S37.5: Optimal digestive responses to changing diet and foraging costs
William H. Karasov
Department of Wildlife Ecology, University of Wisconsin, Madison, WI 53706, USA, e-mail wkarasov@facstaff.wisc.edu
Karasov, W.H. 1999. Optimal digestive responses to changing diet and foraging costs. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2247-2258. Johannesburg: BirdLife South Africa.Predictions from optimal digestion models have now been tested in a variety of circumstances. Major predictions have been rejected over and over again, such as those for Rainbow Lorikeets Trichoglossus haematodus and Cedar Waxwings Bombycilla cedrorum that retention time and digestive efficiency would be inversely related to diet glucose concentration or those for waxwings and Yellow-rumped Warblers Dendroica coronata that retention time and digestive efficiency would be increased when time costs of feeding were increased. Nectar-eaters including Hummingbirds Sephanoides sephanoides, Gurney's Sugarbirds Promerops gurneyi, Black Sunbirds Nectarinia amethystina, and Malachite Sunbirds Nectarinia famosa did respond to altered sucrose concentration according to some predictions but they did not maximise their rate of energy gain. The models = poor fit to the data is likely due to invalid assumptions and/or a misguided framework. Nonetheless, the optimality approached helped us conceptualise a functional model of digestion that can still be used without reference to the concept of optimality. Even if current reactor-based models of gut function prove too simplistic, they have pointed the way to previously unappreciated but important physiological features, and have led us to useful empirical results, including: (1) extraction efficiency does not change in situations where either sugar concentrations or intermeal-intervals are manipulated; (2) when intermeal-intervals are manipulated, retention time does not change; (3) when sugar concentrations are manipulated, retention time either does not change or increases with increasing sugar concentrations. In the ecological realm, this information can improve predictions about relative dispersal distances for seeds of fruits with differing nutrient contents, and it indicates that estimates of digestive efficiency are robust.
INTRODUCTION
A number of optimization models have been suggested as frameworks in studies of digestive physiology, especially in relation to ecology and evolution (Sibly 1981; Penry & Jumars 1987; Cochran 1987; Alexander 1991; Martinez del Rio et al. 1994). Models are useful for several reasons. They show how the important gastrointestinal (GI) tract attributes relate to each other, and thus they are useful tools in mechanistic studies of complex systems. Also, they identify and interrelate those attributes of the GI tract that determine the rate and efficiency of nutrient extraction from food, two parameters of whole-animal and hence ecological importance. The optimization models can be used to generate predictions about the optimal responses of birds to changes in the concentrations of substrates in their foods, a feature of food richness, and to changes in the costs of acquiring those foods. The primary responses of the birds are in how long they retain food in their gut and how efficiently they digest it. If retention time and extraction efficiency are modulated as predicted by the models, then determining the profitability (i.e. energetic gain/costs) of a given food type is complicated because digestive efficiency is not fixed but is instead conditional on the costs of acquiring the food or its richness. Also, differences in retention time could influence ecological features such as maximal feeding and production rates of birds and dispersal distances for seeds they ingest (Karasov 1996).
The optimization approach requires that one choose some optimization criterion (or design objective sensu Penry & Jumars 1987, or 'goal' sensu Schoener 1971). Once a design criterion is selected, it should be possible to predict optimal GI tract structure and/or function. Penry & Jumars (1987) defined their design objective for optimum guts as the maximum conversion of ingested food to assimilable products in minima of time and gut-reactor volume. This design criterion of maximisation of the rate of extracting nutrients was essentially the same criterion adopted by Sibly (1981) in his graphical model. I review here studies that used nectarivorous Rainbow Lorikeets Trichoglossus haematodus (Karasov & Cork 1996), Green-backed Firecrowns Sephanoides sephanoides (Lopez-Calleja et al. 1998), Gurney's Sugarbirds Promerops gurneyi, Black Sunbirds Nectarinia amethystina, and Malachite Sunbirds Nectarinia famosa (Downs 1997), frugivorous Cedar Waxwings Bombycilla cedrorum (McWilliams & Karasov 1998a; Levey & Martinez del Rio 1998), and insectivorous/frugivorous Yellow-rumped Warblers Dendroica coronata (McWilliams & Karasov 1998b) to test major predictions of reactor models based on this criterion. It should be emphasised that under certain conditions an alternative optimization criterion, the maximisation of extraction efficiency, might be more appropriate, and this is considered below.
