S39.1: Waders and their estuarine food supplies: Is predatory impact or predator behaviour the key to understanding carrying capacity?
Philip A.R. Hockey & Jane K. Turpie
Percy FitzPatrick Institute of African Ornithology, University of Cape Town, Rondebosch 7701, South Africa, e-mail phockey@botzoo.uct.ac.za
Hockey, P.A.R. & Turpie, J.K. 1999. Waders and their estuarine food supplies: Is predatory impact or predator behaviour the key to understanding carrying capacity? In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2294-2308. Johannesburg: BirdLife South Africa.
Waders (Charadrii) interact directly and indirectly with their estuarine prey. These interactions have feedbacks to the birds which influence patterns of wader distribution and abundance. These patterns themselves are linked in some poorly understood way to carrying capacity. Attempts to predict wader dispersion and abundance using sediment characteristics have met with limited success. Even classic trophodynamic studies, whilst having some predictive value at coarse spatial scales, generally have failed to provide meaningful insights into carrying capacity. This failure has stemmed in part from methodological deficiencies and in part from an inadequate understanding of bird behaviour. In recent years, however, significant advances have been made in understanding the foraging behaviour of waders, as well as their physiology and energy demands. Behavioural plasticity is sufficiently great, and trophodynamic interactions so complex, that attempts to define carrying capacity using one or other in isolation are likely to fail. One fairly robust model incorporating both predator-prey and behavioural interactions has been developed. However, the model is complex, data-hungry and centres on a single trophodynamic link. One of the major challenges facing wader biologists is to achieve a quantitative understanding of predator and prey interactions in multi-species wader assemblages – which are the norm.
INTRODUCTION
Until the mid-1970s, the majority of estuarine wader research was concentrated in north temperate latitudes: a statistical analysis of recent publications would probably confirm that this is still the case. Even as recently as 1993, Zwarts and Wanink (1993) challenged researchers to quantify seasonal availability of tidal-flat invertebrates from areas other than the Frisian coast!
More recently, extensive ecological research on waders has been carried out in Central and, to a lesser extent, South America, and tropical and subtropical Africa and Australia. This has provided a more balanced view of the waders’ world and, in some cases, has challenged ‘Holarcticentric’ dogma. Tropical-temperate comparisons have become a fruitful field of study and sufficient data are becoming available to attempt preliminary gradient analyses spanning wide latitudinal ranges (e.g. Castro et al. 1992, Hockey et al. 1992, Hockey & Barnes 1997). The full value of recent data from tropical and south temperate latitudes can, however, be realised only because of the thoroughness with which so many north temperate regions (such as the Frisian coast!) have been studied.
The direct and indirect impacts that shorebirds have on their prey supplies form only one element of a complex feedback loop that ultimately determines the distribution and abundance of these birds. One of the greatest challenges facing wader biologists is understanding the nature of the feedback loop between prey and predators and using this knowledge to explain the distribution and abundance of waders, assess the proximity of populations to carrying capacity and predict the effects of future habitat loss or change. Carrying capacity is one of the simplest of concepts and yet its estimation has proven to be one of the most confounding of practical problems. Our success in meeting these challenges will lie to a large extent in whether we are asking the most relevant questions or are guilty of doing ‘more of the same’.
In this review we start by considering efforts that have been made to explain wader distribution and abundance by using sediment characteristics as a surrogate for prey attributes. We then examine a range of impacts that waders may have on their estuarine prey and evaluate how measurement techniques and assumptions affect interpretation of these impacts. We address the extent to which the products of feedback loops from prey to predators, as integrated in the behaviour of the birds themselves, are a necessary adjunct to interpreting trophodynamic data. Finally, we assess how such behavioural studies may help us in our quest to understand carrying capacity.
Can we predict wader abundance and dispersion and is it useful?
Much research over the past few decades has concentrated on explaining the spatial distribution of waders in terms of the spatial distribution of their prey. Indeed, many studies have shown that shorebird densities and prey abundance are correlated over large spatial scales (> 1 km), but similar correlations at scales of 10s or 100s of metres are less easily detected. Intuitively, such small-scale correlations are most likely to be found among small bird species which eat small, abundant prey, such as amphipods. At the Mad River estuary, California, Colwell & Landrum (1993) were able to show that densities of Least Sandpiper Calidris minutilla and Western Sandpiper C. mauri were correlated with amphipod Corophium densities at spatial scales of > 10 m.
More recently, there has been some investigation into whether more simple, abiotic measures can be used as a short-cut to predict wader dispersion: abiotic characteristics are considerably easier and quicker to quantify than are prey attributes. Goss-Custard et al. (1991) compared bird densities, prey abundance and biophysical parameters across several estuaries in southern England. In general, bird density correlated with favoured prey density (a well-established pattern), but sediment characteristics and exposure time were less powerful predictors of bird density.
In a more detailed study on the Wash, England, Yates et al. (1993) found intercorrelations between densities of eight bird species, the densities of their favoured prey and sediment type. Prey densities were, however, better explained by a combination of sediment type and inundation time rather than by sediment type alone, and inundation time alone was important in explaining densities of six of the eight bird taxa considered. Despite the detail of their study, which was conducted in a large embayment (660 km2) rather than a true estuary, they concluded that models based on sediment characteristics alone were likely to fail unless other factors influencing invertebrate distribution and abundance were taken into account.
This (depressing) conclusion is supported by independent studies on beaches (Botton et al. 1994) and estuaries (Kalejta & Hockey 1994). Eggs of the Horseshoe Crab Limulus polyphemus are an important prey of waders on beaches in Delaware Bay, New Jersey. Local wader abundance is tied to the abundance of accessible crab eggs, but is not influenced by sediment type: birds concentrate at shoreline discontinuities which trap crab eggs.
Prey densities or biomass, although having proved to be more reliable predictors of wader dispersion patterns than abiotic factors, still are not consistently reliable. Within the Berg River estuary, South Africa, Curlew Sandpiper Calidris ferruginea and Grey Plover Pluvialis squatarola have diets which overlap considerably in their species complement. However, the dispersion patterns of the two species were determined by different prey attributes. Local abundance of tactilely foraging Curlew Sandpipers was best explained by the numerical density of nereid worms whereas the dispersion of visually-foraging Grey Plovers was determined largely by the biomass density (but not numerical abundance) of the larger of the two common nereids. Neither of the species’ distributions was influenced by substratum type.
