S39.2: Predation by birds on marine tidal flats
Leo Zwarts1 & Bruno J. Ens2
1Rijkswaterstaat-RIZA, PO Box 17, 8200 AA, Lelystad, The Netherlands, e-mail zwablo@tref.nl; 2
Institute for Forestry and Nature Research (BN-DLO), P.O. Box 167, 1790 AD Den Burg, The Netherlands, e-mail b.j.ens@ibn.dlo.nl Zwarts, L. & Ens, B.J. 1999. Predation by birds on marine tidal flats. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2309-2327. Johannesburg: BirdLife South Africa.Waders feeding on intertidal flats have to work hard to find enough prey, despite the fact that their prey usually occur at very high densities. The explanation is that waders ignore a part of their food supply due to the low profitability of many sizes of prey. Moreover, the majority of the remaining profitable prey are not accessible. Hence, waders usually harvest a small and highly variable fraction of their total food supply. The predation pressure by birds on this harvestable fraction of the biomass is also highly variable, but they may occasionally remove all prey. Since different bird species exploit different size classes, bird species taking the smaller size classes may severely depress the food resources in later years of bird species depending on the larger size classes. This is an important phenomenon that needs to be taken into account in both empirical and theoretical studies of interspecific competition.
INTRODUCTION
The early Lotka-Volterra models dealing with resource competition as an important force in structuring biological communities did not contain explicit terms for resource abundances. Instead, fixed competition coefficients represented the negative impact of one species on another. Robert MacArthur and Richard Levins greatly extended this theory and argued that the competition coefficients could be measured simply from the overlap in resource use (MacArthur & Levins 1967; Levins 1968; MacArthur 1972). Their work stimulated many empirical and theoretical investigations and generated much heated debate, but it did not lead to definitive conclusions. Instead, Wiens (1983) concluded that 'until avian ecologists begin the difficult task of measuring resource availability in relation to consumer demands, the arguments [on the importance of competition in structuring bird communities] are likely to remain unresolved'. What remains today is the inspiring vision that the current dynamics of a community cannot be considered separately from its evolutionary past.
MacArthur also pioneered community models that explicitly incorporated the dynamics of the limiting resources (MacArthur 1972), as well as individual-based models of optimal feeding behaviour (MacArthur & Pianka 1966), but he did not stimulate an associated empirical research program. However, this more 'mechanistic approach' to community ecology has been slowly gaining ground ever since. According to Schoener (1986) 'the [mechanistic] approach can be most simply defined as the use of individual-ecological concepts - those of behavioural ecology, physiological ecology, and ecomorphology - as the basis for constructing a theoretical framework with which to interpret the phenomena of community ecology'. Tilman (1990) even thinks that this approach will one day allow us to predict the dynamics and structure of populations in nature. Such a predictive theory 'should explicitly include the manner, method or mode whereby one organism interacts with another, and whereby an organism interacts with its physical environment'. Ens et al. (1994) sketch the necessary research program for the waders and waterfowl that migrate along the East Atlantic Flyway.
In this paper we summarise the results of a ten-year study using this 'mechanistic' or 'individual-based' point of view of the community of waders feeding on the macrozoobenthos of intertidal mud flats along the Frisian coast in the Dutch Wadden Sea. Because of its 'open' nature, described below, our study system is not easily fitted into equations describing the dynamics of both competing predators and their prey. Many of the birds exploit the mudflats only during the non-breeding season, either when passing through during spring and autumn migration, or staying for the winter. Thus, the birds are also subject to population processes and selective forces operating elsewhere. Similarly, many of the macrobenthic animals have a planktonic phase as juveniles, so that there is no direct link between the production of larvae in a local population and the subsequent recruitment at that locality. Recruitment is notoriously erratic and known to depend on climatic factors, while empirical evidence for a relationship with the size of the adult stock is lacking (Beukema 1982b).
Our current, necessarily incomplete, solution to these problems is as follows. For the birds, we take their morphology and aspects of their feeding behaviour as fixed properties. After measuring these properties we can then determine which prey are harvestable. According to the terminology of Zwarts & Wanink (1993), harvestable prey are both available and profitable. Available prey are both detectable (within range of the bird's sensory mechanism), accessible (within reach of the bird's bill) and ingestible (below the maximum size that can be swallowed). Profitability depends on the energy returns per unit time spent handling a prey. For a given food supply the optimal prey choice model, which is based on a generalisation of Holling's disc equation, allows one to calculate the profitability threshold that maximises intake rate (Charnov 1976). For our present purposes, we are especially interested in the prey densities where this maximal intake rate exceeds the minimum intake rate needed to maintain energy balance. Much, but not all, of the necessary information can be extracted from our own detailed studies on foraging behaviour of the various bird species. For the benthos we ignore recruitment itself and focus on changes in availability and the depletion of successive cohorts. Most of the annual growth and reproduction of the macrobenthic animals occurs during summer when the birds are breeding elsewhere. This 'production' then disappears during winter, so that we can compare the elimination of the previously produced biomass with the consumption by the birds, without the need to worry about concomitant production of new biomass. Actually, if depletion was complete, only minimum prey densities would remain at the end of winter.
We therefore address the following questions:
1. On average, do the birds, either the community as a whole or separate bird species, significantly deplete the harvestable biomass?
2. Is food more severely depleted in some years than in others? Because recruitment of the benthic prey is erratic and synchronised over large areas, and because of the long life expectancy of the birds, we may expect that food is limiting in only some years.
3. Is there evidence that species compete for the same foods, i.e. is there evidence that an increase in the consumption by one bird species necessarily detracts from the consumption by another species?
In this exercise we deliberately ignore interference within species and among species as an important mechanism of competition. The importance of interference in determining the 'instantaneous' distribution of feeding birds is beyond doubt, but it is presently not clear whether it also sets an upper limit to the cumulative exploitation of the food supply. Potentially, interference may limit bird densities in areas with high prey densities throughout the winter, so that not all harvestable prey will be harvested by the end of winter. As a result, our test for exploitation competition can also be seen as a test for interference.
METHODS
The study was performed between 1977 and 1986 on intertidal flats along the Frisian coast in the Dutch Wadden Sea. The study area, in total 396 ha, was delimited by long rows of poles, these being the remnants of brushwood groynes made in the 1960s to enhance sedimentation. An extensive description of the study area is given by Zwarts & Blomert (1992) and Zwarts et al. (1996d).
Samples of the macrozoobenthos inhabiting the mudflats were taken in the eastern part of the study area where 73 plots of 0.1 ha were pegged out around one observation tower. One to four samples of the benthic fauna (179 cm2 and 30 cm deep) were taken in these 73 sites in almost every month during the seven years 1980 to 1986 and less frequently in 1978 and 1979. The laboratory procedures used to determine the biomass of the benthic animals have been described by Zwarts (1991). Biomass is expressed as dry flesh without inorganic material (ash-free dry weight or AFDW). The growth of bivalves could sometimes be calculated directly from the length-frequency distribution in the monthly samples, but this was usually impossible because two or even more year classes occurred together. The year classes were identified using the Bhattacharya method for separating cohorts (MPA module of the Compleat ELEFAN software package, version 1.0; Gayanilo et al. 1988). This technique made it possible to estimate the monthly mortality and growth in length for the separate cohorts.
