1999).
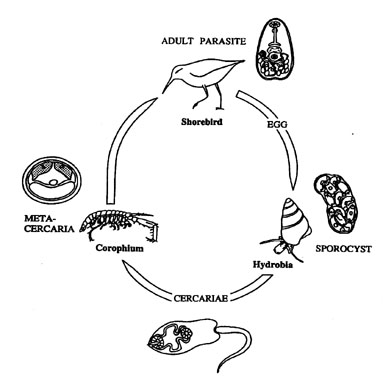
In the case of most helminths, and particularly the Digeneans, the life-cycle (Fig. 1) involves larval transmission between two intermediate host-species. The two hosts, usually from different trophic levels, are used in an obligatory sequence by the parasite in order to reach the target host, where they mature and reproduce (see Dobson 1988, Combes 1991). Parasites have adopted a variety of evolutionary strategies and mechanisms that increase the chances of encounters between the infective stages of the parasites and the hosts. Transmission enhancement strategies are common in the methods used by helminths for reaching definitive hosts within different groups of vertebrate animals (e.g. see Holmes & Bethel 1972; Helluy 1983a, 1983b; Dobson 1988; Hurd 1990; Moore 1984; Milinski 1990; Moore & Gotelli 1990; Combes 1980, 1991). They appear to be examples of convergent evolution, since the same strategies have evolved in three groups of helminths (Cestoda, Trematoda and Acanthocephala) using Chaetognatha, Mollusca, Crustacea and Insecta as intermediate hosts to reach fishes, birds or mammals as final definitive hosts (see Helluy 1983a).
This review provides examples of the various transmission enhancement strategies adopted by helminths that use invertebrates of intertidal environments as intermediate hosts, and shorebirds as final definitive hosts, and attempts to elucidate the consequences for shorebird survival, migration capacity, and reproductive success.
STRATEGIES FOR TRANSMISSION ENHANCEMENT
Five major types are found: pathogenesis, coincidence in time, coincidence in space, prey mimetism, and counter-mimetism, (Combes 1980, 1991). In many cases, transmission involves modification of the normal behaviour of infested intermediate hosts. These strategies include only ethological processes, altered responses or altered behaviour of intermediate hosts, and should be adaptive for the parasites. The mechanisms referred to as manipulation of host behaviour (e.g. Helluy 1983a, 1983b; Hurd 1990; Moore & Gotelli 1990; Combes 1991) are only one type process that increases the chances of parasite transmission.
Inducing pathology and reduced stamina
There are many examples of helminths producing pathological conditions in their intermediate hosts (see Williams 1967; Beck & Beverley-Burton 1968; Smyth & Heath 1970; Holmes & Bethel 1972). The behaviour of a sick host could benefit the parasites (Robb & Reid 1996). Indeed, diseased or weak animals are more vulnerable than healthy ones and a sluggish intermediate host may have difficulty in escaping a predator (Holmes & Bethel 1972; Moore & Gotelli 1990). Transmission to the definitive host may thus be favoured by a strategy by which parasites decrease the stamina of the prey or affect its ability to respond to the predator (Holmes & Bethel 1972). For example, Moore (1984) found that all spiny-headed worms altered intermediate host behaviour in a way that increased their vulnerability to predators.
Reduced vision in fish hosts infested with Diplostomum species
One possible example affecting shorebirds in freshwater habitats is that of some digenean Diplostomum species whose cercariae leave the snails (first intermediate hosts), penetrate suitable fish hosts and migrate to the eye where they develop into metacercariae in the crystalline lens (see Erasmus 1972; Crowden & Broom 1980). The presence of these metacercariae may interfere with vision of fish which depend on it for food detection, thus reducing their feeding efficiency (which needs to be compensated by increasing the time spent feeding) and making them more susceptible to predation by birds which act as definitive hosts (Erasmus 1972; Crowden & Broom 1980; Milinski 1990). Many shorebird species, particularly the Tringa species like the Greater Yellowlegs (T. melanoleuca), occur in freshwater habitats and frequently forage on small fish in shallow waters (see Sperry & Cottam 1944; Reeder 1951; Robert & McNeil 1989; Piersma et al. 1996). The genus Diplostomum is known to infest the alimentary canal of Tringa species (see McNeil et al. 1995, 1996).
Coincidence in time
In some cases, the infecting stage is released when host activity favours infestation (see Combes 1980). This favours transmission between intermediate hosts, or to the definitive ones. In other cases, infested prey and potential final definitive hosts are at their maximum numbers at the same time of the year.
Dogwhelks Thais lapillus and the trematode Parorchis acanthus
Dogwhelks Thais lapillus, molluscs that form part of the diet of Eurasian Oystercatchers Haematopus ostralegus, especially during the autumn and spring (Feare 1971), act in some regions as the first intermediate hosts for the trematode Parorchis acanthus, known in the adult stage to infest several shorebird species (Cable et al. 1960; Loftin 1960; Schmidt & Neiland 1968; Dronen & Badley 1979; Ching 1990; McNeil et al. 1995). Although in most cases predation by birds of the first intermediate host of a trematode does not allow the life cycle to continue, the cercariae may encyst on the shells of molluscs, including Thais, and be infective in this state (see Moore & Gotelli 1990). Infested dogwhelks experience higher predation rates by oystercatchers than uninfested ones, but do not appear to be selected for having slower reaction times in withdrawing into their shell after disturbance (Feare 1971). Dogwhelks are not normally an important prey for oystercatchers in winter as they aggregate in crevices, clefts and pools where they are safe from the birds. However, those infested with P. acanthus delay forming aggregations in the autumn; they are thus vulnerable to the birds for longer and are plentiful on open rocky shores at the time when large numbers of oystercatchers and other shorebirds, including non-immunised juveniles, are arriving from their breeding grounds (Feare 1971). This delay in forming winter aggregations may explain why infested molluscs experience higher predation than uninfested ones and is of survival value to the parasite.