In the Methods section that follows, I outline the basic assumptions and operation of the models and the methods used to test the predictions. Detailed presentations are available elsewhere (Martinez del Rio & Karasov 1990; Karasov & Cork 1996). In Results I summarise the primary responses of avian species to manipulations of nutrient density of foods and the costs of food acquisition. The synthesis in the Discussion considers the major findings in relation to the issue of digestive optimization and offers suggestions for future research.
METHODS
Basic operation of the models
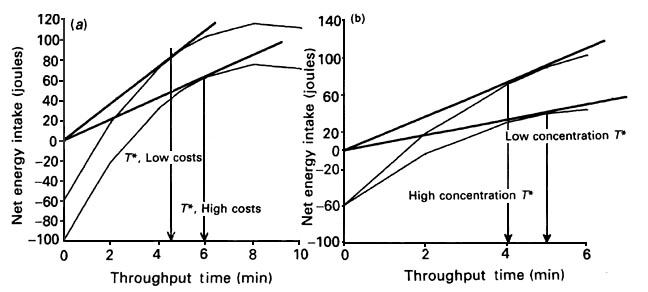
The following is a summary from Martinez del Rio & Karasov (1990). The optimization criteria or 'goal' is maximisation of the rate of extracting nutrients. The boundary conditions in the models are defined by certain benefits and costs. The benefits are determined by the relationship between energetic gain from nutrient absorption as a function of residence time in the intestine. The longer food is retained in the intestine the greater is the contact time between digestive enzymes and nutrient transport mechanisms and the more nutrient is extracted from food, up to the maximum possible. The costs are determined by the energetic expense(s) of obtaining the amount of food required to fill the intestine plus the cost of survival during the time required to process this amount of food. The control variable in the model is the residence time of nutrient in the intestine. We assume intestinal volume is constant so that changes in residence time can only be achieved by altering food intake or processing rate of digesta. The difference between gain and cost is captured graphically in the net energy gain curve, which is the absorbed energy minus costs for obtaining the food and surviving during processing time, plotted as a function of processing time (Fig. 1).
The a priori predictions of the optimization models depend on the shape of the net energy gain curve. Whether it is linear or decelerating towards a maximum is influenced by various details of digestive biochemistry/physiology (Martinez del Rio & Karasov 1990). Currently, the shapes are deduced, and a useful advance would be to find a way to determine their shape by measurement. The gain curves presumably decelerate towards a maximum (as in Fig. 1) for lorikeets drinking glucose solution (Karasov & Cork 1996), waxwings eating sugary fruits (McWilliams & Karasov 1998a), and warblers eating high fat foods (McWilliams & Karasov 1998b) . This is because there is no rate-limiting breakdown step (even for the warblers digesting fat; Place & Stiles 1992) and absorption of these substrates is largely passive, in which case absorption is fastest at initial luminal concentration and slows down as substrate is absorbed and luminal concentration falls. For the hummingbird digesting sucrose at enzyme-saturating concentrations, however, the rate of digestion is relatively constant at all but the lowest luminal concentrations and so the gain curve is probably a linear function of digesta retention time (Martinez del Rio & Karasov 1990). The shapes of the gain curves for the sugarbird and sunbirds are unknown.
In the case of the decelerating gain curve, unless costs are extremely high the optimal amount of energy gained from the food is less than the total possible. (The graphical solution for maximising the net rate of energy gain is the tangent shown in Fig. 1). In other words, the net rate of energy absorption is maximised by expelling some of the digesta prior to complete absorption, and refilling with higher concentration food; i.e. extraction efficiency is considerably less than 100%. This is prediction 1. We predict that Rainbow Lorikeets, Cedar Waxwings, and Yellow-rumped Warblers will have extraction efficiencies notably lower than 100% for their major substrates. However, in the case of the linear gain curve of hummingbirds digesting sucrose, the optimal amount of energy gained is near the maximum, so one predicts nearly complete digestion of sucrose meals. Without information on the shape of the gain curves for the sugarbird or sunbirds, no a priori prediction is made.