Using the relationships between size and flesh weight, determined from all sampling data, we also calculated for each cohort the fluctuation in the biomass of the average individual and for the cohort population as a whole. This allowed us to calculate the production per prey species, separately for each age class. The production can be estimated by adding either the growth increments or the weight losses caused by size-dependent mortality (Crisp 1984). We estimated the production by calculation of the monthly weight loss since this made it possible to indicate to what degree the elimination of biomass due to prey mortality had been determined by the predation pressure of the birds.
The elimination of the prey biomass was determined by the product of two terms: the mean weight of the prey averaged over two consecutive sampling dates and the decrease in the prey density between both sample dates. Both prey weight and the numbers which disappeared, were calculated separately per cohort and recalculated per month if the intervening period was longer than a month. The eliminated biomass per cohort was summed to arrive at the total monthly elimination of biomass per prey species. The 'winter' elimination was calculated for a seven month period (15 August to 15 March of the following year).
The burying depth of the bivalves was measured once or twice a month during the seven years 1980 to1986. The methods have been described by Zwarts & Wanink (1989). The combination of biomass samples and depth measurements was used to describe the annual fluctuation in the biomass actually accessible to birds; see Zwarts & Wanink (1993) for a detailed description of the seasonal and annual variation in biomass and prey accessibility.
There was no human impact on the food supply in the study area. No dredging for Cockles Cerastoderma edule occurred within the study period and only a few people dug for Lugworms Arenicola marina.
Feeding birds were counted, twice a month from 1977 to 1985, at low tide from the top of the sea wall. The birds were dispersed over the feeding area and were counted one by one. The total annual predation pressure by the waders, gulls and Shelduck Tadorna tadorna can be estimated by multiplying bird density and daily food consumption. An Oystercatcher Haematopus ostralegus needs 36 g AFDW per day (Zwarts et al. 1996c), but this increases with 4.5% for each EC below the critical air temperature of 10 EC (Kersten & Piersma 1987). As a consequence the daily consumption increases to 40-45 g in winter and averages 39.4 g. The predation pressure by the other shorebirds was estimated from the relationship between body weight and basal metabolic rate (BMR; Kersten & Piersma 1987), and from the general assumptions that (1) the daily energy expenditure is equivalent to 2.2 x BMR, increasing to 4 x BMR at low temperatures (Kersten & Piersma 1987), (2) the average energy content of flesh is 22 kJ g-1 and (3) 80% of the ingested energy is digested. It should be noted that earlier studies estimated field metabolism as 3 x BMR for non-passerines (Lasiewski & Dawson 1967; Aschoff & Pohl 1970). This produces a daily energy consumption similar to our estimate, because the BMR estimated from the general avian allometric equation is 40% lower than that of Kersten & Piersma (1987) for waders alone.
Prey and size selection and intake rate were studied in detail for two bird species feeding around the towers in the eastern part of the study area: Curlews Numenius arquata (Ens & Zwarts unpubl.; Zwarts & Wanink 1984; Zwarts & Esselink 1989) and Oystercatchers (Hulscher 1982 & unpubl., Blomert et al. 1983, Zwarts & Wanink 1984, Zwarts et al. 1996b & unpubl., Hulsman unpubl.). For the other bird species, prey and size selection was determined by direct observation and pellet analysis (Zwarts 1981; Zwarts & Esselink 1989; Zwarts & Blomert 1992 & unpubl.; Zwarts & Wanink 1993).
RESULTS
Annual production of food resources on the tidal flats
The average standing crop, i.e. the total biomass of all macrobenthic animals averaged over the entire year, was 81 g m-2, of which most was contributed by bivalve species: Cockle Cerastoderma edule, Scrobicularia plana, Baltic Tellin Macoma balthica, Soft-bodied Clam Mya arenaria and Mussel Mytilus edulis (Zwarts & Wanink 1993). The average annual production of these five bivalve species was estimated at 58 g m-2, and including Ragworm Nereis diversicolor, Lugworm Arenicola marina, Mudsnail Hydrobia ulvae and other less common species, 75-80 g m-2. For several reasons, a part of this production is not usable by birds. First, large prey may live out of reach of the bill. Among the bird species occurring in the area, the Curlew has the longest bill, 14 cm. The fraction of Mya living more than 14 cm beneath the surface, and thus safe from all kinds of bird predation, increases from 3% to 99% when the clams grow from 35 to 75 mm long (Zwarts & Wanink 1989). The average contribution of Mya to the total standing crop amounted to 13.3 g m-2, of which 9.1 g m-2, or 72%, referred to clams burying > 14 cm deep. The average annual elimination of Mya was estimated at 9.9 g m-2, of which half is contributed by inaccessible clams. Hence 6% of the total annual elimination referred to mortality of Mya living too deep to be accessible to any predator.
A second source of mortality also not due to predation, occurred during catastrophes. About half of the totally produced cockle flesh disappeared in very short periods. This was due to frost in January and possibly due to parasite infestation in October (Jonsson & Andr
J 1992) and, on an annual basis, amounted to 11.4 g m-2. Dying Cockles begin to gape and come to the surface, so the flesh can be eaten by all predators. There were only two bird species, however, which respond by a sudden increase in their numbers: Herring Gull Larus argentatus and, to a lesser degree, Common Gull Larus canus. Although waders did not aggregate in areas with moribund cockles, the birds already present started to eat this easily accessible food resource. Even a small wader such as a Dunlin Calidris alpina was observed to eat morsels of Cockle flesh. Nevertheless, the amount of flesh consumed by all birds, including gulls, remained very low compared to the high rate at which the flesh disappeared. Assuming that the birds in these periods took only what they needed to meet their daily energy requirements, they consumed 10% of this food supply suddenly on offer. This implies that a total of 10 g m-2 year-1, or another 13% of the total annual elimination was not due to predation. To this may be added the many large Scrobicularia dying when they were 7 years old and the Macoma with a high parasite load that crawl over the surface (Hulscher 1973) but are nevertheless refused by Oystercatchers (Hulscher 1982).Taking all this information together, we estimate that, in total, only 50-60 of the 75-80 g m-2 year-1 was available to predation, or about 70%. This is in the same order of magnitude as the earlier estimation by Beukema (1981) for the Dutch Wadden Sea as a whole. One should realise, however, that both estimates do not include the production of, and the predation on, young spat. New recruitment is underestimated (1) because spatfall extends over several weeks, during which many may be taken by predators and (2) the very young spat are missed altogether because they pass through the 1-mm sieve (e.g. Günther 1991, 1992; Guillou & Tartu 1994). There are two other reasons why actual annual production must be higher than estimated. First, predators usually take the large, most profitable, prey. Consequently, the actual growth of individual prey species, and thus also their production, is underestimated when measurements are based on surviving individuals. Second, predators often take only regenerating parts of their benthic prey, such as the tip of the tail or the siphon (de Vlas 1979, 1985); this biomass had not been added to predation estimates. An extreme example is provided by the study of Moreira (1997), who estimates that in the Tagus estuary (Portugal), half of the total biomass consumption of the birds consists of siphons of Scrobicularia.