Seasonal variation in the trematode prevalence of Corophium volutator
Another example is that of the parasites of the amphipod Corophium volutator, a prey of various shorebird species (Prater 1972; Goss-Custard et al. 1977; Goss-Custard 1979, 1983; Hicklin & Smith 1979; Peer et al. 1986; Durell & Kelly 1990; Mouritsen 1994) and which acts as second intermediate host for the trematodes Levinseniella brachysoma and Maritrema subdolum (Bick 1994). These parasites occur in less than 10% Corophium in the spring, but in almost 100% in late summer and autumn (Bick 1994; see also Mouritsen et al. 1997). Both parasite species are known to infest shorebirds which act as definitive hosts (Cable et al. 1960; Threlfall 1963; Cabot 1969a, 1969b; Ching 1990). This increase in the prevalence of both parasites in the autumn when large numbers of shorebirds, including non-immunised juveniles, are arriving from their breeding grounds may be of survival value for the parasites.
Coincidence in place or habitat between the infested prey and the final host
Another strategy employed by parasites is to act in such a way that the habitat occupied by the prey (intermediate host) overlaps that used by the predator (final definitive host) (see Holmes & Bethel 1972). There are many examples of helminth-infested prey in contrast to non-infested ones frequenting shallower waters near the shore, or swimming near the water surface where birds are feeding (see Holmes & Bethel 1972; Combes 1980; Helluy 1983a; Bartoli & Prévot 1986; Milinski 1990; Moore & Gotelli 1990). This implies movement to locations where potential hosts are more likely to occur (Combes 1980). Such behavioural changes make infested prey more vulnerable to predation by birds (Holmes & Bethel 1972). However, they may not always be a direct result of natural selection but, at least in part, may result from other aspects of the host-parasite association such as, for example, parasite-induced increased oxygen demand forcing the potential prey to move closer to water surfaces (see Milinski 1990; Moore & Gotelli 1990).
Estuarine snails Ilyanassa obsoleta and the trematode Gynaecotyla adunca
Estuarine snails Ilyanassa obsoleta bearing larvae of the trematode Gynaecotyla adunca behave differently to conspecifics lacking this parasite. Indeed, following high tides and especially at night, infested snails leave their non-infested conspecifics at lower tidal levels and crawl up onto beaches and sandbars where cercariae, upon release, encyst as metacercariae in semiterrestrial crustaceans such as sand hopper amphipods (e.g. Talorchestia longicornis and T. megalopthalmia) and fiddler crabs Uca sp. before developing to the adult final stage in shorebirds (Curtis 1987). These amphipods are common prey of a variety of plovers and sandpipers (Rankin 1940; Curtis 1987) and the Uca crabs are preyed upon by Charadrius and Numenius species and the Willet Catoptrophorus semipalmatus (see Strauch & Abele 1979; Thibault & McNeil 1994, 1995; McNeil & Rompré 1995). This trematode genus is known to parasitise shorebirds like the Ruddy Turnstone Arenaria interpres, the Wilson's Plover Charadrius wilsonia, the Sanderling Calidris alba, and the Willet (Hunter 1952; Loftin 1960). The altered behaviour of the snails can be considered as unusual because it favours host-to-host transmission by cercariae rather than enhanced predation on prey infested by metacercariae, the commonly recognised consequence of parasitic modification of host behaviour (see Curtis 1987). The process by which trematodes modify the behaviour of infested snails increases the parasite’s prevalence in the prey of the shorebirds and in the areas where the latter forage, and is therefore beneficial to the parasites.
Location of Macoma balthica infested with Parvatrema affinis
In some parts of their geographical ranges, various shorebird species regularly prey on the mollusc Macoma balthica (Swennen 1969; Prater 1972; Goss-Custard et al. 1977; Pienkowski 1982; Goss-Custard 1983; Hulscher 1982; Worrall 1984; Durell & Kelly 1990; Michaud & Ferron 1990; Lim & Green 1991; Zwarts & Blomert 1992; Mouritsen 1994). Larger Macoma are more heavily infested with the trematode Parvatrema affinis than are smaller ones (i.e. they contain more sporocysts) and are almost all confined to the higher parts of the intertidal zone where most shorebirds forage (Hulscher 1973, 1982; Swennen & Ching 1974; Lim & Green 1991). This distribution may increase the chances of the parasite reaching the adult stage in the final hosts, and can be considered as beneficial to the trematodes. The final stage of P. affinis has been found in various waterbirds, including the European Oystercatcher (Loos-Frank 1971; cited by Swennen & Ching 1974).
Increased conspicuousness and attractiveness
Induced changes in host appearance by increasing conspicuousness may in some cases increase the likelihood of intentional ingestion by the definitive hosts (see Holmes & Bethel 1972, Hurd 1990). Such changes may result from counter-mimetism in some animals (Combes 1980) but in others may be a consequence of parasite-induced changes in behaviour (including hyperactivity and disorientation), making them more vulnerable to predators (Holmes & Bethel 1972, Moore & Gotelli 1990).
U-shaped crawling tracks of Macoma balthica
The classical example quoted in many review papers is that dealing with M. balthica. This small bivalve, regularly preyed upon by shorebirds (see above), is known to make U-shaped crawling tracks during ebb tide on the sediment surface at higher levels of the intertidal zones at various sites along the Dutch coast and in the Wadden Sea, as well as in Hudson Bay (Swennen 1969; Hulscher 1982; Lim & Green 1991). This track-making behaviour has been linked by Swennen (1969), Swennen & Ching (1974), and Hulscher (1973, 1982) with high infestation with P. affinis. Indeed, Swennen (1969) and Hulscher (1973) reported that 100% of the clams that made these crawling tracks were infested with this trematode. Macoma serves as intermediate host, and shorebirds as final definitive hosts, of this parasite. On intertidal sand flats of Hudson Bay, Lim & Green (1991) found that not all crawling specimens were parasitized but that crawling clams were more heavily infested than buried ones. The crawling behaviour can be considered as making clams more conspicuous and thus as a parasite-induced behavioural change promoting the parasites' transmission to the shorebirds that act as final hosts (Swennen 1969; Hulscher 1973, 1982). However, this interpretation was seriously questioned by Mouritsen (1997). Indeed, he found that both crawling and buried Macoma were present on the intertidal flats of the White Sea in Russia, but that all appeared entirely free of trematodes or other types of macroparasites. It is possible that the high parasite prevalence usually observed among crawlers elsewhere is a consequence, rather than the cause, of the behaviour. Crawling specimens could represent a group of retarded animals that, in order to accelerate growth and gonad development, optimise deposit-feeding at the expense of anti-predator behaviour, and therefore move frequently to encounter unexploited food resources (Mouritsen 1997). Thus altered behaviour which incidentally favours parasite transmission efficiency is not always a direct result of natural selection but may sometimes be a side-effect of some of the host requirements such as satisfying its energy demands. Nevertheless, in regions like the Wadden Sea where crawlers dominate among infested individuals, the conspicuous behaviour of M. balthica may indeed favour the transmission of P. affinis to shorebirds and other waterbirds that feed on this clam species.
