S38.1: Foraging ecology and foraging habitat of the American Wood Stork Mycteria americana
Malcolm C. Coulter
Savannah River Ecology Laboratory, PO Drawer E, Aiken, South Carolina 29802, USA; SIS, PO Box 48, Chocorua, N.H. 03817, USA., e-mail coultermc@aol.com
Coulter, M.C. 1999. Foraging ecology and foraging habitat of the American Wood Stork Mycteria americana. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2260-2270. Johannesburg: BirdLife South Africa.The American Wood Stork is found from the SE U.S.A to Argentina and Bolivia; in the U.S.A., it breeds in the southeast, from South Carolina through southern Florida. This is a large waterbird, feeding primarily by tactilocation, foraging in almost all wetlands available where the water depth is appropriate (<50 cm) and where prey is available. It seems to prefer more open wetlands (with little emergent/submergent vegetation or canopy cover) and where the water is still or slow moving. The dynamics of prey availability varies geographically with variation in hydrology and climate. In south Florida Everglades there is a distinct rainy and dry season and even temperatures throughout the year, birds breed at the end of the rainy season and raise young during the dry season when evaporative drawdowns and high concentrations in isolated wetlands. In Georgia, there is no rainy season and no predictable drawdowns and concentrations of prey. Storks feed where there are lower concentrations of prey but feed on larger prey than in southern Florida. They are not present during the winter when temperatures are too low, and breed during spring and summer when temperatures are more suitable.
INTRODUCTION
Birds are often taken as indicators of the health of their natural environments and ability of these environments to work in concert with growing human demands and associated changes to these environments. Storks are good indicators of the health of the wetlands where they are found. Yet, wetlands vary geographically. The dynamics vary with water and rainfall regimes, the responses of algae, water plants, amphibians, fish, and other aquatic prey to these dynamics and to the larger predators on these populations. Our understanding is often concentrated on only a few levels of this hierarchy. But major differences exist at all levels.
Our understanding of the breeding and foraging ecology of American Wood Storks Mycteria americana is based largely on early studies in south Florida. I studied storks at the Birdsville colony in Jenkins County, Georgia from 1984-1991. It is important to compare the results of my studies with those from Florida in order to understand the variation in biology of the storks in response to the different wetland systems and how the population sizes have responded to changes in the wetlands. This paper is a summary of my study in east-central Georgia, comparing my results with the results from the studies in south Florida.
South Florida, an historical perspective
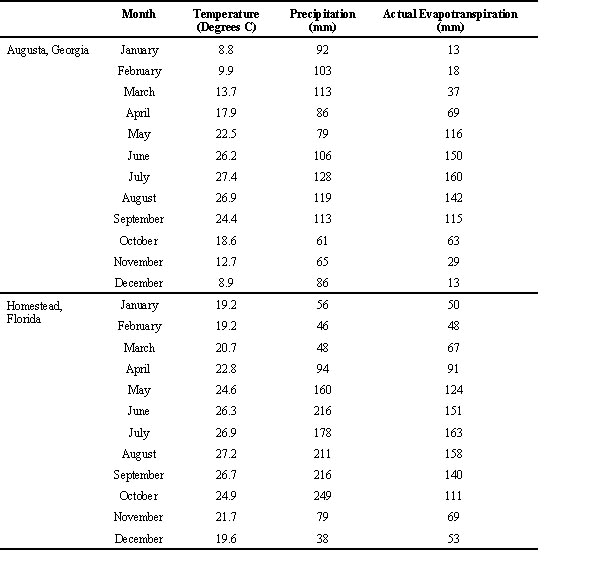
Much of our understanding of wood storks is based on early studies conducted in the vast Everglades wetland of southern Florida (Kahl 1962, 1964, Ogden 1994). The storks are well-adapted to the dynamics of this wetland (see below). Traditionally, four factors in the Everglades have been particularly important for storks: a large continuous wetlands, waterflow, tropical wet-dry season climate and high concentrations of prey during the dry season when evaporation causes wetlands to become isolated and water levels to drop. The Everglades are one single wetland system south of Lake Okeechobee. The water flow in the Everglades emanates largely from Lake Okechobee, flowing south over an extremely low gradient (<2 cm/km) into Florida Bay. The tropical climate includes warm temperatures throughout the year and a rainy season (April-November) followed by a dry season (December-March) (Table 1) (Mather 1964, Willmot et al. 1981). During the dry season, depressions in the Everglades become isolated from the major wetland through evaporation and further evaporation results in high concentrations of prey (Kahl 1964, Kushlan 1976). Densities of 141 potential prey items/ m2 in the Everglades and 40 potential prey items/ m2 in southern coastal Everglades (Ogden et al. 1978). Storks and other wading birds are attracted to these ephemeral areas. Area after area became available to the birds, supporting large numbers of breeding and non-breeding birds. Historically, these factors presented predictable climatic conditions resulting in high concentrations of prey. The average length of the prey in regurgitation samples were 41 mm at the Lane River colony in Florida and 54 mm at the Lane River colony.