The above highlights some of the shortfalls in linking wader dispersion patterns to simple environmental attributes. Whilst sediment studies seem to have little predictive power, quantification of prey abundance is able to explain distribution patterns to some degree. However, such correlative studies are limited in terms of indicating whether waders are food-stressed, and hence close to the carrying capacity of estuaries. Thus, it is hardly surprising that researchers concerned with the latter problem have continued to pursue the more established, though considerably more complex, research route into the impacts of waders on their prey supplies.
Impacts on prey populations
Energy limitation is the premise underlying the majority of studies that have addressed the predatory impacts of waders and is ultimately linked to the concept of, and attempts to define, carrying capacity. The common currencies against which consumption is measured are annual benthic production (e.g. Kalejta 1992a) or standing stock, expressed as numbers, biomass or energy (e.g. Meire et al. 1994). However, predatory impacts can take a number of forms, some direct and some indirect. Direct impacts include removal of invertebrate prey from the sediment and associated short or long-term implications for prey abundance, and demographic and life-history changes in prey populations as a result of predators selecting for attributes such as size, sex or morph. Indirect impacts include modified interactions among invertebrates, e.g. competition or predation, changes in prey behaviour, and changes to sediment characteristics. All of these impacts have potential feedback loops to the predators.
Impacts on prey numbers, biomass and production
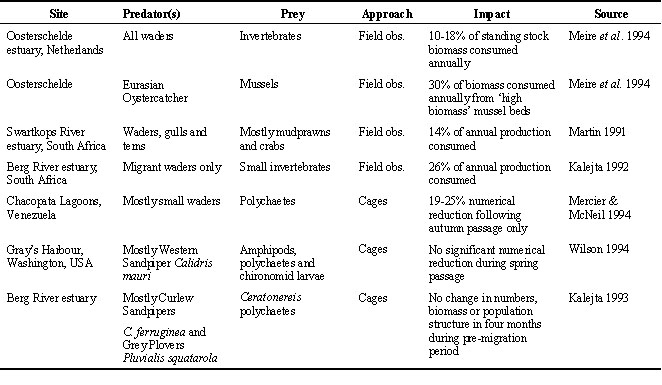
By definition, even if only in the short term, predation results in a reduction in numbers, biomass and reproductive potential of invertebrate prey. This impact is the one that has been measured the most frequently. Most measures of removal rates are compared with standing stock rather than with production, simply because the former is so much easier to measure. Inevitably, this approach will conclude that birds have a net negative impact on prey stocks. However, some studies, even under conditions when predation intensity is high, have been unable to demonstrate empirically any quantitative impact on the standing stock, even in the short term (Botton 1984, Kalejta 1993, Wilson 1994; Table 1). In the Chacopata Lagoons, Venezuela, where bird densities are high and exclusion experiments were run throughout the year, a numerical impact of predation on polychaete densities could be detected only at the time of autumn migration (Mercier & McNeil 1994). Both standing stock and predation rates are fairly easily quantified, but an ecological interpretation of the relationship between these two values is very difficult. This difficulty arises because a measure of standing stock biomass alone has no production component, nor is a large standing stock necessarily indicative of high productivity and vice versa. Without information about production, it is impossible to understand the impacts of predation on prey populations.
In general, high latitude estuaries which are dominated in terms of biomass by large prey, tend to have low production to standing stock biomass (P/B) ratios, whereas tropical and warm temperate estuaries dominated by smaller prey have high P/B ratios (Kalejta & Hockey 1991). Even if there is some predictability about geographical (latitudinal) patterns of P/B ratios, no similar pattern is evident for standing stock biomass densities (Kalejta & Hockey 1991, Piersma et al. 1993). At six temperate estuaries (>50°N) P/B ratios ranged from 1.0 to 1.6, but standing stock biomass varied by an order of magnitude from 10.5 to 140.0 g.m-2 (Kalejta & Hockey 1991). In South Africa, the Swartkops and Berg River estuaries (32-34°S) had standing stocks and P/B ratios of 69.3 g.m-2 and 1.1, and 19.4 g.m-2 and 5.0, respectively (Kalejta & Hockey 1991). The two estuaries are dominated by large and small prey respectively. Thus, the comparison of predation levels with standing stock biomass gives little indication of the significance of the impact of predatory birds, and may lead to overestimation of this significance when the P/B ratio is high. For example, at the Berg River estuary, birds consume 118.5% of the mean annual invertebrate biomass, but this translates to only 26.2% of the annual production (Kalejta & Hockey 1991, Kalejta 1992a). In general, it could be predicted that a greater proportion of the standing stock biomass will be consumed per unit time by birds in productive estuaries than in relatively unproductive estuaries.
Baird et al. (1985) reviewed a series of studies in which predation by estuarine birds had been compared with invertebrate production. They found that consumption rates by birds varied between 6 and 49% of annual production: more recent estimates also fall within this range (e.g. Kalejta 1992a, Hilgerloh 1997). Predatory impacts on single prey species can be higher than this: for example, Baird & Milne (1981) calculated that birds consumed 73% of Mytilus edulis production in the Ythan estuary, Scotland, and local crashes in mytilid populations have been attributed to bird predation more than once (e.g. Nehls 1989, Zwarts & Drent 1981 - in Meire 1993).
Although there is considerable variability in the estimates of the proportion of total production consumed by birds, these estimates are consistently less than 50%. Simplistically, this could be interpreted as meaning that, on average, estuarine bird populations remove far less than the ‘sustainable yield’ of their prey populations, and thus are well below carrying capacity. However, a meaningful interpretation of consumption-production ratios requires knowledge of (a) the availability of prey to birds, and (b) the degree of synchrony between production and predation peaks. These will affect the potential impact that birds can have on their prey populations as well as the number of birds the prey supply can support.