Increased surface activity of Corophium volutator infested with Maritrema subdolum
As mentioned above, the amphipod C. volutator is also an important prey for many shorebirds species. Mouritsen & Jensen (1997) have shown that, apparently due to parasite-induced anaemia, Corophium individuals infested with metacercariae of M. subdolum have increased activity at the surface of tidal flats. A great variety of shorebird species are commonly infested with M. subdolum and other species of Maritrema (Hadley & Castle 1940; Loftin 1960; Cabot 1969a, 1969b; Dronen & Badley 1969; Ching 1974, 1990; Deblock & Canaris 1992; McNeil et al.1995). According to Mouritsen & Jensen (1997), this parasite-induced behavioural change may facilitate transmission of infective stages of M. subdolum to shorebird hosts.
Surface activity of Venerupis aurea infested with Gymnophallus fossarum
The clam Venerupis aurea normally spends its post-larval life buried in the sand sediment. When heavily infested with the metacercariae of Gymnophallus fossarum, V. aurea often reverses its position in the sand, uncovering its ventral side, thus becoming more conspicuous and attractive to the European Oystercatcher. This behavioural change induced by the parasite facilitates the transmission of its infective stage to the oystercatcher in the intestine of which it develops into the adult final stage (Bartoli (1976).
Hyperactivity of Gammarus amphipods infested by the trematode Microphallus papillorobustus
After emerging from snails Hydrobia, the cercariae of the trematode Microphallus papillorobustus penetrate Gammarus amphipods which form part of the diet of various shorebird species (see Rebeck 1964; Bengtson & Svensson 1968; Helluy 1983b; Michaud & Ferron 1990; Holt & Warrington 1996). Helluy (1983a, 1983b) has shown that adult and juvenile G. insensibilis, and juvenile G. aequicauda, infested by the metacercariae, behave abnormally: they exhibit positive phototaxis and negative geotaxis, and thus move to the water surface. In addition, infested G. insensibilis, mechanically triggered by the movements of foraging shorebirds and other waterbirds, aggregate and become hyperactive at the air-water interface, while non-infested individuals remain motionless or swim to the bottom. Such parasite-induced aggregation and hyperactivity make infested amphipods more conspicuous and more vulnerable to shorebird predation and are of survival value to the parasites. Microphallus papillorobustus is known to infest various shorebird species (see Rebeck 1964).
Association between Microphallus papillorobustus and Maritrema subdolum
Helluy (1983b) has shown that M. subdolum frequently infests Gammarus species together with M. papillorobustus. Indeed, the cysts of M. subdolum are present in larger numbers in those Gammarus exhibiting Microphallus-altered behaviour than in normal ones. Shorebirds are commonly infested with M. subdolum and other species of Maritrema (see above). As a consequence, all metacercariae of M. subdolum that are infesting Gammarus species in the presence of metacercariae of M. papillorobustus have increased chances of infesting shorebird species (Helluy 1983b).
The larger size of infested prey makes them more attractive
Parasites might increase their chances of attaining final hosts by increasing the size of intermediate hosts or by selecting the larger ones, which are more attractive to predators. For many shorebirds, larger prey are more profitable than smaller ones and are preferred (see Goss-Custard 1979; Schneider 1981; Zwarts & Drent 1981; Sutherland 1982; Pienkowski 1983; Sutherland & Ens 1987). It has often been reported that parasites enhance the growth of molluscan hosts, leading to 'gigantism', and frequently also to lost fecundity of hosts (see Lim & Green 1991). However, evidence for such parasite-induced gigantism is equivocal in many cases (see Sousa 1983a; Hurd 1990; Mouritsen & Jensen 1994) and will not be discussed here. Cases of enhanced growth have been reported for snails (Cerithidea californica, Hydrobia ulvae, Ilyanassa obsoleta) caused by echinostomatide (Himasthla sp., Echinoparyphium sp.), notocotylide (Catatropis sp.), microphallide (Maritrema, Microphallus) and philopthalmide (Parorchis) trematodes (Sousa 1983a; Mouritsen & Jensen 1994; Curtis 1995). All these trematode species are known to infest shorebird species (Threlfall 1963; Schmidt & Neiland 1968; Cabot 1969a; Dronen & Badley 1979; Tallman et al. 1985; Ching 1990). However, their larval stages, after leaving the snail, need to develop in a second intermediate host before infesting shorebirds (see Sousa 1993b). As a consequence, there is little chance that the 'gigantism' induced in snails by these parasite species affects their prevalence in shorebird species.
However, there are cases in which the tendency for heavier infestations to occur in larger sizes of prey may result in increased parasite prevalence in shorebirds. For example, the larger M. balthica are more heavily infested by P. affinis than smaller ones (see above). In spite of the fact that European Oystercatchers sometimes reject infested Macoma (Hulscher, 1973, 1982), the less experienced juveniles of these and other shorebird species that feed on Macoma may be inclined to select the larger individuals, and thus may increase their risks of being infested with P. affinis. Another example concerns C. volutator. According to Bick (1994), larger C. volutator are more severely infested with L. brachysoma and M. subdolum than smaller ones. Both species are known to infest shorebirds (see above) and larger Corophium are preferred to small ones by many shorebird species (see Goss-Custard 1979; Peer et al. 1986).
The larger size and increased conspicuousness of infested Hydrobia ulvae
The snails H. ulvae are common prey of various shorebird species (see Prater 1972; Goss-Custard et al. 1977; Pienkowski 1992; Worrall 1984; Durell & Kelly 1990; Mouritsen 1994). According to Huxham et al. (1995), the snails larger than 6.1 mm are more heavily infested with the metacercariae of digenean trematodes than the smaller ones, and the snails with metacercarial infestation are more active on the sediment surface than uninfested snails and snails with cercarial infestation. In this way, the parasites benefit both from an increased availability of the snails to the final hosts and the phenomenon of 'gigantism', since the shorebirds probably select the largest snails (see above).