Total consumption by the birds
The Oystercatcher was the most common shorebird species in the study area with 8.4 birds ha-1, averaged over the entire year (Fig. 1A). The average feeding densities of Curlew, Redshank Tringa totanus and Dunlin were only 2.6, 1.7 and 1.1 birds ha-1, respectively, and in other wader species it was < 1 bird ha-1. The total predation pressure by Oystercatchers was estimated as 12 g m-2 year-1. The total predation pressure by all bird species amounted to 22.3 g m-2 year-1, of which 54% can thus be attributed to Oystercatchers (Fig. 1B). This total consumption represents about 30% of the total, and 40% of the available, production.
The estimate of 22 g m-2 year-1 consumed by the waders, gulls and Shelduck together was four times as much as the average predation pressure by birds on the tidal flats in the Dutch Wadden Sea as a whole (Smit 1981). The average total biomass (81 g) and the estimated annual elimination (75-80 g) of all macrobenthic animals were also four times as much as the average values for the entire Dutch Wadden Sea (Beukema 1976, 1981). As already shown by Beukema (1976), the biomass of macrozoobenthos varies with elevation on the tidal flat and with the clay content of the substrate. The highest biomass values are found in mudflats with a mixed substrate and which are situated at around mean sea (tidal) level. This was exactly the situation in our study area along the Frisian coast. Hence, the biomass and production of the benthos, as well as the density of the birds and their levels of predation, in our area do not seem to deviate from those in other highly productive mudflats in the Wadden Sea. The composition of the bird community along the Frisian coast was different, however, from that in the Wadden Sea as a whole. For instance, the Oystercatchers along the Frisian coast took more food than all other bird species together, whereas in the Wadden Sea as a whole, the other bird species take twice as much food as the Oystercatcher.
Prey depletion by Oystercatchers
Since the exploitation of the food resources by Oystercatchers has already been described (Zwarts et al. 1996d), this section includes only a short summary. The Cockle was the major prey of Oystercatchers in our area. More than half of their average annual consumption consisted of cockle flesh. Cockles also contributed about half of the total elimination, summed over the five most common bivalve species. Oystercatchers took 25% of the total elimination by Cockles and Macoma, 22% of Mya, 17% of Scrobicularia but 0% of Mussels. These percentages refer to the entire year, but few Oystercatchers were present in summer. To investigate to what degree the elimination of winter biomass was due to oystercatcher predation, the data were divided into two periods: 15 August to15 March ('winter') and the remaining five months ('summer'). Oystercatchers during the seven winter months took seven times as much food as during the five summer months, 10.5 and 1.5 g year-1 respectively. Total elimination in the winter was twice as high as during the summer, 37.3 and 19 g year-1 respectively. Hence, Oystercatchers were responsible for only 8% of the elimination in summer, but 28% in winter.
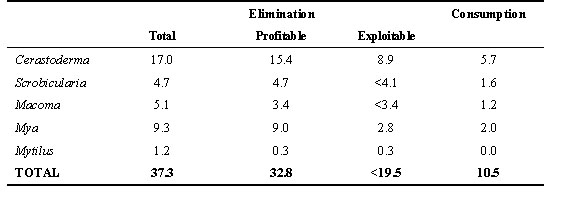
These calculations show that predation by Oystercatchers was not a very important cause of mortality of these bivalve prey, but the risk of a bivalve being taken by an Oystercatcher varied enormously between different prey categories. First, Oystercatchers ignore small, unprofitable prey (Fig. 2). The over-winter elimination of these prey was relatively small, except in Mussels (Table 1). It was more important to take into account the elimination of prey living out of reach of the bill. All Mya > 45 mm were inaccessible for Oystercatchers, as a result of which only 1/3 of the elimination of Mya could be harvested by Oystercatchers. All Scrobicularia were also out of reach of the bill during most of the winters. This reduced the over-winter harvestable elimination of Scrobicularia from 4.7 g to less than 4.1 g m-2. Gaping Cockles during short periods of mass mortality are an example of a food resource that, although harvestable, could not be fully utilised. On average, 4.7 and 4.6 g Cockle biomass m-2 disappeared during mass starvation in October and during ice periods in winter, respectively. The Oystercatchers consumed 1.3 and 1.5 g m-2 of these amounts, respectively. Hence, 3.4 g during the October starvation periods and 3.1 g during the cold spells were, for the Oystercatchers, wasted. This reduced the over-winter elimination of profitable Cockles exploitable by Oystercatchers from 15.4 to 8.9 g m-2. The percentage of the exploitable elimination actually taken by Oystercatchers varied between prey species, being extremely high in Mya and Cockles and low in the other species (Table 1). In the winter period, 3/4 of the Mya biomass and 2/3 of the cockle biomass eliminated could be attributed to oystercatcher predation (Table 1). Mussels were not taken during the study period, because hardly any reached a size at which they were profitable for Oystercatchers.
The average winter predation by Oystercatchers amounted to 10.5 g m-2 (Table 1), but it was only 1.3 g m-2 in 1981/1982 and 23.2 g in 1985/1986, respectively 0.12 and 2.2 times the long-term average. The variation in the total elimination during the seven winter months was less extreme, between 7.3 g m-2 in 1979/1980 and 66.9 in 1985/1986. The total loss of biomass from August to March was larger than the biomass eliminated, due to the loss of condition in individual bivalves, which varied between 15.9 and 89.9 g m-2 in different winters. Figure 18 in Zwarts et al. 1996d plots the total loss of biomass during the seven winter months in relation to the biomass on 15 August. Four types of biomass loss were distinguished. First, loss of body weight in the macrozoobenthos still alive on 15 March, and three types of elimination of biomass: oystercatcher predation, mass starvation of Cockles not consumed by Oystercatchers, and other sources. The higher the biomass, the higher the loss: the macrozoobenthos lost about 20% of their body weight between 15 August and 15 March, and this fraction was independent of the initial density. In contrast, the total elimination was highly positively density dependent which was completely due to the response of the Oystercatchers. Oystercatchers consumed only a few per cent when the food supply was poor but this increased to 17% in winters with a rich food supply. The elimination of prey from causes not due to oystercatcher predation increased only weakly with density, from 10 to 15%.
Competition for food among the bird species
Extensive analysis of the prey fragments found in the pellets and droppings of nine wader and gull species, and direct observation of feeding birds revealed that most bird species in the study area took Shore Crabs Carcinus maenas, Common Shrimps Crangon crangon and Ragworms. In the case of Shore Crabs it could be shown that Curlews and Herring Gulls took crabs with carapaces twice and three times as large as those of crabs taken by Redshank and Greenshank Tringa nebularia (Zwarts 1981). Differences in size selection were also found in shorebirds feeding on bivalves, as shown by the three examples given below.
Species feeding on Mya
The only bird species besides the Oystercatcher that selected large bivalves was the Curlew. This species extracted the flesh from Mya >25 mmin length, thus taking the size classes living out of reach of the Oystercatcher's bill (Zwarts & Wanink 1984). The Oystercatchers took Mya the year before Curlews started to eat them, and because they took so many of these prey (Fig. 5 in Zwarts et al. 1996d) severely depleted this potential food resource for Curlews. This was possibly the only circumstance in which Oystercatchers preyed upon an invertebrate species potentially important for another bird species. The reverse occurred quite often.