The American Wood Stork is well adapted to this dynamics because it feeds by tactilocation, allowing the stork to take advantage of high densities of prey (Kahl 1964). It feeds by feeling movement in the water without need to see the fish, allowing the storks to feed in murky water and at night (Coulter 1987).
Wood Storks traditionally bred at the end of the rainy season, raising their chicks during the dry season (Ogden 1994). In South Florida, storks traditionally bred in large colonies south of the Everglades. They would fly long distances in large groups to feed in large flocks in these ephemeral pounds where the fish were concentrated (Kahl 1964, Kushlan 1976). They frequently flew >100 km (Kahl 1964, J. Ogden, pers. comm.) and fed in large flocks (Kushlan .1976, Ogden pers. comm.).
The Everglades is a young habitat, probably existing for fewer than 5,000 years (Gleason and Stone 1994, Wanless et al. 1994). While the storks are well-adapted to take advantage of the high concentrations of prey and have traditionally nested in large numbers in the Everglades, they are not necessarily specifically adapted to this habitat; it is likely that their variable biology allows them to thrive in many different wetland systems.
Recent changes
Major efforts to control to the waterflow in the wetlands, increasing from 1950s have modified the dynamics of the wetlands (Light and Dineen 1994). These changes tended to decrease the high concentrations of prey by equalising the waterflow during much of the year. This has resulted in the loss of early-season feeding habitat, equalised flow pattern, and unnatural and reduced flow of fresh water to Florida Bay. The wetlands no longer support the large numbers of breeding and non-breeding birds that were traditionally found in this area. The stork colonies have formed later in the year with varying but smaller numbers of birds than prior to the 1950s and 1960s (Ogden 1994). With large decreases in the size of the stork colonies in southern Florida there has been a decline in the U.S. breeding population. In 1984, the U.S. breeding population was listed as endangered (Bentzein 1984).
Simultaneous with the decrease in breeding numbers in south Florida; there has been an increase in the number of colonies and the numbers of storks breeding farther north in Florida (Ogden et al. 1987). Storks began breeding in Georgia in 1965 (Harris 1995) and South Carolina in 1981 (Murphy 1995). By 1993-1995, the U.S. breeding population was estimated at 5,768-7,853 pairs of which approximately 33% of the storks bred in Georgia and South Carolina (U.S. Fish and Wildlife Service 1996).
Wetlands in East-central Georgia and other northern parts of the storks’ range.
In east-central Georgia, the climate and wetland dynamics available to storks has similarities and differences with those available in south Florida. As much as 20% of the area surrounding the colony wetlands (Coulter et al. 1987, Hodgson et. al. 1988), but many of these are very disconnected from each have dynamics that operate independently. Water flow operates through the watersheds on a greater gradient than found in south Florida.
The climate in east-central Georgia is subtropical. Temperature varies significantly between the hot summer (April-September) and cooler winter months (October-March) while rainfall is almost even throughout the year (Table 1) (Mather 1964, Wilmott et al. 1981). As a result, there is high evapotranspiration during the hot summer, but very low evapotranspiration during the cool season. The result is a wet-dry seasonality dominated by seasonal changes in evapotranspiration rather than rainfall differences as in south Florida. As part of this study, I examined whether the climate and high evapotranspiration would result in predictable drawdowns and concentrations of prey.