SIGNIFICANCE OF HELMINTH INFESTATION OF INVERTEBRATES FOR SHOREBIRD PREDATORSSuspected benefits from parasite-induced 'gigantism'
The larger size of some infested prey (see above), in addition to making them more attractive, can make them more profitable if larger size is induced by somatic growth. Indeed, larger size may sometimes mean more food. For example, the cercariae of trematodes such as Maritrema sp., Microphallus sp. and Himasthla sp. produce enhanced shell and somatic growth in H. ulvae, their first intermediate host (see Mouritsen & Jensen 1994), which is commonly preyed upon by various shorebird species (see Prater 1972; Goss-Custard et al. 1977; Pienkowski 1982; Durell & Kelly 1990). As a consequence, shorebirds might get some indirect advantage from the helminths that infest their invertebrate prey, for the risks of being infested by these helminth species are low, since the shorebirds are predating on the first and not the second intermediate hosts. In addition, Lafferty (1992) considers that, when the parasites are only slightly pathogenic and energetic costs of parasitism are moderate, final hosts also may actually benefit from eating parasite-modified prey.
Organ malfunction, disease and mortality due to helminth infestation
The likelihood of helminth infestation is higher in juvenile birds (Borgsteede et al. 1988; Hoeve & Scott 1988; Goater 1989; McNeil et al. 1995, 1996). Helminths may normally be considered as having low pathogenicity and do not always exert a negative effect on the fitness of their hosts (see Reid 1991). However, by definition, parasites damage their hosts and have negative effects on them at least some of the time during their life-cycle (see Holmes & Zohar 1990) and any parasites that use the tissues or food of their hosts may very well have negative effects, but the extent of these greatly depends on the number of parasites (infestation intensity) (Cheng 1973; Reid 1991; Goater & Holmes 1997). Many of the negative effects of helminths on hosts are concentrated on juveniles (Goater & Holmes 1997). Pathogenic helminths depend upon the host for nourishment which may lead to nutrient deficiencies, produce toxic substances with local or general action detrimental to the host, damage the host's intestinal mucosa, cause intestinal obstruction, have irritative and inflammatory actions, produce enteritis and diarrhoea, sometimes undertake complex migrations through various organs of the host, produce anaemia and haemorrhage, pave the way for pathogenic microbes and viruses, sensitise the hosts to other diseases, and even result in death of hosts (Watson 1960; Dogiel 1964; Ulmer 1971; Cheng 1973). Many of these detrimental effects may apply to migratory birds, as recognised by Wehrmann (1909).
Trematode infestation and oversummering of migratory shorebirds in their non-breeding areas
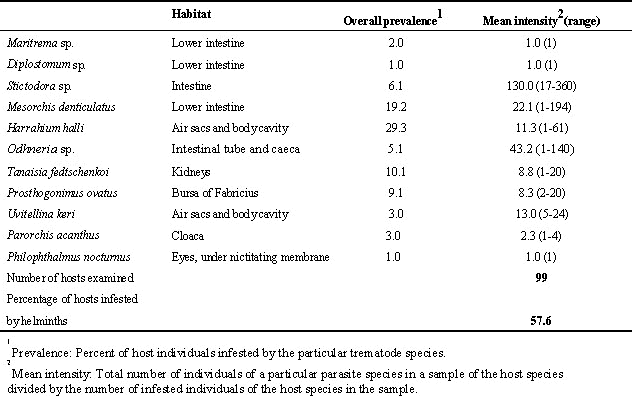
Various hypotheses have been proposed to explain oversummering (remaining on 'wintering' grounds during the boreal summer) in boreal-breeding shorebirds (see McNeil 1970; McNeil et al. 1994). Although most oversummering birds are approximately one year old, the age as such does not appear to be the causative factor (see McNeil et al. 1994). Indeed, some individuals of species known to oversummer do migrate to the breeding grounds and start breeding at the end of their first year of life (McNeil et al. 1994). In oversummering individuals, pre-migratory moult and fattening either do not take place, or are delayed (see McNeil 1970; McNeil et al. 1994). The relationship between digenean trematode infestation, absence of or delay in developing premigratory condition, and oversummering, first suggested by McNeil (1970), has been examined in Greater Yellowlegs oversummering in northeastern Venezuela (McNeil et al. 1995, 1996). Of the individuals collected, 57.6% were infested with one to four digenean species. Eleven genera were found (Table 1); specimens of Prosthogonimus ovatus and of Maritrema, Stictodora, Odhneria, and Diplostomum were present only in juveniles. The 26 adults collected in August, September and October shortly after their arrival on the 'wintering' ground had an average trematode infestation intensity (14.69) significantly higher than that (0.25) of the 8 juveniles collected in September, October and November. In contrast, the average parasite load of juveniles (68.69) collected in March, April, May and June tended to be higher than that of adults (7.00) collected during the same interval, but the difference was not significant at the 5% level. Infestation intensity in the air sacs was significantly higher in first-year birds than in adults. The prevalence of digenean infestation (in adult and juvenile birds pooled, due to the small size of the samples) increased steadily from November, shortly after the arrival of juveniles, to April-May. In fact, between mid-February and mid-March, when yellowlegs carried their highest premigratory fat loads, there was a significant inverse exponential relationship between fat loads and parasite loads (McNeil et al. 1995, 1996).
The location of parasites as well as their numbers (see above) explain to some extent their pathogenicity. Most parasites that infested Greater Yellowlegs were found in the intestine, air sacs and the body cavity. All helminths found in the air sacs, particularly Harrarium halli, belonged to the Cyclocoelidae. Cyclocoelids have severe effects on their hosts (McLaughlin 1977; Feizullaev 1985; Hoeve & Scott 1988). For example, Cyclocoelum mutabile penetrates the intestine and enters the body cavity, then penetrates the liver and, after a period of development, leaves the liver and becomes established in the air sacs of the avian host where it matures and causes in extremely severe damage (McLaughlin 1977).