Knot, Oystercatcher and Curlew feed on successive ages of Mya, respectively some months, 1.5 year and >2 years old. There is hardly any overlap in the size classes taken by the three bird species (Fig. 3A). Calculated over all sampling dates, the average density in the study area was 57 Mya m-2 and the average biomass 13.77 g m-2. Half of the numbers, but only 1.5% of the biomass, were too small to be profitable for Oystercatchers. In contrast, 9% of the numbers, but 70% of the biomass were out of reach of the Curlew's bill, and thus safe from all predation (Fig. 3A).
The observed decrease in Mya density could be attributed to the predation pressures from Oystercatcher and Curlew (Zwarts & Wanink 1984; Fig. 3B). Since both bird species were scarce between April and August, mortality of Mya was low in late spring and early summer and high in the rest of the year. Knot occurred in large numbers in late summer only. We know, however, that Knot did not contribute much to the mortality of the spat, because the birds hardly visited the study area in August in the years when there was heavy spatfall of Mya (Fig. 15 in Zwarts & Wanink 1993), probably because they preferred to feed on the extensive beds of Mussel spat on the lower shore. Hence it is much more likely that the high mortality of Mya spat was due to juvenile Shore Crabs which occurred in extremely high density (76 m-2) in late summer. Moreover, it has been shown experimentally that small Mya are easy, highly profitable, prey for Shore Crabs (Blomert & Zwarts unpubl.). Hence, it very likely that Shore Crabs in the field selected the small Mya found at, or just below, the surface.
Although Knot hardly exploited the spat of Mya during our study period, they are known to do so in other circumstances. For example, all Knot present in the Mokbaai on the Frisian island of Texel fed on Mya spat in October 1994. A flock of 30 Knot returned for more than a week to the same patch of 1000 m2 bare mudflat between some mussel ridges. Their feeding behaviour was studied in detail during 6 consecutive days by Blomert & Zwarts (unpubl.). Since each Knot consumed 2500 spat per day, the flock removed 75 spat m-2 day-1. The prey density was 4000 spat m-2. Thus, such exploitation could continue at this level for maximally two months. It is unknown whether the birds remained to feed at this particular patch, but the first sampling of the benthos after the winter 1994/1995 revealed that none of the Mya spat was left (Ens unpubl.). Hence it is likely that Knot may severely deplete this potential food resource of Oystercatchers.
Species feeding on Macoma
Two wader species, Knot and Bar-tailed Godwit Limosa lapponica, were observed to take medium-sized Macoma which still need one or two growing seasons to become profitable for Oystercatchers. The predation pressure by both species was low (Fig. 1), since they were not common and occurred each year in only short periods: the Knot in August and the Bar-tailed Godwit in May. As Fig. 2 shows, there is hardly any overlap in the size classes of Macoma taken by Knot and Oystercatcher. Knot feed in dense flocks of 100-300 birds ha-1 and since each individual consumes about 10 g AFDW day-1 (Piersma et al. 1994), they may exert a heavy predation pressure. For example, when Knot feed in a typical density of 200 birds ha-1 and select Macoma 10 mm long, which contain 10 mg AFDW, they remove 10 Macoma m-2 per low water period. Assuming that the birds return to the same spot day after day, they might consume about 600 Macoma m-2 10 mm long within a month, and thus almost completely eliminate the local Macoma populations within some months. It is not the habit of Knot, however, to feed for many days on exactly the same spot. Instead, they roam over the intertidal feeding areas and visit even the richest areas during only a few low water periods (Zwarts et al. 1992). In our study area, the predation pressure by Knot was so low (Fig. 1B) that, although they took Macoma, and thus potential prey for Oystercatchers, they hardly effected the food supply harvestable by Oystercatchers. This might be different in other years and other sites, however.
Macoma is not a major prey for Herring Gulls, but in April, May and June we saw several times that birds took Macoma by 'puddling', first described by Medcof (1949) for Herring Gulls taking small Mya. We never saw this feeding method used during the rest of the year, probably because prey have to be very shallow to bring them to the surface in this way. Herring Gulls have no problem in swallowing the largest Macoma, so there is no reason to assume that they will reject the larger size classes.
Species feeding on Mytilus
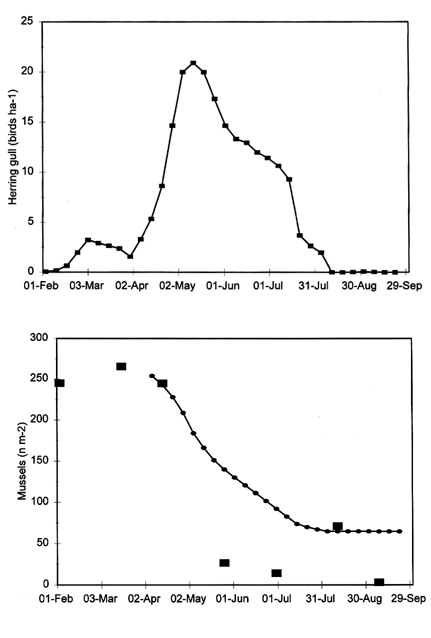
The effect of Herring Gulls Larus argentatus on the food supply of the Oystercatcher could be dramatic. The Herring Gull took a large array of prey species, including large Shore Crabs in early summer and moribund bivalves during, and after, frost periods. Herring Gulls were observed to select second year Mussels during several months in the summer of 1985. The birds were present in large numbers between mid April and mid July (Fig. 4A). Because Mussels occur in clumps, it is difficult to get an accurate estimate of the density at which they occur but, obviously, the second year Mussels decreased from about 250 m-2 in early April to less than 30 from June onwards (Fig. 4B). To estimate the number of second year Mussels removed by Herring Gulls, the bird density (Fig. 4A) was multiplied by the presumed number of prey taken. Since Herring Gulls need 60 g dry flesh per day (Spaans 1971 and assuming that AFDW is 17% relative to fresh weight), they would have had to consume, daily, 7500 Mussels in March, when the average prey weighed 8 mg, 3000 of 20 mg in April and 1200 of 50 mg from May to July. The observed decline in the prey density may be compared to the predicted decrease of the second year Mussels assuming that all Herring Gulls foraged on this prey only and performed no size selection. The few Herring Gulls present in March and early April cannot have foraged on the small Mussels, because the prey densities were fairly constant until mid April. It seems most likely that the Herring Gulls started to exploit the Mussels when they reached 13 mm in length and weighed more than 20 mg. The few Mussels still remaining in September, at the end of the growing season, measured 28 mm. Mussels of this size would offer Oystercatcher an exploitable food resource (Fig. 3 in Zwarts et al. 1996d), although the birds have to wait another year before the prey become profitable. In fact, none of the Mussels of this year class was given the opportunity to be eaten by Oystercatchers because Herring Gulls had already taken them.