Hypotheses.
During my studies, I compared my results with those from south Florida. While both areas are influenced by wet-dry season variation, there may be larger differences among the wetlands. Based on studies in south Florida, I wondered what differences existed in the dynamics of the wetlands between south Florida and Georgia and how these differences could affect the dynamics of the wetlands in the two areas, the differences in food availability and how these might influence the biology of the storks in the two areas.
My study concentrated on the storks and also on the wetland factors that might influence the storks. I compared the results of my studies with the studies in south Florida. I developed the following hypotheses:
That the storks begin to breed at the end of the wet season and raise their young during the dry season when potential prey is more concentrated at feeding areas.
That there is a seasonal decline in water levels and a simultaneous increase in prey densities as a result of evaporative concentration. And that this is seasonally predictable seasonally each year.
That storks use the breeding colony as an information centre in the sense of Ward and Zahavi (1973), to locate foraging sites as they seem to in south Florida.
METHODS
Storks from the Birdsville colony near Millen, Georgia, Jenkins County, Georgia, were studied from 1984-1989 (Coulter 1990). This colony is in a 567 ha cypress swamp at Big Dukes Pond (32o52’ N, 82o03’ W), 13 km north-west of Millen. Foraging areas were located in the areas around the colony in east-central Georgia (Coulter et al. 1993).
Storks were followed in a fixed-wing aircraft from the rookery to foraging sites (Bryan and Coulter 1987, Bryan et al. 1995), referred to here as 'random sites'. During each flight a single bird was followed but the number of birds that joined it and seemed to be travelling with it were also counted. For analysis, the number of birds that left the colony, the maximum number travelling together at any time during the flight, and the number that arrived at the foraging site were calculated. The number of storks and other long-legged wading birds at the site when the followed bird arrived was recorded.
A ground crew was directed into the area by the air crew. A permanent stake was placed to mark the spot where the stork was first observed feeding. Vegetation and water quality characteristics at the site were sampled (Coulter and Bryan 1993).
Densities of fish, crustacean and amphibian prey (items/m2) and biomass (g/m2 wet weight) were determined by using a 1-m2 throw-trap (Kushlan 1981, Depkin et al. 1993). Analyses for this considered only those species known to be eaten by storks or closely related species and only individuals longer than 24 mm because all regurgitated items were over this length. Density and biomass were strongly correlated so throughout this paper the emphasis is n the patterns of potential prey density, similar trend having been found for biomass. Habitat characteristics (water quality and habitat) were sampled as described by Coulter and Bryan (1993).
In 1986 and 1987, we compared wetlands where storks were observed foraging with similar habitats where they were not observed. After following a bird to a foraging site, we located from the plane a paired area of similar habitat nearby where storks were not foraging. I refer to these other sites as 'alternative wetlands.' These sites were similar in distance and direction as their paired random sites. The alternate sites were sampled in the same way that random sites were sampled.
Among the 'random sites', 4 'resample sites' were chosen to be visited at roughly 3-wk intervals during the breeding seasons of 1985-1989. During each visit, water depth at the stake was measured and potential prey was sampled to calculate potential density and biomass. Sites 055, 067, 070 were discovered in 1984 while site 219 was found in 1985. Site 055 is a pond of about 1500 m2 at the edge of an agricultural field. There is no woody vegetation, but the herbaceous vegetation becomes very thick during the summer. During wet periods, the pond is connected by water to a hardweood swamp. Aquatic fauna probably colonise the site from this swamp after dry periods: the site became completely dry in 1985 and 1988. Site 067 is an agricultural pond of about 3000 m2 that is unconnected to other wetlands: it became dry in 1987 and lost its fish fauna but in 1987 crayfish (which may have survived the dry period in the substrate), tadpoles and a few fish (put into the pond by the landowner) were sampled. Site 070 is an agricultural wetland with scattered hardwoods which spreads into nearby low areas and may have an areas of 4-5 ha after we periods although during the breeding season, it is only slightly larger that sites 055 and 067. Although the pond was never dry, it was reduced to a single, small pond each year. Site 219 is an open hardwood swamp with an area of about 60 ha where there has always been water, although in 1988, it was dry except for some deep ditches in the swamp.