It is therefore quite possible that, in addition to causing enteritis, anaemia and death of some individuals, helminths may also prevent or delay normal moult and pre-migratory fattening in some shorebirds on their wintering grounds, and hence be an important factor responsible for their oversummering (McNeil 1970; McNeil et al. 1994, 1995). Digestive disturbances resulting from intestinal helminth infestation are also expected to result in similar effects.
Birds may develop some degree of immunity to reinfestation with particular species of trematodes (see Macy 1973; Huffman & Roscoe 1986; Wallace & Pence 1986; Hoeve & Scott 1988). Partial immunity would explain, at least in part, (1) why juveniles Greater Yellowlegs tended to be more highly infested with digeneans at the time of or just before to the normal spring migration period, (2) why adults were also parasitised to some extent, and (3) why adults, which constitute the bulk of those 'scheduled' for migrating north, were less infested with trematodes in spite of the fact that the additional feeding associated with fat accumulation almost certainly exposed them to greater risk of parasitic infestation.
CONCLUSIONSIt seems likely that trematode infestation may be a significant factor responsible for oversummering in at least some shorebird species, although probably not in all species, and can interfere with their migration, and ultimately with their reproductive success. The likelihood of helminth infestation might be higher in those shorebird species breeding in boreal and low Arctic and wintering in freshwater and coastal lagoons and wetlands than in those breeding in the high Arctic regions and wintering in coastal marine environments. Indeed, high Arctic tundra and marine shores seem to be relatively parasite-free. Shorebirds breeding at rather low latitudes and/or being found in habitats other than coastal marine appear to have higher loads of parasites. According to Piersma (1997), the use of relatively parasite-poor high Arctic breeding habitats would go hand-in-hand with energetically costly longer migration distances, restriction to seashore environments in the non-breeding season, and low investment in general immunocompetence.
Trematodes may normally be considered as having low pathogenicity, except when habitat conditions are such that birds can acquire heavy worm burdens (Reid 1991). Some helminth species can become a problem when crowding or flocking of many individuals of the same host species occur (Dogiel 1964; Schmidt & Roberts 1985). Over the years, a great deal of pressure has been placed on tropical wetlands and coastal habitats, much of it at the expense of shorebirds (McNeil et al. 1985, 1990). Increased crowding in shorebirds on the wintering grounds may lead to their more rapid infestation by digenean helminths through a more rapid contamination of some of their invertebrate prey, the intermediate hosts, though no evidence is currently available to support this hypothesis. If the trend continues and shorebirds are forced into smaller and less suitable habitats, parasitism is likely to increase and the natural balance between hosts and parasites might become disturbed. The long term implication is that shorebird populations will decline as a result.
ACKNOWLEDGEMENTSThis study was financed by NSERC (Canada), Université de Montréal and Universidad de Oriente, Venezuela, through the collaboration agreement between both universities. We thank Peter R. Evans and Kim N. Mouritsen for useful suggestions for improving the manuscript.
REFERENCESBartoli, P. 1976. Modification de la croissance et du comportement de Venerupis aurea parasité par Gymnophallus fossarum P. Bartoli, 1965 (Trematoda, Digenea). Haliotis 7: 23-28.
Bartoli, P. & Prévot, G. 1986. Stratégies d’infestation des hôtes cibles chez les trématodes marins parasites de Larus cachinnans michaellis de Provence. Annales de parasitologie humaine et comparée 61: 533-552.
Beck, J.W. & Beverley-Burton, M. 1968. The pathology of Trichuris, Capillaria and Trichinella infestations. Helminthological Abstracts 37: 1-26.
Bengtson, S.-A. & Svensson, B. 1968. Feeding habits of Calidris alpina L. and C. minuta Leisl. (Aves) in relation to the distribution of marine shore invertebrates. Oikos 19: 152-157.
Bick, A. 1994. Corophium volutator (Corophiidae: Amphipoda) as an intermediate host of larval Digenea – An ecological analysis in a coastal region of the southern Baltic. Ophelia 40: 27-36.
Borgsteede, F.H.M., van Den Broek, E. & Swennen, C. 1988. Helminth parasites of the digestive tract of the Oystercatcher, Haematopus ostralegus, in the Wadden Sea, The Netherlands. Netherlands Journal of Sea Research 22: 171-174.
Bush, A.O., Aho, J.M. & Kennedy, C.R. 1990. Ecological versus phylogenetic determinants of helminth parasite community richness. Evolutionary Ecology 4: 1-20.
Cable, R.M., Connor, R.S. & Balling, J.W. 1960. Digenetic trematodes of Puerto Rican shore birds. Scientific Survey of Porto Rico and the Virgin Islands, New York, New York Academy of Sciences 17 (part 2): 187-255.
Cabot, D. 1969a. Helminth parasites from Charadriiform birds at Galway Bay, Co. Galway. Proceedings of the Royal Irish Academy 68: 149-159.
Cabot, D. 1969b. A new tapeworm, Hymenolepis arenariae n. sp. (Cyclophyllidea: Hymenolepididae) from the intestine of the turnstone, Arenaria interpres L., (Aves: Charadriiformes) from Galway Bay, Ireland. Irish Naturalists's Journal 16: 135-138.
Cheng, T.C. 1973. General parasitology. New York; Academic Press.
Ching, H.L. 1974. Two new species of Maritrema (Trematoda: Microphallidae) from the Pacific Coast of North America. Canadian Journal of Zoology 52: 865-869.
Ching, H.L. 1990. Some helminth parasites of Dunlin (Calidris alpina) and Western Willet (Catoptrophorus semipalmatus inornatus) from California. Journal of Helminthology 57: 44-50.
Combes, C. 1980. Les mécanismes de recrutement chez les Métazoaires parasites et leur interprétation en termes de stratégies démographiques. Vie et Milieu 30: 55-63.
Combes, C. 1991. Ethological aspects of parasites transmission. American Naturalist 138: 866-880.
Crowden, A.E. & Broom, A.E. 1980. Effects of the eyefluke, Diplostomum spathaceum, on the behaviour of dace (Leuciscus leuciscus). Animal Behaviour 28: 287-294.
Curtis, L.A. 1987. Vertical distribution of an estuarine snail altered by a parasite. Science 235: 1509-1511.
Curtis, L. 1995. Growth, trematode parasitism, and longevity of a long-lived marine gastropod (Ilyanassa obsoleta). Journal of the Marine Biological Association of the United Kingdom 75: 913-925.