This example shows that Herring Gulls may remove all Mussels of a year class before these prey reach the lower acceptance size threshold for Oystercatcher. The potential impact of Herring Gulls is especially large if they take very small spat. Although such spat do not contain much flesh, they form a harvestable prey for Herring Gulls, since they can be swallowed in clumps (Sibly & McCleery 1983). If, for instance, Herring Gulls eat Mussel spat of 3 mm, containing about 0.2 mg flesh, each individual bird might remove 300,000 spat per day, given a daily consumption of 60 g dry flesh. Herring Gulls feed on mussel beds at an average density of 50 birds ha-1 (Fig. 7 in Zwarts & Drent 1981), and this may even be higher after heavy spatfall. This implies that, even at an average density of 50 birds ha-1, the gulls would be able to remove 1500 Mussels of 3 mm m-2 day-1 and thus completely eliminate a strong year class of 50,000 spat m-2 within a month. Herring Gulls not only take Mussel spat, but also larger size classes. Harris (1965) found that Herring Gulls took Mussels up to 23 mm in length. It is likely that larger ones may be swallowed, however, because the average diameter of a pellet is 28 mm (Wietfield 1977) or even 32 mm (Ehlert 1957), equivalent to a circumference of 47 mm. Using the equations given by Zwarts & Blomert (1992), this would mean that Herring Gulls may swallow Mussels to a maximum of 33 mm long. Even if Herring Gulls take Mussels up to this upper size threshold, there is still hardly any overlap with the size classes taken by Oystercatchers, because 30 mm is about the lower acceptance threshold for Oystercatchers.
The locations of Mussel beds scarcely change from year to year (Dankers & Koelemaij 1989; Obert & Michaelis 1991), but local variations in age structure, density and biomass of Mussels are extremely large, mainly due to erratic spatfall. A mussel bed near Schiermonnikoog, studied between 1967 and 1979, attracted large numbers of Oystercatchers before the winter of 1973/74, but not in later years, because the density of Mussels >40 mm was too low to be exploited by Oystercatchers (Zwarts & Drent 1981). In the good years, the Oystercatchers foraged at a density of 100 birds ha-1 and removed about 40% of the biomass per year. They depleted their own food resource and there was no replacement of large Mussels by growth of smaller ones because Herring Gulls had removed these. Hence, Oystercatchers left the mussel beds and switched to feed on surrounding mudflats (Zwarts & Drent 1981).
DISCUSSION
Role of birds in the intertidal food web
Beukema (1981) estimated the total annual predation pressure on the macrozoobenthos of the Dutch Wadden Sea by birds, fish, crabs, shrimps and man to be 13-16 g m-2 which is equivalent to 65-80% of the annual somatic secondary production of 20 g m-2. However, Smit (1981) estimated that all bird species together did not take more than 4.1 g m-2 . Hence, the role of waders in the intertidal food web is not as dominant as the impressive numbers of waders on the roosts might suggest. The relatively low predation pressure by birds is not surprising, however, if one realises that shorebirds feed at low tide at densities of only about 10 birds ha-1, on average, whereas shrimps and Shore Crabs may occur at average densities 1000 to 10000 times as high (Beukema 1991, 1992a). Field data and exclosure experiments confirm that epibenthic predators are able to remove a high proportion of the benthic stock (e.g. Reise 1978; Jensen & Jensen 1985; Sanchez-Salazar et al. 1987). The studies summarised in this paper, as well as several other studies, show that waders feeding in the tidal zone usually reduce the density of their prey populations by 20-40% during a winter, although on occasion by more than 90% (reviewed by Meire 1993).The generally limited role of waders in the intertidal food web in terms of biomass consumption might lead one to suspect that they are not limited by their food supply, but such a conclusion would be premature.
Are bird numbers limited by their food supply on the tidal flats?
Many workers have attempted to answer the question whether the numbers of shorebirds feeding on intertidal flats are limited by their food supply. One approach has been to measure the total consumption by the birds and comparing this to the total production of all their prey animals, such as was done for the Ythan estuary (Scotland) by Milne & Dunnett (1972) and Baird & Milne (1981). A fundamental shortcoming of such studies is that production is not independent of the consumption. A higher predation pressure will often enhance the total production of the macrozoobenthos. Recruitment and growth have been shown to be negatively density-dependent (Hancock 1973; Beukema & de Vlas 1979; Brock 1980; André & Rosenberg 1991; Jensen 1992; McGrorty et al. 1990). Moreover, elimination of long-lived species, such as Lugworms and Cockles, not only creates more room for their own recruitment, but also for other, usually short-lived, species, such as the amphipod Corophium volutator (Flach 1992). This results in an increase in the total production of the macrozoobenthos, because the population turn-over is higher in short-lived species (Robertson 1979). Consequently, a higher predation pressure may simply result in a higher production. There is no simple solution to this problem.
A second less fundamental shortcoming of production/consumption studies is that waders depending on the intertidal zone face large year-to-year variations in their food supply (Beukema et al. 1993). Hence it is not sufficient to know the average production, but also the variation in annual production (Baird et al. 1985) and the fraction disappearing as a result of disease or severe winter weather and hence unavailable to predators. As our study shows, this problem can be solved by continuing measurements over a great many years.
Third, some species of prey can grow so large that they become no longer harvestable by waders. Thus, it is important to study size selection by the birds as well as to measure the production of each size class of the benthos, as in this study. The second and the third problems may often combine in the Wadden Sea: many benthic species reproduce successfully after a severe winter (Beukema 1982b), so that huge numbers of young benthic animals occur on the mudflats and are easy prey for many birds. However, after 5 to 10 years, old specimens of long-lived species, such as Mya and Scrobicularia, constitute a dominant fraction of the biomass production but can no longer be harvested by the birds. This occurred on the Balgzand (Dutch Wadden Sea) with Mya (Beukema 1976; Beukema et al. 1978) and with Scrobicularia in our study area in the eastern part of the Dutch Wadden Sea (Fig. 4 in Zwarts & Wanink 1993; Fig. 16 in Zwarts et al. 1996d).
Clearly, measures of the predation pressure by shorebirds on tidal-flat invertebrates may help to answer the question whether food limits bird numbers only if the measurements are continued over a long period and are supplemented by detailed measurements on the harvestable fractions of the prey. In addition, measurements have to be collected on the daily consumption by the birds because this will indicate whether, and how often, the local harvestable food supply is insufficient to guarantee their daily food requirements. This approach was used by Zwarts et al. (1996d). They were able to show that Oystercatchers need an intake rate of 1 mg s-1 during feeding to meet their daily energy requirements. During the summer, the intake rate varied between 2 and 4 mg s-1 and thus was always high enough. In winter, however, the intake rate was below this critical level during four of the ten years. Hardly any Oystercatchers remained in the study area during these four poor winters, because they moved to feeding areas further offshore. Regrettably, we did not have information on the feeding conditions for the birds in these alternative feeding areas. Nevertheless, it could be shown that the winter mortality in years with a low intake rate was twice as high as in years with a high intake rate (due to a good food supply in our study area).