The statistical package STATA was used. All statistical tests were 2-tailed. Frequency distributions were examined for normality (D’Agostino et al. 1990, Royston 1991). If distributions were not normal, non-parametric statistical tests were used. Confidence intervals in this paper are + 1 standard deviation.
RESULTS
Breeding
The phenology was similar in all years of the study, 1984-1991. Storks returned to Jenkins county each year in late February. The colony began to form in early March. Eggs were laid from late March through mid May. Young began fledging in late June. The last fledglings left the colony by mid-August (Coulter 1993, Coulter and Bryan 1995). The timing was very similar in all years. The storks raised their chicks during the summer months when evapotranspiration was greatest.
Foraging sites
Storks were followed from the colony to foraging sites between 0.3 and 63.1 km from the colony (0 =12.04 +10.6 km). The foraging sites were in a variety of wetlands (Table 2), most wetlands available. We sampled potential prey species at the sites to which storks were followed.
Most foraging sites were located in bottomland hardwoods and cypress swamps (Table 1). Most sites were in Bottomland hardwoods and cypress swamps and in lesser frequencies in ponds and marshes, and fewer in rarer and more ephemeral wetlands (ditches, flooded roads, etc.).
When the frequency of sites in major habitats was compared with the expected frequency of those expected by habitat analysis based on satellite imagery, more sites were among the more open sites (ponds and marshes) and fewer sites were among the sites with trees (x 2 3=56.97, P< 0.001) (Coulter et al. 1987, Hodgsen et al. 1988).
Even at foraging sites, the habitat tended to be open with little canopy cover, submergent or emergent vegetation. Woody stems were sparse (Coulter and Bryan 1993). However, habitat characteristics did not differ significantly between foraging sites and 'alternate sites'.
The densities of potential at foraging sites varied from 0.07-249.75 items/m2 (median=2.67, 0 =7.82+20.98, n=186 sites) and the biomass varied from 0.28-479.43 g/ m2 (median=9.02, 0 =21.15+46.27, n=186) (Coulter and Bryan 1993). The mean length of potential prey at sites was 49.9+21.9 mm (n=5191). The storks ate larger prey than those available. The mean length of prey in regurgitations was 84.7 mm. The difference was significant (Mann-Whitney U-test, P<0.01) (Depkin et al. 1992).
Changes in water depth and prey density and resample sites
At four resample sites, we measured water depth and prey density at 3-week intervals during the breeding seasons (March-August) from 1985-1989. Among 102 sampling efforts, the water level decreased 64 (63%) and increased 38 (37%) of samplings (Table 3) (Coulter 1992). Prey density was negatively related to water depth, increasing on 77% of samplings when water levels decreased, and potential prey density decreased on 61% of samplings when water levels increased.
The number of times that there was a decrease in water level at these sites varied among years. The percents of sampling observations when a water decrease was recorded were 50%, 82%, 75%, 64% and 33% for 1985-1989, respectively. The percents of samplings when an increase in potential prey density was recorded were 50%, 57%, 63%, 48% and 75% for these same years.
Numbers of birds travelling to foraging sites.Storks usually left the colony alone. The median number of birds leaving the colony was 1 stork (range=1-16, 0 =1.6+1.7 storks, n=211 foraging trips). While travelling the followed bird was occasionally joined by others. The median of the maximum number of birds travelling together was 2 stork (range=1-19, 0 =2.8+2.9 storks, n=211 foraging trips). The median number of storks landing together at the foraging site was 1 stork (range=1-15, 0 =1.7+1.9 storks, n=211 foraging trips). The differences between these were statistically significant (Kruskal-Wallis test, P<0.001). The number of storks at the site when the followed bird arrived varied from 1-171 (median=2, 0 =6.3+15.8 storks, n=192 sites). Sixty-eight (35%) of the sites had no storks present; 31 (16%) had a single stork present.