The second prediction is that retention time in the small intestine and hence extraction efficiency are inversely related to nutrient concentration. This prediction, which applies to birds exhibiting the decelerating gain curve, is displayed graphically in Fig. 1b. Hummingbirds, however, should exhibit a positive relationship between gut retention time and sugar concentration, and almost complete digestion of sucrose independently of sugar concentration.
The third prediction for birds with a decelerating gain curve is that when costs of food acquisition are increased, the residence time of nutrient in the intestine will also increase if the bird is maximising the net rate of energy gain. In other words, as food becomes more expensive to acquire, the food should be held longer in the intestine (Fig. 1a) and thus nutrients in the food will be more thoroughly digested. For hummingbirds, which are predicted always to digest sucrose completely, the cost of food acquisition should have no effect on digestive features.
These predictions can be generated for digestive systems that are modelled as batch reactors that digest food discontinuously (e.g. Cochran 1987) or plug-flow reactors that digest food continuously (e.g. Martinez del Rio & Karasov 1990; Levey & Martinez del Rio 1998).
Testing the predictions
Birds are habituated to cages and an appropriate diet. In some studies birds are switched to test foods only hours before measurements are made (e.g. Levey & Martinez del Rio 1998) whereas in others birds are offered test foods several days prior to tests (e.g. Karasov & Cork 1996). Special observation cages with one-way glass are typically used to reduce birds' behavioural stress associated with human observer presence (see Afik & Karasov (1995) for full description). Specific experimental manipulations, involving alterations in the concentration of a key nutrient or in energy expenditure, are described below. The primary parameters measured in tests of about 4 to 6 h duration are feeding rate, digestive efficiency, and measure(s) of residence time of food in the digestive tract. Feeding rate is measured gravimetrically and residence time is measured using indigestible markers, whereas digestive efficiency can be measured either gravimetrically or by the inert indicator ratio method (Karasov 1990). The optimality models predict responses in intestinal residence time, which has been measured in many of the studies as either the transit time (time of first appearance of the indigestible marker), or as the difference between mouth-to-anus total mean retention time (TMRT; Warner 1981) and some measure of stomach residence time. Whereas the determination and meaning of TMRT are relatively straightforward, the measures of intestinal and stomach residence time are approximations subject to various assumptions (Karasov & Cork 1996; McWilliams & Karasov 1998a,b). Considering this, as well as the expectation that changes in intestinal residence time and TMRT will be positively correlated, and the fact that TMRT has been measured in most studies, I report in this review mainly the data on TMRT.
RESULTS
Testing predictions about the completeness of digestion
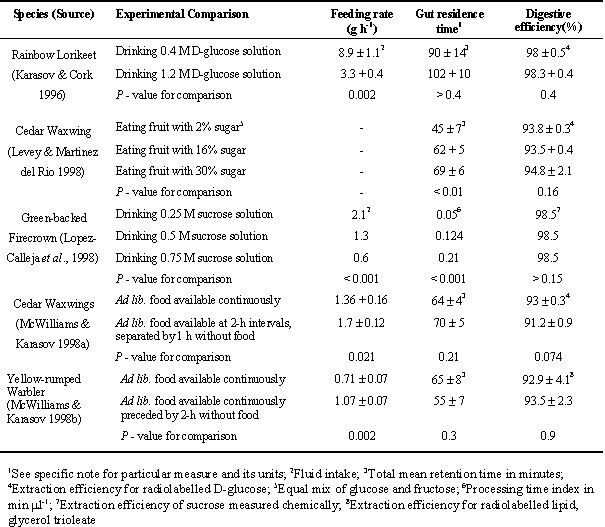
For hummingbirds with a linear gain curve, digestion of sugar should be uniformly very high, which is the consistent observation (Karasov 1990; Lopez-Calleja et al. 1998). In the case of birds with a decelerating gain curve, extraction efficiency is predicted to be considerably less than 100%. This prediction was clearly rejected for lorikeets digesting glucose, but results in other studies seemed consistent with the prediction. In the case of lorikeets, for whom glucose absorption is primarily by a passive mechanism (Karasov & Cork 1994) and thus the prediction applies (above), absorption of 14C-labelled D-glucose was 98%. However, Cedar Waxwings, American Robins Turdus migratorius, and Yellow-rumped Warblers, all of whom share the same glucose absorptive mechanism with lorikeets (Afik et al. 1997) extracted only about 90% of radiolabelled glucose (Karasov & Levey 1990; Afik & Karasov 1995) which is in apparent agreement with the prediction. But studies in which digestive efficiency is determined by measuring glucose directly in food and excreta (rather than isotopic activity) have reported digestive efficiencies of 98% for Cedar Waxwings and American Robins (Witmer 1998; Lepczyk et al. 1998b). Possibly, the absorption of the radiolabelled glucose is also as high as 98%, but some 14C is excreted in the form of a metabolite such as uric acid, which lowers the apparent absorption. Such an isotope effect on calculated digestive efficiency was demonstrated in the case of 3H-labelled vs. 14C labelled D-glucose (McWilliams & Karasov 1998a). Experiments in which extraction efficiency is measured simultaneously by the chemical and radiolabel method can resolve this issue. For now, it seems premature to conclude that, in apparent agreement with Prediction #1, some birds are somewhat inefficient digesting glucose.