Probably the most interesting result of our study is the suggestion that bird species that show little overlap in prey choice may in actual fact be strong competitors. Bird species that ate the smaller size classes of a particular prey species significantly depleted the food stocks for bird species that depended on the larger size classes. This happened when Herring Gulls ate so many small Mussels that no large Mussels remained for Oystercatchers to feed on in later years. It also happened in Mya. If the Oystercatchers had not taken so many small ones, many more could have grown to a size where they could have been harvested by Curlews. Even if the dynamics of the prey is incorporated in models of interspecific competition, it is rarely the case that the prey is allowed a distinct life history, where different predators feed on different life history stages. Van der Meer (1997) has started to explore the theoretical consequences of this phenomenon and finds, not surprisingly, that the competitive effects are unidirectional. When the recruitment of the prey is not affected by the size of the reproductive stock, the density of the predator feeding on the large prey is depressed by the density of a different predator feeding on the small prey, but there is no effect in the reverse direction. The most interesting prediction is that increasing year-to-year variability in prey recruitment reduces the deleterious competitive effects of the small-prey specialist on the large-prey specialist. Thus, both empirical and theoretical studies underline the need to include the life history of the prey in studies of interspecific competition between predators.
ACKNOWLEDGEMENTS
Peter Evans commented on a earlier draft of this paper.
REFERENCES
André, C. & Rosenberg, R. 1991. Adult-larval interactions in the suspension-feeding bivalves Cerastoderma edule and Mya arenaria. Marine Ecology Progress Series 71: 227-234.
Aschoff, J. & Pohl, H. 1970. Der Ruheumsatz von Vögeln als Funktion der Tageszeit und der Körpergrösse. Journal für Ornithologie 111: 38-47.
Baird, D. & Milne, H. 1981. Energy flow in the Ythan estuary, Aberdeenshire, Scotland. Estuarine Coastal Shelf Science 13: 455-472.
Baird, D., Evans, P.R., Milne, H. & Pienkowski, M.W. 1985. Utilization by shorebirds of benthic invertebrate production in intertidal areas. Oceanography Marine Biology Annual Review 23: 573-597.
Beukema, J.J. 1976. Biomass and species richness of the macro-benthic animals living on the tidal flats of the Dutch Wadden Sea. Netherlands Journal of Sea Research 10: 236-261.
Beukema, J.J. 1979. Biomass and species richness of the macrobenthic animals living on a tidal flat area in the Dutch Wadden Sea: effects of a severe winter. Netherlands Journal of Sea Research 13: 203-223.
Beukema, J.J. 1980. Calcimass and carbonate production by molluscs on the tidal flats in the Dutch Wadden Sea: I. the tellenid bivalve Macoma balthica. Netherlands Journal of Sea Research 14: 323-338.
Beukema, J.J. 1981. The role of the larger invertebrates in the Wadden Sea ecosystem. In: N. Dankers et al. (eds) Invertebrates of the Wadden Sea, p. 211-221. Balkema, Rotterdam.
Beukema, J.J. 1982a. Calcimass and carbonate production by molluscs on the tidal flats in the Dutch Wadden Sea: II. the edible cockle, Cerastoderma edule. Netherlands Journal of Sea Research 15: 391-405.
Beukema, J.J. 1982b. Annual variation in reproductive success and biomass of the major macrozoobenthic species living i a tidal flat area of the Wadden Sea. Netherlands Journal of Sea Research 16: 37-45.
Beukema, J.J. 1985. Zoobenthos survival during severe winters on high and low tidal flats in the Dutch Wadden Sea. In: J.S. Gray & M.E. Christiansen (eds.) Marine biology of polar regions and effects of stress on marine organsms, p. 351-361. John Wiley, Chichester.
Beukema, J.J. 1989a. Long-term changes in macrozoobenthic abundance on the tidal flats of the western part of the Dutch Wadden Sea. Helgoländer Meeresuntersungen 43: 405-415.
Beukema, J.J. 1989b. Bias in estimates of maximum life span, with an example of the edible cockle, Cerastoderma edule. Netherlands Journal of Sea Research 39: 79-85.
Beukema, J.J. 1991. The abundance of Shore Crabs Carcinus maenas (L.) on a tidal flat in the Wadden Sea after cold and mild winters. Journal experimental marine Biology Ecology 153: 97-113.
Beukema, J.J. 1992a. Dynamics of juvenile shrimp Crangon crangon in a tidal-flat nursery of the Wadden Sea after mild and cold winters. Marine Ecology Progress Series 83: 157-165.
Beukema, J.J. 1992b. Expected changes in the Wadden Sea benthos in a warmer world: lessons from periods with mild winters. Netherlands Journal of Sea Research 30: 73-79.
Beukema, J.J. 1993. Increased mortality in alternative bivalve prey during a period when the tidal flats of the Dutch Wadden Sea were devoid of mussels. Netherlands Journal of Sea Research 31: 395-406.
Beukema, J.J. & J. de Vlas 1979. Population parameters of the Lugworm, Arenicola marina, living on tidal flats in the Dutch Wadden Sea. Netherlands Journal of Sea Research 13: 331-353.
Beukema, J.J., de Bruin, W. & Jansen, J.J.M. 1978. Biomass and species richness of the macrobenthic animals living on the tidal flats of the Dutch Wadden Sea: long-term changes during a period with mild winters. Netherlands Journal of Sea Research 12: 58-77.
Beukema, J.J, Essink, K., Michaelis, H. & Zwarts, L. 1993. Year-to-year variability in the biomass of macrobenthic animals on tidal flats of the Wadden Sea: how predictable is this food source for birds? Netherlands Journal of Sea Research 31: 319-330.
Blomert, A.-M., Engelmoer, M. & Logemann, D. 1983. Voedseloecologie van de scholekster op het Friese wad. Report Rijkdienst voor de IJsselmeerpolders, Lelystad.
Brock, V. 1980. Notes on relations between density, settling, and growth of two sympatric cockles, Cardium edule (L.) and C. glaucum (Bruguière). Orphelia Supplement 1: 241-248.
Crisp, D.J. 1984. Energy flow measurements. In: N.A. Holme & A.D. McIntyre (eds.) Methods for the study of marine benthos, p. 284-372. Blackwell Scientific Publications, Oxford.
Dankers, N. & Koelemaij, K. 1989. Variations in the mussel population of the Dutch Wadden Sea in relation to monitoring of other ecological parameters. Helgoländer Meeresuntersungen 43: 529-535.
Dare, P.J. 1975. Settlement, growth and production of the mussel, Mytilus edulis L., in Morecambe Bay, England. Fisheries Investigation London (Series II) 28: 1-25.
Ens, B.J., Piersma, T. & Drent, R.H. 1994. The dependence of waders and waterfowl migrating along the East Atlantic Flyway on their coastal food supplies: what is the most profitable research program? Ophelia Suppl. 6: 127-151.
Ehlert, W. 1957. Zur Ernährung der Silbermöwe (Larus argentatus) in der Vorbrutzeit. Ornithologische Mitteilungen 9: 201-203.
Flach, E.C. 1992. The influence of four macrozoobenthic species on the abundance of the amphipod Corophium volutator on tidal flats in the Wadden Sea. Netherlands Journal of Sea Research 29: 379-394.