DISCUSSION
In east-central Georgia, the landscape was dominated by an abundance of wetlands that were hydrologically independent from each other and in which availability of potential stork prey also varied independently. During the study the storks bred during the summer when evapotranspiration rates were highest and when the water level in most wetlands decreased and prey density increases. However this pattern was not as predictable as it had been historically in southern Florida. In Georgia, among the resample sites, the average yearly percent of observations when a decrease in water was recorded was 65% and the percent of observations when an increase in potential prey density was recorded was 59%.
Prey densities at foraging sites in Georgia averaged 7.82 items/ m2 , less than 141 and 40 items/ m2 in the Everglades and the southern Florida coast, respectively. Yet the length of prey in Georgia were larger than prey consumed in south Florida: 84.7 mm length of prey in regurgitations in Georgia compared with 41 mm and 54 mm in regurgitations from two south Florida colonies.
Storks in Georgia, feeding in wetlands with lower concentrations of larger fish than available in Florida, foraged differently than reported in south Florida. They fed in much smaller groups of birds, usually on or two birds feeding together. Furthermore, they didn’t seem to use the breeding colony as an information centre. Instead, they travelled singly or in small groups to foraging sites and fed in small groups at foraging sites.
This comparison between east-central Georgia and south Florida suggests important points. In making these comparisons, I have concentrated on what is important to storks. However, these have more far-reaching implications to other aspects of the wetlands:
Storks, and probably many birds, are highly variable. They can adapt their biologies to very different environmental situations. Storks have historically done well in south Florida and are currently doing well farther north, including east-central Georgia.
The health of wetlands and the dynamics of wetlands vary geographically. The Georgia wetlands differ from the Everglades. The most important factors to the storks in one wetland may be very different from those that are important in another wetland. In east-central Georgia, the birds feed in wetlands where there are low densities of large prey whereas in Florida, there is a seasonally predictable concentration of prey in ephemerally concentrated wetlands. In east-central Georgia they fly singly or in small groups to the foraging sites where they feed in small numbers, whereas in Florida, they travel and feed in much larger flocks.
It is important to study the wetland dynamics as well as the birds in order to determine the factors that are important to the birds. If only the birds are studied, then it is likely that important aspects of the wetlands may be ignored. Preservation and restoration depend on an understanding of the wetlands.
ACKNOWLEDGEMENTS
I am grateful to the Savannah River Ecology Laboratory for support throughout this work. I am lucky to have worked with a dedicated, enthusiastic and hard-working staff: A. L. Bryan, Jr., (1984-1989), S. L. Coe (1986-1988), F. C. Depkin (1986-1988), T. L. Gentry (1988-1989), L. C. Huff (1984-1986), S. D. Jewell (1984-1985), W. B. Lee (1984), L. S. McAllister (1984), D. E. Manry (1989), K. L. Montgomery (1988-1989), L. A. Moreno (1988), M. A. Rubega (1986), D. J. Stangohr (1987), W. J. Sydeman (1985), N. K. Tsipoura (1987), J. M. Walsh (1985-1987), B. E. Young (1986) and D. P. Young (1989). J. Meyers who directed the stork project in June 1983-April 1984, laid the groundwork for many of the sampling methods used. D. Alexander, B. Beaushears, P. J. Clark, L. Hall, E. Johnson and M. Wease of Augusta Aviation piloted the aircraft during the aerial surveys over the years. I also thank M. H. Smith, J.W. Gibbons and W. D. McCort for indispensable support throughout this project. This research was supported by the United States Department of Energy, Savannah River Operations contract DE-AC0976SROO-819 with the University of Georgia, Institute of Ecology Savannah River Ecology Laboratory.
REFERENCES
Bentzein, M.M. 1984. Endangered and threatened wildlife and plants: U.S. population of the Wood Stork determined to be endangered. Federal Register 49(40)(2/28/84): 7332-7335.