Deblock, S. & Canaris, A. 1992. Contribution à l'étude des Microphallidae Travassos, 1920 (Trematoda). XLII. – De six espèces d'Afrique du Sud dont une d'un genre nouveau. Annales de Parasitologie Humaine et Comparée 67: 204-218.
Dobson, A.P. 1988. The population biology of parasite-induced changes in host behavior. Quarterly Review of Biology 63: 139-165.
Dogiel, V.A. 1964. General parasitology. Edinburgh; Oliver & Boyd.
Dronen, N.O., Jr. & Badley, J.E. 1979. Helminths of shorebirds from the Texas Gulfcoast. I. Digenetic trematodes from the Long-billed Curlew, Numenius americanus. Journal of Parasitology 65: 645-649.
Durell, S.A.E. Le V. dit & Kelly, C.P. 1990. Diets of Dunlin Calidris alpina and Grey Plover Pluvialis squatarola on the Wash as determined by dropping analysis. Bird Study 37: 44-47.
Erasmus, D.A. 1972. The biology of trematodes. London; Edward Arnold.
Feare, C.J. 1971. Predation of limpets and dogwhelks by Oystercatchers. Bird Study 18: 121-129.
Feizullaev, N.A. 1985. Pathogenic effect of trematodes of Cyclocoeloidea on the hosts. Parasitologia 19: 248-250 (In Russian).
Goater, C.P. 1989. Patterns of helminth parasitism in the Oystercatcher, Haematopus ostralegus, from the Exe Estuary, England. Ph.D. Dissertation, University of Exeter, Exeter, UK.
Goater, C.P. & Bush, A.O. 1988. Intestinal helminth communities in Long-billed Curlews: the importance of congeneric host-specialist. Holarctic Ecology 11: 140-145.
Goater, C.P. & Holmes, J.C. 1997. Parasite-mediated natural selection. In: Clayton, D.H. & Moore, J. (eds) Host-parasite evolution. Oxford; Oxford University Press: 9-29.
Goss-Custard, J.D. 1979. The energetics of foraging by Redshank, Tringa totanus. Studies in Avian Biology 2: 247-257.
Goss-Custard, J.D. 1983. Spatial and seasonal variations in the food supply of waders Charadrii wintering in the British Isles. Proceedings of the Third Nordic Congress of Ornithology 1981: 85-96.
Goss-Custard, J.D., Jones, R.E. & Newberry, P.E. 1977. The ecology of the Wash. I. Distribution and diet of waking birds (Charadrii). Journal of Applied Ecology 14: 681-700.
Hadley, C.E. & Castle, R.M. 1940. Description of new species of Maritrema Nicoll 1907, Maritrema arenaria, with studies of the life history. Biological Bulletin 78: 338-348.
Helluy, S. 1983a. Relations hôte parasite du trématode Microphallus papillorobustus (Rankin, 1940). II. Modifications du comportement des Gammaraus hôtes intermédiaires et localisation des métacercaires. Annales de Parasitologie Humaine et Comparée 58: 1-17.
Helluy, S. 1983b. Un mode de favorisation de la transmission parasitaire: la manipulation du comportement de l'hôte intermédiaire. Revue d'Écologie (Terre et Vie) 38: 211-223.
Hicklin, P.W. & Smith, P.C. 1979. The diets of five species of migrant shorebirds in the Bay of Fundy. Proceedings of the Nova Scotia Institute of Sciences 29: 483-488.
Hoeve, J. & Scott, M.E. 1988. Ecological studies on Cyathocotyle bushiensis (Digenea) and Sphaeridiotrema globulus (Digenea), possible pathogens of dabbling ducks in southern Québec. Journal of Wildlife Diseases 24: 407-421.
Holmes, J.C. & Bethel, W.M. 1972. Modification of intermediate host behaviour by parasites. Zoological Journal of the Linnean Society 51 (Supplement 1): 123-149.
Holmes, J.C. & Zohar, S. 1990. Pathology and host behaviour. In: Barnard, C.J. & Behnke, J.M. (eds) Parasitism and host behaviour. London; Taylor and Francis: 34-63.
Holt, P. & Warrington, S. 1996. The analysis of faeces and regurgitated pellets for determining prey size: problems and bias illustrated for Green Sandpipers Tringa ochropus feeding on Gammarus. Wader Study Group bulletin 79: 65-68.
Huffman, J.E. & Roscoe, D.E. 1986. Acquired resistance in Mallard ducks (Anas platyrhynchos) to infection with Sphaeridiotrema globulus (Trematoda). Journal of Parasitology 72: 958-959.
Hulscher, J.B. 1973. Burying-depth and trematode infection in Macoma balthica. Netherlands Journal of Sea Research 6: 141-156.
Hulscher, J.B. 1982. The Oystercatcher Haematopus ostralegus as a predator of the bivalve Macoma balthica in the Dutch Wadden Sea. Ardea 70: 89-152.
Hunter, W.S. 1952. Contributions to the morphology and life-history of Gynaecotyla adunca (Linton, 1905) (Trematoda: Microphallidae). Journal of Parasitology 38: 308-314.
Hurd, H. 1990. Physiological and behavioural interactions between parasites and invertebrate hosts. Advances in Parasitology 29: 271-319.
Huxham, M., Raffaelli, D. & Pike, A.W. 1995. The effect of larval trematodes on the growth and burrowing behaviour of Hydrobia ulvae (Gastropoda: Prosobranchiata) in the Ythan estuary, north-east Scotland. Journal of Experimental Marine Biology and Ecology 185: 1-17.
Jensen, K.T. & Mouritsen, K.N. 1992. Mass mortality in two common soft-bottom invertebrates, Hydrobia ulvae and Corophium volutator - the possible role of trematodes. Helgoländer Meeresuntersuchungen 46: 329-339.
Lafferty, K.D. 1992. Foraging on prey that are modified by parasites. American Naturalist 140: 854-867.
Lim, S.S.L. & Green, R.H. 1991. The relationship between parasite load, crawling behaviour, and growth rate of Macoma balthica (L.) (Mollusca, Pelecypoda) from Hudson Bay, Canada. Canadian Journal of Zoology 69: 2202-2208.