Gayanilo, F.C. Jr, Soriano, M. & Pauly, D. 1988. A draft guide to the Compleat ELEFAN. ICLARM Software 2. International Center for Living Aquatic Resources Management, Manila.
Goss-Custard, J.D. 1969. The winter feeding ecology of the Redshank Tringa totanus. Ibis 111: 338-356.
Goss-Custard, J.D. 1977. The ecology of the Wash III. Density-related behaviour and the possible effects of a loss of feeding grounds on wading birds (Charadrii). Journal of applied Ecology 14: 721-739.
Goss-Custard, J.D., McGrorty, S. & dit Durell, S.E.A. le V. 1996a. The effect of Oystercatchers Haematopus ostralegus on shellfish populations. Ardea 84A: 453-468.
Guillou, J. & Tartu, C. 1994. Post-larval and juvenile mortality in a population of the edible Cockle Cerastoderma edule (L.) from northern Brittany. Netherlands. Journal of Sea Research 33: 103-111.
Günther, C.-P. 1991. Settlement of Macoma balthica on an intertidal sandflat in the Wadden Sea. Marine Ecology Progress Series 76: 73-79.
Günther, C.-P. 1992. Settlement and recruitment of Mya arenaria L. in the Wadden Sea. Journal of experimental marine Biology and Ecology159: 203-215.
Hancock, D.A. 1971. The role of predators and parasites in a fishery for the mollusc Cardium edule L. Proceedings Advanced Study Inst. Dynamics Numbers Popul. Oosterbeek, p. 419-439.
Hancock, D.A. 1973. The relationship between stock and recruitment in exploited invertebrates. Rapports et processes verbaux de la reunion du conseil pour la exploration de la mer 164: 113-131.
Harris, M.P. 1965. The food of some Larus gulls. Ibis 107: 43-53.
Hulscher, J.B. 1973. Burying-depth and trematode infection in Macoma balthica. Netherlands Journal of Sea Research 6: 141-156.
Hulscher, J.B. 1982. The oystercatcher (Haematopus ostralegus) as a predator of the bivalve Macoma balthica in the Dutch Wadden Sea. Ardea 70: 89-152.
Jensen, K.T. 1992. Dynamics and growth of the cockle, Cerastoderma edule, on an intertidal mud-flat in the Danish Wadden Sea: effects of submersion time and density. Netherlands Journal of Sea Research 28: 335-345.
Jensen, K.T. & Jensen, J.N. 1985. The importance of some epibenthic predators on the density of juvenile benthic macrofauna in the Danish Wadden Sea. Journal experimental marine Biology Ecology 89: 157-174.
Jonsson, P.R. & André, C. 1982. Mass mortality of the bivalve Cerastoderma edule on the Swedish west coast caused by infestation with the digenean trematode Cercaria cerastodermae I. Orphelia 36: 151-157.
Kersten, M. & Piersma, T. 1987. High levels of energy expenditure in shorebirds; metabolic adaptations to an energetically expensive way of life. Ardea 75: 175-187.
Lasiewski, R.C. & Dawson, W.R. 1967. A reexamination of the relation between standard metabolic rate and body weight in birds. Condor 69: 13-23.
Levins, R. 1968. Evolution in changing environments. Princeton University Press, Princeton.
MacArthur, R.H. 1972. Geographical Ecology: Patterns in the Distribution of Species. Harper & Row, New York.
MacArthur, R.H. & Pianka, E.R. 1966. On the optimal use of a patchy environment. American Naturalist 100: 603-609.
MacArthur, R.H. & Levins, R. 1967. The limiting similarity, convergence and divergence of coexisting species. American Naturalist 101: 377-385.
McGrorty, S., Clarke, R.T., Reading, C.J. & Goss-Custard, J.D. 1990. Population dynamics of the mussel Mytilus edulis density changes and regulation of the population in the Exe estuary, Devon. Marine Ecology Progress Series 67: 157-169.
Medcof, J.C. 1949. 'Puddling' - A method of feeding by herring gulls. Auk 66: 204-205.
Meer, J. van der 1997. A handful of feathers: studies on the estimation and modelling of temporal and spatial fluctuations in the numbers of birds and their prey. PhD dissertation, University of Groningen.
Meire, P.M. 1993. The impact of bird predation on marine and estuarine bivalve populations: a selective review of patterns and underlying causes. In: R.F. Dame (ed.) Bivalve filter feeders in estuarine and coastal ecosystems process, p. 197-243. Springer-Verlag, Heidelberg.
Moreira, F. 1997. Diet of Black-headed Gulls Larus ridibundus on emerged intertidal areas in the tagus estuary (Portugal): predation or grazing? Journal of Avian Biology 26: 277-282.
Obert, B. & Michaelis, H. 1991. History and ecology of the mussel beds (Mytilus edulis L.) in the catchment area of a Wadden Sea tidal inlet. In: M. Elliott & J-P. Ducrotoy (eds.) Estuaries and coasts: spatial and temporal intercomparisons, p. 185-194. Olsen & Olsen, Fredensborg.
Piersma, T., Verkuil, Y. & Tulp, I. 1994. Resources for long-distance migration of Knots Calidris canutus islandica and C. c. canutus: how broad is the temporal exploitation window of benthic prey in the western and eastern Wadden Sea? Oikos 71: 393-407.
Reading, C.J. & McGrorty, S. 1978. Seasonal variations in the burying depth of Macoma balthica (L.) and its accessibility to wading birds. Estuarine and coastal marine Science 6: 135-144.
Reise, K. 1978. Experiments on epibenthic predation in the Wadden Sea. Helgoländer Meeresuntersungen 51: 55-101.
Robertson, A.I. 1979. The relationship between annual production: biomass ratios and life spans for marine macrobenthos. Oecologia 38: 193-202.
Sanchez-Salazar, M.E., Griffiths, C.L. & Seed, R. 1987. The interactive roles of predation and tidal elevation in structuring populations of the edible cockle, Cerastoderma edule. Estuarine Coastal Shelf Science 25: 245-260.
Sibly, R.M. & McCleery, R.H. 1983. The distribution between feeding sites of herring gulls breeding at Walney Island, U.K. Journal of Animal Ecology 52: 51-68.
Schoener, T.W. 1986. Mechanistic approaches to community ecology: a new reductionism? American Zoologist 26: 81-106.
Smit, C.J. 1984. Production of biomass by invertebrates and consumption by birds in the Dutch Wadden Sea areas. In: W.J. Wolff (ed.) Ecology of the Wadden Sea, 2, part 6, p. 290-301. Balkema, Rotterdam.
Spaans, A.L. 1971. On the feeding ecology of the herring gull Larus argentatus in the northern part of the Netherlands. Ardea 59: 73-188.
Tilman, D. 1990. Constraints and tradeoffs: toward a predictive theory of competition and succession. Oikos 58: 3-15.
Vlas, J. de 1979. Annual food intake by Plaice and Flounder in a tidal flat area in the Dutch Wadden Sea, with special reference to consumption of regenerating parts of macrobenthic prey. Netherlands Journal Sea Research 13: 117-153.