Bryan, A.L., Jr. & Coulter, M.C. 1987. Foraging flight characteristics of Wood Storks in east-central Georgia, U.S.A. Colonial Waterbirds 10: 157-161.
Bryan, A.L., Jr., Coulter, M.C. & Pennycuick, C.J. 1995. Foraging strategies and energetic costs of foraging flights by breeding Wood Storks. Condor 97: 133-140.
Coulter, M.C. 1987. Wood Storks of the Birdsville Colony and swamps of the Savannah River Site. 1989 annual report. SREL-38/UC-66e. Savannah River Ecology Laboratory, Aiken, SC, USA.
Coulter, M.C. 1990. Wood Storks of the Birdsville Colony and swamps of the Savannah River Site. 1986 annual report. SREL-31/UC-66e. Savannah River Ecology Laboratory, Aiken, SC, USA.
Coulter, M.C. 1992. Foraging ecology of American Wood Storks Mycteria americana in east-central Georgia, USA. Proc. VIII Pan-African Ornithological Congress: 411-416.
Coulter, M.C. 1993. Wood Storks of the Birdsville Colony and swamps of the Savannah River Site. General overview of research findings, 1983-1990. SREL-42/UC-66e. Savannah River Ecology Laboratory, Aiken, SC, USA.
Coulter, M.C. & Bryan, Jr., A.L. 1993. Foraging ecology of Wood Storks (Mycteria americana) in east-central Georgia. I. Characteristics of Foraging Sites. Colonial Waterbirds 112: 237-243.
Coulter, M.C. & Bryan, Jr., A.L. 1995. Factors affecting reproductive success of Wood Storks (Mycteria americana) in east-central Georgia. Auk 112: 237-243.
Coulter, M.C., Bryan, Jr., A.L. Mackey, Jr., H.E., Jensen, J.R. & Hodgson, M.E. 1987. Mapping of Wood Stork foraging habitat with satellite data. Colonial Waterbirds 10: 178-180.
Coulter, M.C., Rodgers. Jr., J.A., Ogden, J.C. & Depkin, F.C. In press. The American Wood Stork (Mycteria americana). In: Poole, A. & Gill, F.G. (eds) The Birds of North America. The Academy of Natural Sciences, Philadelphia PA, and the Americana Ornithologists’ Union, Washington, D. C.
Critchfield, H.J. 1974. General Climatology, Third Edition. Prentice-Hall, Inc., Inc., Inglewood Cliffs, N. J., U.S.A.
D’Agostino, R.B., Belander, A. & D’Agostino, R. B., Jr. 1990. A suggestion for using powerful and informative tests of normality. American Statistician. 44: 316-321.
Depkin, F.C., Coulter, M.C. & Bryan Jr, A.L. 1992. Food of nestling Wood Storks in east-central Georgia. Colonial Waterbirds 15: 219-225.
Dusi, J.L. & Dusi, R.T. 1968. Evidence for the breeding of the wood stork in Alabama. Alabama Birds 16:14-16.
Gleason, J.P. & Stone, P. 1994. Age, origin, and landscape evolution of the Everglades peatland. In: Davis, S. & Ogden, J.C. (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach, FL, USA: 149-197.
Harris, M.J. 1995. The status of the Wood Stork in Georgia. P. In: Proceedings of the Wood Stork Symposium. The Georgia Conservancy. Savannah Georgia: 34-46.
Hodgson, M.E., Jensen, J.R., Mackey Jr., H.E. & Coulter, M.C. 1988. Remote sensing of wetland habitat: a Wood Stork example. Photogrammetric Engineering and Remote Sensing 53: 1075-1080.
Kahl, M.P. 1962. Bioenergetics of growth of nestling Wood Storks. Condor 64: 169-183.
Kahl, M.P. 1964. Food ecology of the Wood Stork (Mycteria americana) in Florida. Ecological Monographs 34: 97-117.
Kushlan, J.A. 1976. Wading bird predation in a seasonally fluctuating pond. Auk 93: 464-476.