Predatory impacts of birds on estuarine invertebrates are likely to be greatest when the proportion of prey that is available is high. The greater the proportion of prey unavailable to birds, the greater is likely to be the stability of the predator-prey relationship because of refuge effects. The buffering effects on prey which have a refuge from predation are substantial and have been well documented for rocky shore invertebrates experiencing intense predation from oystercatchers (Hockey & Branch 1984, Bosman & Hockey 1988, Bosman et al. 1989). Hockey et al (1992) used invertebrate and bird data from the Berg River estuary, South Africa, to model stable and unstable soft-sediment predation dynamics. A small change in the ratio of available/unavailable prey biomass from 70:30 to 80:20, while holding predation pressure constant, destabilised the predator-prey interaction such that the available prey biomass approached zero in little more than 24 months.
The unavailable fraction of prey is important not only in buffering predation effects, but also feeds back to the predator populations. The importance of this was highlighted in a study of Eurasian Oystercatchers Haematopus ostralegus eating mussels in the Oosterschelde estuary, Netherlands (Craeymeersch et al. 1986). The oystercatchers consumed 40% of Mytilus edulis production. However, the birds consumed 60% of the production in their favoured 30-50 mm size category. Within this size category, 40% of the mussels are unavailable to birds. Thus, even though the oystercatchers ate a relatively small proportion of the total mussel production, their numbers were food limited in that year.
As with standing stock, there appears to be little predictability about the proportions of prey stocks that are potentially harvestable by birds: even within a single prey type, availability can vary temporally (Zwarts & Wanink 1993) or spatially. Using the specific example of Red Knot Calidris canutus, Piersma et al. (1993) found in tropical West Africa that 23-84% of the biomass of six bivalve species was available to the birds. In the cold temperate Dutch Wadden Sea, 5-22% of the biomass of two bivalve species was available to knots. In Roebuck Bay, in tropical NW Australia, only 2-3% of the biomass of two bivalve species was available, while 52% of the biomass of a third was potentially harvestable by Red Knots. In exceptional cases, where both prey abundance and availability are very high (e.g. horseshoe crab eggs in Delaware Bay - Castro & Myers 1993), abundance may substitute for availability, but such instances probably are rare. The importance of understanding the relationship between prey abundance and availability is highlighted empirically by Zwarts & Ens (1999).
The potential impacts that birds can have on their prey populations and vice versa are also affected by the timing of predation events relative to seasonal patterns of invertebrate productivity. The greater the synchrony between production and predation peaks, the greater the loss to predators that invertebrate populations can withstand without becoming destabilised (Hockey et al. 1992). In the Northern Hemisphere, migrating shorebirds are resident on estuaries during the winter months when invertebrate productivity is very low: in the Southern Hemisphere peaks of predation and production coincide. Thus, an estuary in the Southern Hemisphere should have a higher carrying capacity than an equivalent northern estuary with the same level of invertebrate production. However, there is no evidence that the proportion of annual invertebrate production consumed is greater in southern estuaries (Hockey et al. 1992). This may be due either to a limited ability to quantify invertebrate productivity and availability to birds accurately, or simply a paucity of such studies from the Southern Hemisphere.
Demographic and community level impacts
Size selective predation by estuarine birds has the potential to modify the size – and hence age-structure of prey populations. This in turn may affect their reproductive behaviour, competitive interactions and population dynamics; i.e. influence their life history. Counter-intuitively, selective predation by Semipalmated Sandpipers Calidris pusilla on large amphipods Corophium volutator results in increased amphipod densities. This is because the removal of competitively dominant adult Corophium increases the survivorship of juveniles (Matthews et al. 1992).
Predator-induced changes in prey population sizes may alter the strength, or even direction, of interactions within invertebrate foodwebs. From a theoretical standpoint, it could also be predicted that predators will target the most abundant prey, leading to an increased evenness in invertebrate community structure: this prediction has been borne out in at least one study (Schneider 1978).
There have been several demonstrations of shorebirds affecting invertebrate community structure on rocky shores (e.g. Hockey & Bosman 1987) where the effects of predation by birds are relatively easy to see. In soft sediments, observing such processes is much more difficult, and so also is ascribing community change to predation. One exception to this generalisation is predation on epibenthic bivalves, especially mytilids. Predation dynamics of these have been reviewed by Meire (1993). Given that many studies of soft sediment infauna have been unable to demonstrate even a numerical effect of intertidal predation by birds, it is perhaps not surprising that relatively little is known about how (or whether) shorebird predators affect invertebrate community structure. It is unclear as yet over what time-frame experiments should run to detect such effects (and establish whether they are, indeed, representative of the natural situation).
Indirect effects on invertebrates
The indirect effects of bird predation in estuaries are likely to be frequent and mostly undocumented (because most researchers have failed to look for them). Birds which crop bivalve siphons (more strictly grazing than predation) have an indirect effect by making ‘cropped’ bivalves more vulnerable to subsequent predation because they do not bury themselves as deeply as undamaged animals (Moreira 1995).
An even more subtle indirect effect (of waders preying on the amphipod Corophium volutator) was identified by Daborn et al. (1993). When predation pressure on Corophium was low, sediment cohesion was due in part to secretions of polysaccharides by benthic diatoms whose abundance was regulated by grazing pressure from Corophium. When Corophium densities were reduced by birds, this grazing pressure decreased and diatom abundance and hence polysaccharide secretion and sediment cohesion increased. Whether this in turn had any feedback effects on foraging birds is unknown.
Predatory impacts as a route to understanding carrying capacity and distribution: persevere or pursue alternatives?
From the perspective of the wader biologist, attempts to understand the impacts of waders on their prey populations are ultimately directed at understanding the constraints that prey populations place on wader numbers, behaviour and community structure. The quantification by direct observation of prey removal by birds is relatively straightforward. Recent advances in understanding of the physiology of waders (e.g. geographical and seasonal variations in metabolic rate - Piersma et al. 1995, 1996) are further refining the accuracy with which we can predict the energy removal from estuaries by individual species, although it is surprising that more emphasis has not been placed on isotopic measurement of field metabolic rates (cf. Nagy 1987). Despite these advances, we are still a long way from accurate determination of the basic numerical and biomass effects of predation by multi-species wader assemblages on multi-species estuarine prey assemblages. Impacts on prey will be determined in part by the abundance and species assembly of the predator community but also by the population and behavioural response of prey to their predators. Complicating the picture are prey attributes not (or not obviously) linked to predation such as seasonal variation in burrowing depth or asynchrony in reproductive cycles (e.g. Kalejta 1992b) and predator foraging attributes linked to e.g. age and dominance (Goss-Custard & West 1997).