Testing predictions about optimal response to changing food sugar concentration
Tests of Prediction #2: In the case of birds with a decelerating gain curve, retention time in the small intestine and hence extraction efficiency are inversely related to nutrient concentration
This prediction was tested using Rainbow Lorikeets (n = 6) drinking artificial nectar (Karasov & Cork 1996) and Cedar Waxwings (n = 6) eating artificial fruit (Levey & Martinez del Rio 1998). The tests were preceded by tissue- and whole animal-level studies that established that glucose was absorbed by both active and passive mechanisms (Karasov & Levey 1990; Karasov & Cork 1994) and therefore the birds were thought to exhibit a decelerating gain curve. Then, in whole animal experiments mouth-to-anus retention time was measured with an aqueous-phase marker (polyethylene glycol 4000 in lorikeets, sodium ferrocyanide in waxwings), and extraction efficiency was measured by the inert indicator ratio technique. All predictions were rejected. In lorikeets, extraction efficiency was uniformly very high (98 %; Table 1) and was not influenced by glucose concentration. Retention time increased significantly with increasing sugar concentration in Cedar Waxwings (Table 1). There was a trend in lorikeets for stomach emptying to slow when drinking more concentrated solution, which would cause an increase in retention time in the GI tract (Karasov & Cork 1996), as in the waxwings, rather than the predicted decrease. Thus, these results for lorikeets and waxwings were not consistent with the premise of maximisation of net energy gain.
Tests of Prediction #2: In the case of birds with a linear gain curve, retention time in the small intestine and hence extraction efficiency are positively related to nutrient concentration
This prediction was tested with Green-backed Firecrowns (n = 5 to 15) drinking artificial nectar (Lopez-Calleja et al. 1998). Feeding rate and digestive efficiency were measured gravimetrically, and the inverse of excretion rate was taken to be an index positively related to gut retention time. Several of the model predictions were supported. Hummingbirds exhibited almost complete assimilation of sucrose (99%), and the meal processing index increased linearly with sugar concentration (Table 1). An important prediction was falsified, however. Hummingbirds did not increase their rate of energy gain when fed on more concentrated sugar solutions, but maintained relatively constant daily energy intakes. This response to richer sugar solutions, which was also apparent in the lorikeet, is easily observed by comparing across diets in each experiment the product of sugar concentration and feeding rate (Table 1). This result was not consistent with the premise of maximisation of net energy gain.
Responses by Gurney's Sugarbirds, Black Sunbirds, and Malachite Sunbirds to increased food sugar concentration were very similar to those of the hummingbird (Downs 1997). Intake rates by volume were greater at low sugar concentrations, such that energy intakes per 90 min were similar, irrespective of diet, which supports the idea of regulated, rather than maximised, energy intake for these nectarivorous birds. All species were efficient at energy extraction, excreting 1% or less sucrose equivalent irrespective of the initial sugar concentration of each diet. Transit times of solutions ingested increased with an increase in sugar concentration.