Kushlan, J.A. 1981. Quantitative sampling of fish populations in shallow freshwater environments. Trans. Amer. Fish Soc. 103: 348-352.
Kushlan, J.A. 1986. Responses to wading birds to seasonally fluctuating water levels: strategies and their limits. Colonial Waterbirds 9: 155-162.
Light, S.S. & Dineen, J.W. 1994. Water control in the Everglades: a historic perspective. In: Davis, S. & Ogden, J.C. (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach, FL, USA: 47-84.
Mather J.R., Ed. 1964. Average Climatic Water Balance Data for the Continents. Part VII. United States. C.W. Thornthwaite Associates, Laboratory of Climatology, Centerton, New Jersey, Technical Report No. 7: 419-615.
Murphy, T.M. 1995. The status of the Wood Stork in South Carolina. P; 30-33 in Proceedings of the Wood Stork Symposium. The Georgia Conservancy. Savannah Georgia.
Oberholser, H. C. 1938. The Bird Life of Louisiana. Louisiana Dept of Conserv. Bull. 28.
Oberholser, H.C. & Kincaid Jr., E.B. 1974. The Bird Life of Texas. University of Texas Press, Austin, Texas.
Ogden, J.C., Kushlan, J.A. & Tilmant, J.T. 1978. The food habits and nesting success of Wood Storks in Everglades National Park in 1974. United States National Park Service Natural Resources Report 16.
Ogden, J.C. 1994. A comparison of wading bird nesting colony dynamics (1931-1946 and 1974-1989) as an indication of ecosystem conditions in the southern Everglades. In: Davis, S. & Ogden, J.C. (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach, FL, USA: 533-570
Ogden, J.C., McCrimmon Jr., D.A., Bancroft, G.T. & Patty, B.W. 1987. Breeding populations of the Wood Stork in the southeastern United States. Condor 89: 752-759.
Royston, P. 1991. Comment on sg3.4 and an improved D’Agostino Test. Stata Tech. Bull. 3: 23-24.
U.S. Fish and Wildlife Service. 1996. Revised recovery plan for the U.S. breeding population of the wood Stork. U.S. Fish and Wildlife Service. Atlanta, Georgia.
Ward, P. & Zahavi, A. 1973. The importance of certain assemblages of birds as 'information centres' for food-finding. Ibis 115: 517-534.
Wanless, H.R., Parkinson, R.W. & Tedesco, L.P.. 1994. Sea level control on stability of Everglades wetlands. In: Davis, S. & Ogden, J.C. (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach, FL, USA: 199-223
Willmott, C.J., Mather, J.R. & Rowe, C.M. 1981. Average monthly and annual surface air temperature and precipitation data for the world. Part 2. The Western Hemisphere. Publications in Climatology. Vol. XXXIV. C. W. Thornthwaite Associates, Elmer, New Jersey.
Table 1. Monthly climatic variables for Augusta, Georgia and Homestead, south Florida. Data from Mather 1964 and Wilmott et al. 1981.

Table 2. Foraging Habitats available to and used by Wood Storks in east-central Georgia
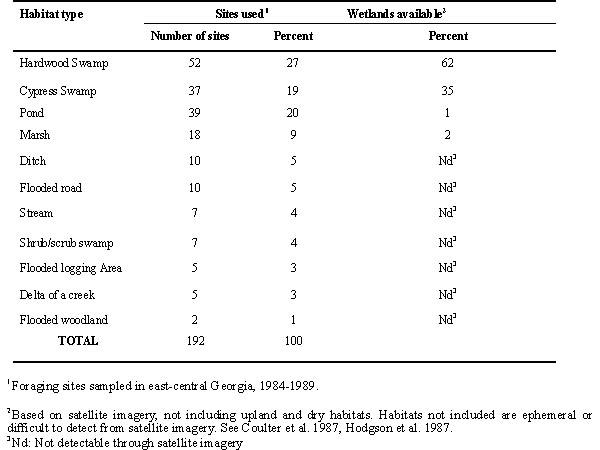
Table 3. Changes in water depth and densities of potential prey at resample sites in east-central Georgia, 1985-1989.