Loftin, H. 1960. An annotated check-list of trematodes and cestodes and their vertebrate hosts from northwest Florida. Quarterly Journal of the Florida Academy of Sciences 23: 302-314.
Loos-Frank, B. 1971. Zur Kenntnis der gymnopalliden Trematoden des Nordseeraumes. IV. Übersicht über die gymnophalliden Larven aus Mollusken der Gezeitenzone. Zeitschrift für Parasitenkunde 36: 206-232.
Macy, R.W. 1973. Acquired resistance in ducks to infection with the psilostome trematode Sphaeridiotrema globulus (Rudolphi, 1814). Journal of Wildlife Diseases 9: 44-46.
Mariaux, J. 1989. Cestodes d'oiseaux de Côte-d'Ivoire. III. Sur quelques parasites de Charadriiformes. Revue suisse de Zoologie 96: 541-559.
McLaughlin, J.D. 1977. The migratory route of Cyclocoelum mutabile (Zeder) (Trematoda: Cyclocoelidae) in the American Coot, Fulica americana (Gm.). Canadian Journal of Zoology 55: 274-279.
McNeil R. 1970. Hivernage et estivage d'oiseaux aquatiques nord-américains dans le nord-est du Venezuela (mue, accumulation de graisse, capacité de vol et routes de migration). Oiseau et Revue Française d'Ornithologie 40: 185-302.
McNeil, R. & Rompré, G. 1995. Day and night feeding territoriality in Willets Catoptrophorus semipalmatus and Whimbrel Numenius phaeopus during the non-breeding season in the tropics. Ibis 137: 169-176.
McNeil, R., Ouellet, H. & Rodríguez S., J.R. 1985. Urgencia de un programa de conservación de los ambientes costeros (lagunas, planicies fangosas, laderas costeras y manglares) del Norte de América del Sur. Boletin de la Sociedad Venezolana de Ciencias Naturales 143: 449-474.
McNeil, R., Limoges, B. & Rodríguez S., J.R. 1990. Corocoro colorado (Eudocimus ruber) y otras aves acuáticas coloniales de las lagunas, ciénagas y salinas de la costa centro-oriental de Venezuela. International Waterfowl Research Bureau Special Publication 11: 28-45.
McNeil, R, Díaz, M.T. & Villeneuve, A. 1994. The mystery of shorebird over-summering: a new hypothesis. Ardea 82: 143-152.
McNeil, R., Díaz, M.T., Casanova, B. & Villeneuve, A. 1995. Trematode parasitism as a possible factor in over-summering of Greater Yellowlegs (Tringa melanoleuca). Ornitología Neotropical 6: 57-65.
McNeil, R., Díaz, M.T., Casanova, B., Villeneuve, A. & Thibault, M. 1996. Trematode infestation as a factor in shorebird oversummering: A case study of the Greater Yellowlegs (Tringa melanoleuca). Bulletin of the Scandinavian Society of Parasitology 6: 114-117.
Michaud, G. & Ferron, J. 1990. Sélection des proies par quatre espèces d'oiseaux limicoles (Charadrii) de passage dans l'estuaire du Saint-Laurent lors de la migration vers le sud. Canadian Journal of Zoology 68: 1154-1162.
Milinski, M. 1990. Parasites and host decision-making. In: Barnard, C.J. & Behnke, J.M. (eds) Parasitism and host behavior. London: Taylor & Francis; 95-116.
Møller, A.P. & Erritzøe, J. 1999. Host immune defence and migration in birds. Evolutionary Biology (in press).
Moore, J. 1984. Parasites that change the behavior of their host. Scientific American 250 (5): 108-115.
Moore, J. & Gotelli, N.J. 1990. A phylogenetic perspective on the evolution of altered host behaviours: a critical look at the manipulation hypothesis. In: Barnard, C.J. & Behnke, J.M. (eds.) Parasitism and host behaviour. London; Taylor & Francis: 193-229.
Mouritsen, K.N. 1994. Day and night feeding in Dunlins Calidris alpina: choice of habitat, foraging technique and prey. Journal of Avian Biology 25: 55-62.
Mouritsen, K.N. 1997. Crawling behaviour in the bivalve Macoma balthica: the parasite-manipulation hypothesis revisited. Oikos 79: 513-520.
Mouritsen, K.N. & Jensen, K.T. 1994. The enigma of gigantism: effect of larval trematodes on growth, fecundity, egestion and locomotion in Hydrobia ulvae (Pennant) (Gastropoda: Prosobranchia). Journal of Experimental Marine Biology and Ecology 181: 53-66.
Mouritsen, K.N. & Jensen, K.T. 1997. Parasite transmission between soft-bottom invertebrates: temperature mediated infection rates and mortality in Corophium volutator. Marine Ecology Progress Series 151: 123-134.
Mouritsen, K.N., Jensen, T & Jensen, T. 1997. Parasites on an intertidal Corophium-bed: factors determining the phenology of microphallid trematodes in the intermediate host populations of the mud-snail Hydrobia ulvae and the amphipod Corophium volutator. Hydrobiologia 355: 61-70.
Peer, D.L., Linkletter, L.E. & Hicklin, P.W. 1986. Life history and reproductive biology of Corophium volutator (Crustacea: Amphipoda) and the influence of shorebird predation on population structure in Chignecto Bay, Bay of Fundy, Canada. Netherlands Journal of Sea Research 20: 359-373.
Pienkowski, M.W. 1982. Diet and energy intake of Grey and Ringed plovers, Pluvialis squatarola and Charadrius hiaticula, in the non-breeding season. Journal of Zoology (London) 197: 511-549.
Pienkowski, M.W. 1983. The effects of environmental conditions on feeding rates and prey-selection of shore plovers. Ornis Scandinavica 14: 227-238.
Piersma, T. 1997. Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos 80: 623-631.
Piersma, T., van Gils, J. & Wiersma, P. 1996. Family Scolopacidae (sandpipers, snipes and phalaropes). In: del Hoyo, J, Elliott, A. & Sargatal, J. (eds.) Handbook of birds of the world. Vol. 3; Barcelona; Lynx Ediciones.: 444-533.
Prater, A.J. 1972. The ecology of Morecambe Bay. III. The food and feeding habits of knots (Calidris canutus L.) in Morecambe Bay. Journal of Applied Ecology 9: 179-194.