We are even further from understanding the links between prey community characteristics (e.g. biomass, demographic structure, community structure and behaviour), prey availability and wader community characteristics. Without an understanding of how these feedback links from prey to predators operate, we are no closer to solving the mystery of how bird populations relate to carrying capacity or fully understanding the determinants of distribution patterns.
Feedback effects are likely to be most detectable if the predator population is close to an energetically determined carrying capacity (rather than one determined by e.g. roost site availability). We define carrying capacity as meaning ‘one-in, one-out’ (Goss-Custard 1985) whereby for every newly arriving bird, one bird either emigrates or dies. Many migrant waders have very large nonbreeding ranges and experience large inter-annual fluctuations in global population sizes as a result of variable breeding performance (Summers & Underhill 1987), and in local population sizes due to mortality or emigration during severe weather (Ferns 1994). Given these conditions, the concept of a global carrying capacity (at least on the nonbreeding grounds) probably is nebulous, and individual sites may frequently not reach carrying capacity because the supply of recruits is too small for resources to be fully utilised (Goss-Custard & West 1997).
The best-studied estuarine shorebird-prey interaction is undoubtedly that between Eurasian Oystercatchers and mussels Mytilus edulis on the Exe estuary, U.K. (Goss-Custard et al. 1995a,b; Goss-Custard & West 1997 and references therein). After more than two decades of intensive behavioural and ecological studies, researchers have now been able to develop a model to predict the carrying capacity of the estuary for oystercatchers (Clarke & Goss-Custard 1996). The model has recently been extended to predict how migratory sub-populations of oystercatchers with different reproductive rates might respond to habitat loss (dit Durell et al. 1997). The Exe estuary model has shown that even though interference among oystercatchers is high, the population size is still about 22% below the estuarine carrying capacity. This finding has important implications for modelling likely effects of habitat loss, because such models have usually implicitly assumed that bird populations in estuaries are already at carrying capacity (e.g. Yates et al. 1996). Like the feedback loops between predator and prey, these models thus contain an element of circular reasoning that rests on an untested assumption.
The Exe estuary oystercatcher model could not have been developed using a simple trophodynamics approach (even though it is centred on a single trophodynamic link). Rather, its success was dependent on a detailed understanding of the birds’ behaviour (including dominance hierarchies, age effects and the consequences of interference). In more complex food webs, trophodynamic analysis, which has underpinned community ecology for decades, is even less likely to generate robust predictions of predator response.
Foraging behaviour of estuarine waders
The foraging behaviour and performance of any bird ultimately is driven by the balance between energy requirements and expenditure. Modern laboratory and field techniques allow accurate measurement of both basal and field metabolic rates. In recent years these techniques have been increasingly applied in evaluating aspects of wader energetics at different stages of their annual cycles (e.g. Castro et al. 1992, Evans et al. 1992, Piersma & Poot 1993, Wiersma et al. 1993, Driedzic et al. 1993, Van der Meer & Piersma 1994, Piersma et al. 1996). This field of research is relatively new and rapidly developing, and most emphasis has been placed on physiological aspects of energetics rather than ecological ones. There are exceptions to this generalisation: Castro et al. (1992) investigated the energetic costs of overwintering by Sanderlings Calidris alba at different latitudes, showing that birds spending the nonbreeding season in warm, tropical regions incurred lower energetic costs than those at higher latitudes.
In general, however, single-species, single-locality studies of foraging behaviour have remained the order of the day. Studies have concentrated on explaining variation in foraging behaviour in relation to different prey types or sizes (Boates & Goss-Custard 1992, Moreira 1994a,b), changing environmental conditions (Cullen 1994), age (Hockey et al. 1996), sex (dit Durell et al. 1993) or morphology (Hockey et al. in press) of the birds, and the incidence or otherwise of aggression and territoriality (Turpie 1995). Several studies have linked behaviour to energy intake, the latter derived either from direct observation or from indirect dietary reconstruction (e.g. Boates & Goss-Custard 1992, dit Durell et al. 1993, Turpie & Hockey 1993, Moreira 1994a,b, Turpie 1995, Hockey et al. 1996, Turpie & Hockey 1996, 1997). Our understanding of the relationship between intraspecific interference and energy intake rates has been further improved by the use of sophisticated models (Stillman et al. 1996, 1997).
Most waders do not forage continuously throughout the tidal cycle. Large birds eating large prey are often able to capture prey at a much faster rate than they can process (digest) them. The ratio of ingestion to processing rate in oystercatchers is as high as 4. Because of the constraints imposed by digestion (the digestive bottleneck), birds cannot forage at maximum intake rates for more than about two hours (Kersten & Visser 1996). Intake rates vary seasonally but almost always substantially exceed processing rates (Zwarts et al. 1996). If oystercatchers are time-stressed (e.g. by artificially reduced tidal exposure time) they can increase their intake rates to the point where they would only need to forage for about four hours in every 24 h (Swennen et al. 1989). Combined, these observations led Kersten & Visser (1996) to conclude that ‘oystercatchers rarely face situations of food shortage’. Turpie & Hockey (1997) studied the foraging of Grey Plovers and Whimbrels at several sites in the Indian Ocean and south-east Atlantic. Net intake rates of Grey Plovers ranged from 0.53-1.38 kJ.min-1, and foraging times from 186-325 min per daytime tidal exposure period. Corresponding values for Whimbrels at the same sites were 0.67-1.83 and 162-283. However, for neither species was there a significant relationship between intake rate and foraging time. The proportions of exposure time spent foraging were, however, more constant – 60-73% for Grey Plovers and 52-70% for Whimbrels.
At Mida Creek, Kenya, adult Crab Plovers needed to forage for only 1.8-4.2 h per 24 h to satisfy their daily energy demands (10-24% of available foraging time). Smaller plovers at Mida Creek needed to forage for 19-62% of available time (Hockey et al. 1996, in press).