Vlas, J. de 1985. Secondary production by siphon regeneration in a tidal flat population of Macoma balthica. Netherlands Journal of Sea Research 19: 147-164.
Wiens, J.A. 1983. Avian community ecology: an iconoclastic view. In: A.H. Brush & G.A. Clark (eds.), Perspectives in ornithology, p. 355-403. Cambridge University Press, Cambridge.
Wietfield, J. 1977. Untersuchungen an Speiballen der Silbermöwe (Larus argentatus) im Naturschutzgebied Grosser Knechtsand (Elbe-Weser-Mündung). Vogelwelt 98: 221-229.
Zwarts, L. 1981. Habitat selection and competition in wading birds. In: C.J. Smit & W.J. Wolff (eds) Birds of the Wadden Sea, p. 271-279. Balkema, Rotterdam.
Zwarts, L. & Blomert, A-M. 1992. Why knot Calidris canutus take medium-sized Macoma balthica when six prey species are available. Marine Ecology Progress Series 83: 113-128.
Zwarts, L. & Drent, R.H. 1981. Prey depletion and the regulation of predator density: oystercatchers (Haematopus ostralegus) feeding on mussels (Mytilus edulis). In: N.V. Jones & W.J. Wolff (eds.) Feeding and survival strategies of estuarine organisms. Plenum Press, New York, p. 193-216.
Zwarts, L. & Esselink, P. 1989. Versatility of male curlews (Numenius arquata) preying upon Nereis diversicolor: deploying contrasting capture modes dependent on prey availability. Marine Ecology Progress Series 56: 255-269.
Zwarts, L. & Wanink, J. 1984. How oystercatchers and curlews successively deplete clams. In: P.R. Evans, J.D. Goss-Custard, W.G. Hale (eds.) Coastal waders and wildfowl in winter, p. 69-83. Cambridge University Press, Cambridge.
Zwarts, L. & Wanink, J. 1989. Siphon size and burying depth in deposit- and suspension-feeding benthic bivalves. Marine Biology 100: 227-240.
Zwarts, L. & Wanink, J.H. 1993. How the food supply harvestable by waders in the Wadden Sea depends on the variation in energy content, body weight, biomass, burying depth and behaviour of tidal-flat invertebrates. Netherlands Journal of Sea Research 31: 441-476.
Zwarts, L., Blomert, A.-M. & Wanink, J.H. 1992. Annual and seasonal variation in the food supply harvestable by knot Calidris canutus staging in the Wadden Sea in late summer. Marine Ecology Progress Series 83: 129-139.
Zwarts, L., Cayford, J.T., Hulscher, J.B., Kersten, M., Meire, P.M. & Triplet, P. 1996a. Prey size selection and intake rate. In: J.D. Goss-Custard (ed.) Behaviour and ecology of the Oystercatcher: from individuals to populations, p. 30-55. Oxford University Press, Oxford.
Zwarts, L., Ens, B.J., Goss-Custard, J.D., Hulscher, J.B., dit Durell, S.A.E. le V. 1996b. Causes of variation in prey profitability and its consequences for the intake rate of the Oystercatcher Haematopus ostralegus. Ardea 84A: 229-268.
Zwarts, L., Ens, B.J., Goss-Custard, J.D., Hulscher, J.B. & Kersten, M. 1996c. Why Oystercatchers Haematopus ostralegus cannot meet their daily energy requirements in a single low water period. Ardea 84A: 269-290.
Zwarts, L., Wanink, J.H. & Ens, B.J. 1996d. Predicting seasonal and annual fluctuations in the local exploitation of different prey by Oystercatchers Haematopus ostralegus: a ten-year study in the Wadden Sea. Ardea 84A: 401-440.
Table 1. Average over-winter consumption by Oystercatchers on tidal flats along the Frisian coast (Dutch Wadden Sea) (g m-2), compared to the average annual elimination of biomass (g m-2) between 15 August and 15 March (1) of all cohorts combined ('total'), (2) of the size classes large enough to be profitable for Oystercatchers (profitable prey are Cockles > 10 mm long, Scrobicularia > 13 mm long, Macoma > 11 mm long, Mya > 17 mm and Mussel > 25 mm), and (3) of exploitable prey i.e. profitable, accessible (Mya and Scrobicularia) and not dying in large numbers during extremely short periods.

Fig. 1. (A) Average density (birds ha-1 year-1) and (B) estimated total predation pressure (g m-2 year-1) by different waders and other shorebirds on tidal flats along the Frisian coast (Dutch Wadden Sea).
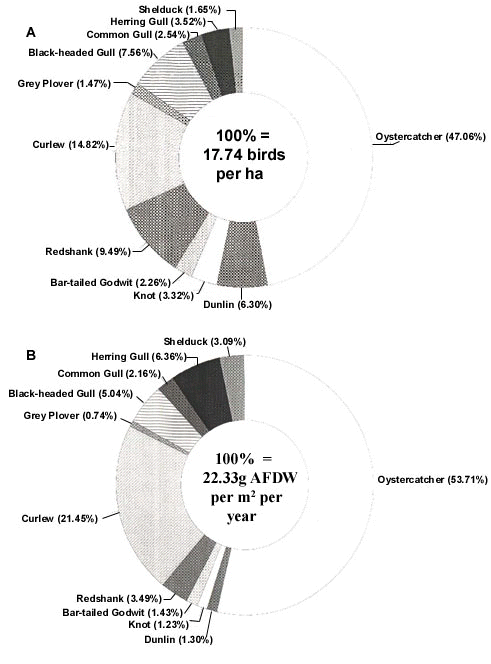
Fig. 2. Macoma. Average size selection by Knot (from Zwarts & Blomert 1992) and Oystercatcher (from Zwarts et al. 1996a). The size selection is given as relative prey risk, i.e. the ratio between relative number in the prey population and the relative number of prey taken, and expressed relative to the size class with the highest prey risk (set to 100).
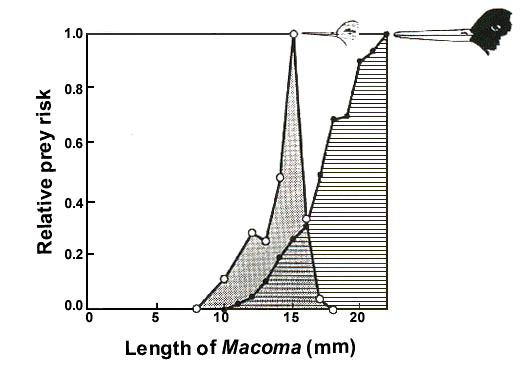
Fig. 3. Mya. (A) Burying depth as a function of shell size (from Zwarts & Wanink 1989); the range within which 80% of the clams are found is indicated. (B) Density as a function of shell size (year class 1979; Frisian coast; from Zwarts et al. 1996d); The fraction of prey harvestable by Knot, Oystercatcher and Curlew are indicated.
Fig. 4. (A) Feeding density of Herring Gulls and (B) actual (filled squares) and predicted (line joining filled circles) decline in the density of second year Mussels in spring 1985 on tidal flats along the Frisian coast. The predicted decrease is based on the assumption that each Herring Gull took Mussels only from early April onwards and in total 60 g AFDW day-1.