Rankin, J.S., Jr. 1940. Studies on the trematode family Microphallidae Travassos, 1921. IV. The life cycle and ecology of Gynaecotyla nassicola (Cable and Hunninen, 1938) Yamaguti, 1939. Biological Bulletin 79: 439-451.
Rebeck, J. 1964. Recherches systématiques, biologiques et écologiques sur les formes larvaires de quelques trématodes de Camargue. Thèse de doctorat d'état, Université de Marseille, Marseille, France.
Reeder, W.G. 1951. Stomach analysis of a group of shorebirds. Condor 53: 43-45.
Reid, W.M. 1991. Cestodes and trematodes. In: Calnek, B.W. (ed.) Diseases of poultry. Ames; Iowa State University Press: 764-778.
Robb, T. & Reid, M.L. 1996. Parasite-induced changes in the behaviour of cestode-infected beetles: adaptation or simple pathology? Canadian Journal of Zoology 74: 1268-1274.
Robert, M. & R. McNeil. 1989. Comparative day and night feeding strategies of shorebirds species in a tropical environment. Ibis 131: 69-79.
Schmidt, G.D. & Neiland, K.A. 1968. Hymenolepis (Hym.) deblocki sp. n., and records of other helminths from Charadriiform birds. Canadian Journal of Zoology 46: 1037-1040.
Schmidt, G.D. & Roberts, L.S. 1985. Foundations of parasitology. Toronto; Mosby.
Schneider, D.C. 1981. Size-selective predation on mysids by birds (plovers). Marine Ecology Progress Series 5: 223-224.
Smyth, J.D. 1962. Introduction to animal parasitology. London; English Universities Press.
Smyth, J.D. & Heath, D.D. 1970. Pathogenesis of larval cestodes in mammals. Helminthological Abstracts 39: 1-22.
Sousa, W.P. 1983a. Host life history and the effect of parasitic castration on growth: a field study of Cerithidea californica Haldeman (Gastropoda: Prosobranchia) and its trematode parasites. Journal of Experimental Marina Biology and Ecology 73: 273-296.
Sousa, W.P. 1983b. Interspecific antagonism and species coexistence in a diverse guild of larval trematode parasites. Ecological Monographs 63: 103-128.
Sperry, C.C. & Cottam, C. 1944. The Greater and Lesser yellow-legs as fish eaters. Wilson Bulletin 56: 45.
Storer, T.I. & Usinger, R.L. 1957. General zoology. New York; McGraw-Hill Book.
Strauch, J.G., Jr. & Abele, L.G. 1979. Feeding ecology of three species of plovers wintering on the Bay of Panama, Central America. Studies in Avian Biology 2: 217-230.
Sutherland, W.J. 1982. Do Oystercatchers select the most profitable cockles? Animal Behaviour 30: 857-861.
Sutherland, W.J. & Ens, B.J. 1987. The criteria determining the selection of mussels Mytilus edulis by Oystercatchers Haematopus ostralegus. Behaviour 103: 187-202.
Swennen, C. 1969. Crawling-tracks of trematode infected Macoma balthica (L.). Netherlands Journal of Sea Research 4: 376-379.
Swennen, C. & Ching, H.L. 1974. Observations on the trematode Parvatrema affinis, causative agent of crawling tracks of Macoma balthica. Netherlands Journal of Sea Research 8: 108-115.
Tallman, E., K.C. Corkum & D.A. Tallman. 1985. The trematode fauna of two intercontinental migrants: Tringa solitaria and Calidris melanotos (Aves: Charadriiformes). American Midland Naturalist 113: 374-383.
Thibault, M. & McNeil, R. 1994. Day/night variation in habitat use by Wilson's Plovers in northeastern Venezuela. Wilson Bulletin 106: 299-310.
Thibault, M. & McNeil, R. 1995. Predator-prey relationship between Wilson's Plovers and fiddler crabs in northeastern Venezuela. Wilson Bulletin 107: 73-80.
Threlfall, W. 1963. Factors concerned in the mortality of some birds which perished in Anglesey and Northern Caernarvonshire during the winter of 1963, with special reference to parasitism by helminths. Annals and Magazine of Natural History 13: 721-737.
Threlfall, W. 1968. Helminth parasites of some birds of Newfoundland. Canadian Journal of Zoology 46: 909-913.
Ulmer, M.J. 1971. Site-finding behaviour in helminths in intermediate and definitive hosts. In: Fallis, A.M. (ed.) Ecology and physiology of parasites . Toronto; University of Toronto Press: 123-159.
Wallace, B.M. & Pence, D.B. 1986. Population dynamics of the helminth community from migrating Blue-winged Teal: loss of helminths without replacement on the wintering grounds. Canadian Journal of Zoology 64: 1765-1773.
Watson, J.M. 1960. Medical helminthology. London; Baillière Tindall and Cox.
Wehrmann (Née Brodsky), S. 1909. Sur l'action pathogène des Helminthes des oiseaux. Archives de Parasitologie (Paris) 13: 204-238.
Williams, H.H. 1967. Helminth diseases of fish. Helminthological Abstracts 36: 261-295.
Worrall, D.H. 1984. Diet of the Dunlin Calidris alpina in the Severn Estuary. Bird Study 31: 203-212.
Zwarts, L. & Blomert, A.-M. 1992. Why knot Calidris canutus take medium-sized Macoma balthica when six prey species are available. Marine Ecology Progress Series 83: 113-128.
Zwarts, L. & Drent, R.H. 1981. Prey depletion and the regulation of predator density: Oystercatchers (Haematopus ostralegus) feeding on mussels (Mytilus edulis). In: Jones, N.V. & Wolff, W.J. (eds) Feeding and survival strategies of estuarine organisms. New York; Plenum: 193-216.
Table 1. Prevalence and mean intensity of digenean trematodes infesting Greater Yellowlegs in northeastern Venezuela (Taken from McNeil et al. 1995).

Fig. 1. Typical example for the life cycle of digenean microphallid trematodes such as Maritrema subdolum and Microphallus claviformis with intertidal invertebrates as first and secondary intermediate hosts, and shorebirds as final definitive ones (Adapted from Mouritsen et al. 1997).