The results of Swennen et al.’s (1989) experiments with time-stressed oystercatchers have challenged the dogma that waders always try to maximise instantaneous intake rates. This does not necessarily mean that they do not maximise their overall energy assimilation rate. As long as the average (and not necessarily instantaneous) ingestion rate exceeds the digestion rate, it is not necessary to maximise instantaneous intake rates in order to maximise the overall rate of energy assimilation. Indeed, the pressure to maximise intake rates may be greatest under time limitation or food limitation, which could lead to exactly the opposite pattern of energy intake rate than would be predicted from the premise of foraging rate maximisation. This creates problems in interpreting comparative field measurements of intake rates because it is difficult to assess whether birds are maximising intake rates. If birds did always maximise instantaneous intake rates, then inter-site or seasonal comparisons of intake rates would provide a useful indication of the ease with which birds find food and hence of relative food availability. If not, then interpretation of differences in intake rates in terms of the relationship between birds and their prey will require measures of effort in relation to intake rates.
The study of foraging behaviour and effort in relation to energy intake rates may indicate the extent to which birds are or are not food stressed. However, there is no a priori reason to assume that foraging conditions will be the same by night as by day. For example, a study in Venezuela demonstrated that prey availability can change dramatically over diel time scales (McNeil et al. 1995). Swimming organisms were 3 – 30 times more available by night than by day, and infaunal amphipods, isopods and polychaetes were more than ten times as abundant at the surface by night as by day. If such differences result in comparable differences in predator responses, conclusions about estuarine carrying capacity extrapolated from daytime observations of behaviour may be quite wrong. In recognition of this, McNeil et al. (1992) highlighted the lack of data on day vs night energy intake rates as a major deficiency in our knowledge.
The widespread occurrence of nocturnal foraging has been realised only relatively recently. Its probable importance has been demonstrated by the occurrence of retinal adaptations for nocturnal foraging among certain waders which are obligate visual foragers (De Azuaje et al. 1993). Preference for nocturnal foraging could result from increased food availability, reduced risk of predation (e.g. from diurnal raptors), a reduced incidence of kleptoparasitism, or less disturbance (McNeil et al. 1992). Whether it is a ‘preferred’ or simply a ‘top-up’ strategy, nocturnal foraging clearly has to be included in studies of foraging behaviour and energy intake that aim to understand estuarine predator-prey relationships. The behaviour of nocturnally foraging birds is likely to differ from daytime behaviour and it may not be possible to extrapolate directly from one to the other. It could be predicted that, relative to their daytime foraging efficiency, the efficiency of nocturnal foraging would be maximal in obligate tactile foragers, minimal in obligate visual foragers and intermediate in facultative foragers. In support of this, Dunlins Calidris alpina switch from predominantly visual foraging by day to tactile foraging by night (Mouritsen 1993, 1994). Similarly it has been shown that nocturnal intake rates cannot be inferred from measures of foraging effort such as step or peck rates, as the relationship between these variables and intake rates changes at night (Sutherland 1982, McNeil & Robert 1988, Robert & McNeil 1989, Turpie & Hockey 1993). With improved night viewing equipment researchers have been able to confirm the importance of nocturnal foraging to waders and have demonstrated difference in habitat use between day and night (e.g. Mouritsen 1994, Rohweder & Baverstock 1996, Rompré & McNeil 1994, Thibault & McNeil 1994). However, we are aware of only three studies that have directly quantified the energy intake rates of waders at night (Zwarts & Dirksen 1990, Turpie & Hockey 1993, 1996). One species common to all three studies, the Whimbrel Numenius phaeopus, was found to be far less successful at night than during the day when foraging on crabs in Mauritania, whereas it was equally successful by day and night when foraging mainly on mudprawns at the Swartkops estuary, South Africa. There are two possible reasons for this difference. Firstly, surfacing mudprawns are sluggish and easy to catch whereas crabs on the surface are active and more difficult to catch. Secondly, the average sizes of crabs eaten in Mauritania (17-24 mm) are much smaller than the average size of prawns eaten in South Africa (50 mm): prey size may influence the detection ability of the birds.
We are still a long way from understanding the energetic implications or importance of nocturnal foraging in shorebirds. We do know that many species forage at night, that some non-territorial species use different habitats by night and day, and that they may forage for as long (or longer) by night than day and achieve comparable instantaneous energy intake rates. The energetic aspect of nocturnal foraging is undoubtedly an important target area for future research. Such research will provide not only a more holistic appreciation of wader ecology, but may also be of paramount importance in setting conservation priorities.
DISCUSSIONAt first glance, this short review may read more like a litany of sorrows than a celebration of recent successes, but this is far from the case. In recent years, substantial progress has been made in several areas. These include the importance of quantifying the available prey fraction (and how this feeds back to predator numbers), understanding the factors influencing foraging rhythms and how these link to optimality theory, and identifying prey base attributes that do or do not have elements of geographic predictability. Our understanding of wader energetics and physiology has grown dramatically, but as yet, very few studies have been able to quantify directly the elements of nocturnal energetics, although the importance of nocturnal foraging is widely appreciated.
As more and more studies of wader foraging have become available, the diversity of interactions and influences identified has also multiplied. In terms of its contribution to the development of community ecological theory, estuarine wader research has always been very appealing because most trophic and other interactions occur in a near two-dimensional environment. However, it is becoming increasingly clear that even though the third environmental dimension (the sediments and associated infauna) may be only a few centimetres deep, small differences in organismal distribution within this stratum can have profound impacts on predator-prey interactions.
As our understanding of the complexity of estuarine trophodynamics (and non-trophic interactions which influence these) increases, the elusive spectre of carrying capacity becomes ever more wraith-like. Applying the concept of carrying capacity to an entire estuarine wader assemblage is unrealistic – it is very unlikely that all species would be constrained by the same limiting factor at the same time. Nevertheless, in seeking to establish the carrying capacity for even a single species, some understanding is required of the way in which other species impinge on the focal population’s niche space.
Any implicit assumption that the carrying capacity of an estuary for any particular species is constant is also fundamentally flawed. Dynamic changes occur in estuaries that affect prey populations but are quite unrelated to the activities of birds. In addition, the source pool of birds potentially able to recruit to estuaries varies from year to year. Above average bird numbers in one year could have the effect of reducing carrying capacity in a subsequent year, and vice versa.
Despite the plethora of potential pitfalls, the fact remains that biologists will increasingly be asked to make predictions about the effects of future changes to estuaries. To do this successfully, the implications of some of the new findings reviewed here will have to be investigated thoroughly. Some short-cut avenues that have been explored, such as correlations between bird numbers and sediment characteristics, seem to have limited potential. Ultimately, the most sensitive indicator of a bird’s foraging environment is the bird itself. We propose that if shortcuts to predicting carrying capacity are to be found, they will lie in the appropriate analyses of bird behaviour as much or more than in pursuing traditional trophodynamic approaches.
REFERENCESBaird, D. & Milne, H. 1981. Energy flow in the Ythan estuary, Aberdeenshire, Scotland. Estuarine, Coastal and Shelf Science 13: 455-472.
Baird, D., Evans, P.R. Milne, H. & Pienkowski, M.W. 1985. Utilization by shorebirds of benthic invertebrate production in intertidal areas. Oceanography and Marine Biology Annual Review 23: 573-597.
Boates, J.S. & Goss-Custard, J.D. 1992. Foraging behaviour of Oystercatchers Haematopus ostralegus specializing on different species of prey. Canadian Journal of Zoology 70: 2398-2404.
Bosman, A.L. & Hockey, P.A.R. 1988. Life-history patterns of populations of the limpet Patella granularis: the dominant roles of food supply and mortality rate. Oecologia 75: 412-419.
Bosman, A.L., Hockey, P.A.R. & Underhill, L.G. 1989. Oystercatcher predation and limpet mortality: the importance of refuges in enhancing the reproductive output of prey populations. Veliger 32: 120-129.
Botton, M.L. 1994. Effects of Laughing Gull and shorebird predation on the intertidal fauna at Cape May, New Jersey. Estuarine, Coastal and Shelf Science 18: 209-220.
Botton, M.L., Loveland, R.E. & Jackobsen, T.R. 1994. Site selection by migratory shorebirds in Delaware Bay, and its relationship to beach characteristics and abundance of Horseshoe Crab (Limulus polyphemus) eggs. Auk 111: 605-616.
Castro, G. & Myers, J.P. 1993. Shorebird predation on eggs of Horseshoe Crabs during spring stopover on Delaware Bay. Auk 110: 927-930.
Castro, G., Myers, J.P. & Ricklefs, R.E. 1992. Ecology and energetics of Sanderlings migrating to four latitudes. Ecology 73: 833-844.
Clarke, R.T. & Goss-Custard, J.D. 1996. The Exe estuary oystercatcher-mussel model. In: Goss-Custard, J.D. (ed.) The Oystercatcher: from individuals to populations. Oxford; Oxford University Press: 390-393.
Colwell, M.A. & Landrum, S.L. 1993. Nonrandom shorebird distribution and fine-scale variation in prey abundance. Condor 95: 94-103.
Craeymeersch, J.A., Herman, P.M.J. & Meire, P.M. 1986. Secondary production of an intertidal mussel (Mytilus edulis L.) population in the Eastern Scheldt (SW-Netherlands). Hydrobiologia 133: 107-115.
Cullen, S.A. 1994. Black-necked Stilt foraging site selection and behaviour in Puerto Rico. Wilson Bulletin 106: 508-513.
Daborn, G.R., Amos, C.L., Brylinsky, M., Christan, H., Drapeau, G., Faas, R.W., Grant, J., Long, B., Paterson, D.M., Perillo, G.M.E. & Piccolo, M.C. 1993. An ecological cascade effect: migratory birds affect stability of intertidal sediments. Limnology and Oceanography 38: 225-231.
De Azuaje, L.M.R., Tai, S. & McNeil, R. 1993: Comparison of Rod/Cone Ratio in three species of shorebirds having different nocturnal foraging strategies. Auk 110: 141-145.
Dit Durell, S.E.A. Le V., Goss-Custard, J.D. & Caldow, R.W.G. 1993. Sex-related differences in diet and feeding method in the Oystercatcher Haematopus ostralegus. Journal of Animal Ecology 62: 205-215.
Dit Durell, S.E.A. Le V., Goss-Custard, J.D. & Clarke, R.T. 1997. Differential response of migratory sub-populations to winter habitat loss. Journal of Applied Ecology 34: 1155-1164.
Driedzic, W.R., Crowe, H.L., Hicklin, P.W. & Sephton, D.H. 1993. Adaptations in pectoralis muscle, heart mass and energy metabolism during premigratory fattening in Semipalmated sandpipers (Calidris pusilla). Canadian Journal of Zoology 71: 1602-1608.
Evans, P.R., Davidson, N.C., Uttley, J.D. & Evans, R.D. 1992. Premigratory hypertrophy of flight muscles: an ultrastructural study. Ornis Scandinavica 23: 238-243.
Ferns, P.N. 1994. The Severn Estuary’s changing wader populations during the last two decades. Biological Journal of the Linnaean Society 51: 219-227.
Goss-Custard, J.D. 1985. Foraging behaviour of wading birds and the carrying capacity of estuaries. In: Sibly, R.M. & Smith, R.H. (eds), Behavioural eEcology: ecological consequences of adaptive behaviour. Oxford; Blackwell Scientific: 169-188.
Goss-Custard, J.D., Caldow, R.W.G., Clarke, R.T., dit Durell, S.E.A. Le V. & Sutherland, W.J. 1995a. Deriving population parameters from individual variations in foraging behaviour. I. Empirical game theory distribution model of Oystercatchers Haematopus ostralegus feeding on mussels Mytilus edulis. Journal of Animal Ecology 64: 265-276.
Goss-Custard, J.D., Caldow, R.W.G., Clarke, R.T. & West, A.D. 1995b. Deriving population parameters from individual variations in foraging behaviour. II. Model tests and population parameters. Journal of Animal Ecology 64: 277-289.
Goss-Custard, J.D., Warwick, R.M., Kirby, R., McGrorty, S., Clarke, R.T., Pearson, B., Rispin, W.E., dit Durell, S.E.A. Le V. & Rose, P.J. 1991. Towards predicting wading bird densities from predicted prey densities in a post-barrage Severn estuary. Journal of Applied Ecology 28: 1004-1026.
Goss-Custard, J.D. & West, A.D. 1997. The concept of carrying capacity and shorebirds. In: Goss-Custard, J.D., Rufino, R. & Luis, A. (eds) Effect of habitat loss and change on waterbirds; London; The Stationery Office: 52-62.
Hilgerloh, G. 1997. Predation by birds on blue mussel Mytilus edulis beds of the tidal flats of Spiekeroog (southern North Sea). Marine Ecology Progress Series 146: 61-72.
Hockey, P.A.R. & Barnes, K.N. 1997. Latitudinal patterns in the structure of coastal wader assemblages and associated implications of habitat loss. In: Goss-Custard, J.D., Rufino, R. & Luis, A. (eds), Effects of habitat loss and change on waterbirds; London; The Stationery Office: 68-74.
Hockey, P.A.R. & Bosman, A.L. 1987. Stabilising processes in bird-prey interactions on rocky shores. In: Chelazzi, G. & Vannini, M. (eds), Behavioural adaptation to intertidal life; New York & London; Plenum Press; 297-315.
Hockey, P.A.R. & Branch, G.M. 1984. Oystercatchers and limpets: impacts and implications. A preliminary assessment. Ardea 72: 199-206.
Hockey, P.A.R. Navarro, R.A., Kalejta, B. & Velasquez, C.R. 1992. The riddle of the sands: why are shorebird densities so high in southern estuaries? American Naturalist 140: 961-979.
Hockey, P.A.R., Plagányi, É.E., Turpie, J.K. & Phillips, T.E. 1996. Foraging behaviour of Crab Plovers Dromas ardeola at Mida Creek, Kenya. Ostrich 67: 33-44.
Hockey, P.A.R., Turpie, J.K., Plagányi, É.E., Phillips, T.E. 1998. Scaling patterns in the foraging behaviour of sympatric plovers: effects of body size and diet. Journal of Avian Biology, in press.
Kalejta, B. 1992a. Time budgets and predatory impacts of waders at the Berg River estuary, South Africa. Ardea 80: 327-342.
Kalejta, B. 1992b. Distribution, biomass and production of Ceratonereis erythraeensis (Fauvel) and Ceratonereis keiskama (Day) at the Berg River Estuary, South Africa. South African Journal of Zoology 27: 121-129.
Kalejta, B. 1993. Intense predation cannot always be detected experimentally: a case study of shorebird predation on nereid polychaetes in South Africa. Netherlands Journal of Sea Research 31: 385-393.
Kalejta, B & Hockey, P.A.R. 1991. Distribution, abundance and productivity of benthic invertebrates at the Berg River Estuary, South Africa. Estuarine Coastal and Shelf Science 33: 175-191.
Kalejta, B. & Hockey, P.A.R. 1994. Distribution of shorebirds at the Berg River estuary, South Africa, in relation to foraging mode, food supply and environmental features. Ibis 136: 233-239.
Kersten, M. & Visser, W. 1996. The rate of food processing in the Oystercatcher: food intake and energy expenditure constrained by a digestive bottleneck. Functional Ecology 10: 440-448.
Martin, A.P. 1991. Feeding ecology of birds on the Swartkops estuary, South Africa. PhD thesis, University of Port Elizabeth, South Africa.
Matthews, S.L., Boates, J.S. & Walde, S.J. 1992. Shorebird predation may cause discrete generations in an amphipod prey. Ecography 15: 393-400.
McNeil, R. & Robert, M. 1988. Nocturnal feeding strategies of some shorebirds in a tropical environment. Acta Congressus Internationalis Ornithologici 19: 2328-2336.
McNeil, R., Díaz, O.D. & Ildefonso, L.A. 1995. Day- and night-time prey availability for waterbirds in a tropical lagoon. Canadian Journal of Zoology 73: 869-878.
McNeil, R., Drapeau, P., Goss-Custard, J.D. 1992. The occurrence and adaptive significance of nocturnal habitats in waterfowl. Biol. Rev. 67: 381-419.
Meire, P.M. 1993. The impact of bird predation on marine and estuarine bivalve populations: A selective review of patterns and underlying causes. In: Danne, R.F. (ed), Bivalve filter feeders in estuarine and coastal ecosystem processes; Berlin; Springer-Verlag: 197-243.
Meire, P.M., Schekkerman, H. & Meininger, P.L. 1994. Consumption of benthic invertebrates by waterbirds in the Oosterschelde estuary, SW Netherlands. Hydrobiologia 282/283: 525-546.
Mercier, F. & McNeil, R. 1994. Seasonal variations in intertidal density of invertebrate prey in a tropical lagoon and effects of shorebird predation. Canadian Journal of Zoology 72: 1755-1763.
Moreira, F. 1994a. Diet and feeding rates of Knots Calidris canutus in the Tagus estuary (Portugal). Ardea 82: 133-136.
Moreira, F. 1994b. Diet, prey-size selection and intake rates of Black-tailed Godwits Limosa limosa feeding on mudflats. Ibis 136: 349-355.
Moreira, F. 1995. Diet of Black-headed Gulls Larus ridibundus on emerged intertidal areas in the Tagus estuary (Portugal): predation or grazing? J. Avian Biol. 26: 277-282.
Mouritsen, K.N. 1993. Diurnal and nocturnal prey detection by Dunlins Calidris alpina. Bird Study 40: 212-215.
Mouritsen, K.N. 1994. Day and night feeding in Dunlins Calidris alpina: choice of habitat, foraging technique and prey. Journal Avian Biology 25: 55-62.
Nagy, K.E. 1987. Field metabolic rate and food scaling in mammals and birds. Ecological Monographs 57: 111-128.
Nehls, G. 1989. Occurrence and food consumption of the Common Eider, Somateria mollissima in the Wadden Sea of Schleswig-Holstein. Helgoländer Meeresunters 43: 385-393.
Piersma, T. & Poot, M. 1993. Where waders may parallel penguins: spontaneous increase in locomotor activity triggered by fat depletion in a voluntarily fasting Knot. Ardea 81: 1-8.
Piersma, T., Cadée, N. & Daan, S. 1995. Seasonality in basal metabolic rate and thermal conductance in long-distance migrant shorebird, the Knot (Calidris canutus). Journal of Comparative Physiology B. 165: 37-45.
Piersma, T., De Goeij, P. & Tulp, I. 1993. An evaluation of intertidal feeding habitats from a shorebird perspective: towards relevant comparisons between temperate and tropical mudflats. Netherlands Journal of Sea Research 31(4): 503-512.
Piersma,T., Bruinzeel, L., Drent, R., Kersten, M., Van Der Meer, J. & Wiersma, P. 1996. Variability in basal metabolic rate of a long-distance migrant shorebird (Red Knot, Calidris canutus) reflects shifts in organ sizes. Physiological Zoology 69: 191-217.
Robert, M. & McNeil, R. 1989. Comparative day and night feeding strategies of shorebird species in a tropical environment. Ibis 131: 69-79.
Rohweder, D.A. & Baverstock, P.R. 1996. Preliminary investigation of nocturnal habitat use by migratory waders (Order Charadriiformes) in northern New South Wales. Wildlife Research 23: 169-184.
Rompré, G. & McNeil, R. 1994. Seasonal changes in day and night foraging of Willets in northeastern Venezuela. Condor 96: 734-738.
Schneider, D.C. 1978. Equalization of prey numbers by migratory shorebirds. Nature (London) 271: 353-354.
Staine, K.J. & Burger, J. 1994. Nocturnal foraging behaviour of breeding Piping Plovers (Charadrius melodus) in New Jersey. Auk 111(3): 579-587.
Stillman, R.A., Goss-Custard, J.D. & Caldow, R.W.G. 1997. Modelling interference from basic foraging behaviour. Journal of Animal Ecology 66: 692-703.
Stillman, R.A., Goss-Custard, J.D., Clarke, R.T. & dit Durell, S.E.A.Le V. 1996. Shape of the interference function in a foraging vertebrate. Journal of Animal Ecology 65: 813-824.
Swennen, C., Leopold, M.F. & de Bruijn, L.L.M. 1989. Time-stressed Oystercatchers, Haematopus ostralegus can increase their intake rate. Animal Behaviour 38: 8-22.
Summers, R.W. & Underhill, L.G. 1987. Factors relating to breeding production of Brent Geese Branta b. bernicla and waders (Charadrii) on the Taimyr Peninsula. Bird Study 34. 161-171.
Sutherland, W.J. 1982. Do oystercatchers select the most profitable cockles? Animal Behaviour 30: 857-861.
Thibault, M. & McNeil, R. 1994. Day/night variation in habitat use by Wilson’s Plovers in Northeastern Venezuela. Wilson Bulletin 106: 299-310.
Turpie, J.K. 1995. Non-breeding territoriality: causes and consequences of seasonal and individual variation in Grey Plover Pluvialis squatarola behaviour. Journal of Animal Ecology 64: 429-438.
Turpie, J.K. & Hockey, P.A.R. 1993. Comparative diurnal and nocturnal foraging behaviour and energy intake of premigratory Grey Plovers Pluvialis squatarola and Whimbrels Numenius phaeopus in South Africa. Ibis 135: 156-165.
Turpie, J.K. & Hockey, P.A.R. 1996. Foraging ecology and seasonal energy budgets of estuarine Grey Plovers Pluvialis squatarola and Whimbrels Numenius phaeopus at the southern tip of Africa. Ardea 84: 57-74.
Turpie, J.K. & Hockey, P.A.R. 1997. Adaptive variation in the foraging behaviour of Grey Plover Pluvialis squatarola and Whimbrel Numenius phaeopus. Ibis 139: 289-298.
Van Der Meer, J. & Piersma, T. 1994. Physiologically inspired regression models for estimating and predicting nutrient stores and their composition in birds. Physiological Zoology 67: 305-329.
Wiersma, P., Bruinzeel, L. & Piersma, T. 1993. Energiebesparing bij wadvogels: over de kieren van de Kanoet. Limosa 66: 41-52.
Wilson, W.H.Jr. 1994. The effects of episodic predation by migratory shorebirds in Gray’s Harbor, Washington. Journal of Experimental Marine Biology and Ecology 177: 15-25.
Yates, M.G., Goss-Custard, J.D. & Rispin, W.E. 1996. Towards predicting the effect of loss of intertidal feeding areas on overwintering shorebirds (Charadrii) and Shelduck (Tadorna cana): refinements and tests of a model developed for the Wash, east England. Journal of Applied Ecology 33: 944-954.
Yates, M.G., Goss-Custard, J.D., Mcgrorty, S., Lakhani, K.H., Dit Durell, S.E.A. Le V., Clarke, R.T., Rispin, W.E., Moy, I., Yates, T., Plant, R.A. & Frost, A.J. 1993. Sediment characteristics, invertebrate densities and shorebird densities on the inner banks of the Wash. Journal of Applied Ecology 30: 599-614.
Zwarts, L. & Dircksen, S. 1990. Digestive bottleneck limits the increase of food intake of Whimbrels preparing their departure from the Banc d' Arguin, Mauritania, in spring. Ardea 78: 257-278.
Zwarts, L. & Ens, B.J. 1999. Predation by birds on marine tidal flats. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2309-2327. Johannesburg: BirdLife South Africa.
Zwarts, L. & Wanink, J.H. 1993. How the food supply harvestable by waders in the Wadden Sea depends on the variation in energy density, body weight, biomass, burying depth and behaviour of tidal-flat invertebrates. Netherlands Journal of Sea Research 31: 441-476.
Zwarts, L., Ens, B.J., Goss-Custard, J.D., Hulscher, J.B. & Dit Durell, S.E.A. le V. 1996. Causes of variation in prey profitability and its consequences for the intake rate in oystercatchers Haematopus ostralegus. Ardea 84: 229-268.
Table 1. Summary of selected recent studies that have estimated or measured predatory impact of estuarine waders.