Testing predictions about optimal response to increased cost of food acquisition
These tests were undertaken with captive birds held under conditions in which they were likely to maximise their net energy gain. To induce hyperphagia, Cedar Waxwings were acclimated to -20oC (McWilliams & Karasov 1998a) and Yellow-rumped Warblers were initially held on short day length and then exposed to longer day length (McWilliams & Karasov 1998b). The waxwings were fed a fruit-like diet and the warblers an insect-like diet. In a repeated measures design, birds were exposed to either of two different feeding regimes before the test periods. Control birds were provided continuous access to food ad libitum, but experimental birds had food available ad libitum for 2 h intervals separated by 1 - 2 h without food. The intervals without food were chosen to ensure that the birds had digested and excreted most but not all of the food from the previous feeding period before being allowed to feed again. In terms of the optimal digestion models, the feeding regime with food periodically available was thought to simulate the ecological situation where a bird finds rich food patches but then experiences non-feeding periods as it searches for new, rich food patches. Effectively, search costs were increased. The prediction was that during the test periods experimental birds would exhibit longer retention times and higher digestive efficiency than control birds (Fig. 1).
Waxwings (n = 10) and warblers (n = 7) were each fed according to the two feeding regimens. During trials of 4 - 5 h duration, feeding rate was measured gravimetrically, mouth-to-anus retention time was measured with an inert marker, and extraction efficiency (of glucose for waxwings, of lipid for warblers) was measured by the inert indicator ratio technique. During the actual trial periods the experimental waxwings were maintained on their regime of interval feeding, but the warblers were provided food continuously following a pre-test period of interval feeding. Experimental birds, during the intervals they had food, ate it faster than control birds, by 25% for waxwings and 51% for warblers (Table 1). Surprisingly, however, there were no significant differences in retention time or digestive efficiency between experimentals and controls in either species (Table 1). Thus, all predictions were rejected in both species.
DISCUSSION
The results from tests of predictions from the digestive optimization models have implications for both physiologists interested in modelling the digestive system as well as for ecologists interested in extrapolating results on digestion rate and efficiency from laboratory studies to the free-living situation. I first discuss how the results are inconsistent with the predictions of the optimal digestion model and with the assumption that birds are energy maximisers. I then review how, nonetheless, we have learned much by adopting the approach.
Regulation of digestion: prediction from the optimization model and conventional physiology
Predictions from the optimal digestion model have now been tested in a variety of circumstances: effects of food quality have been studied in Rainbow Lorikeets (Karasov & Cork 1996), hummingbirds (Lopez-Calleja et al. 1998), and Cedar Waxwings (Levey & Martinez del Rio, 1998) at room temperature and at steady state; effects of costs of feeding have been studied in cold-acclimated Cedar Waxwings (McWilliams & Karasov 1998a) and in Yellow-rumped Warblers in migratory-state as induced by manipulations of day length (McWilliams & Karasov,1998b). In the latter two tests of the model birds were forced to increase their energy expenditure and so were more likely to be aiming to maximise their rate of energy gain. Major predictions have been rejected over and over again, such as those for lorikeets and waxwings that retention time and digestive efficiency would be inversely related to nutrient concentration or those for waxwings and warblers that retention time and digestive efficiency would be increased when costs were increased. Hummingbirds did respond to altered nutrient concentration according to predictions, as regards retention time and digestive efficiency, but they and the other nectarivores did not maximise their rate of energy gain.
Did the optimality model fail because of feature(s) or constraint(s) not taken into account or because of an improperly selected optimization criterion? One feature not taken into account in the original model (Martinez del Rio & Karasov 1990) was possible water exchange between luminal contents and blood, which could have several implications. If hyperosmotic sugar solutions equilibrate osmotically in the small intestine, then water will be excreted from the GI tract along with sugar not absorbed. Theoretically, this could dehydrate the bird and therefore could have been an important constraint in the evolution of a digestion control system that maximises net rate of energy absorption by trading-off extraction efficiency to achieve very high digesta flow and hence nutrient absorption rate. Also, intestinal osmotic equilibration would tend to make differences in sugar concentrations in the lumen smaller than they are in foods, depending upon how quickly equilibration occurs and how much other solute is coupled to the water flow from blood into intestine lumen. Thus, differences in sugar concentration in experimental tests of the model might be considerably smaller than we assume. The next generation of optimal digestion models will rectify the omission of osmotic forces (Levey & Martinez del Rio 1998).
While these concerns relate mainly to tests of responses to altered food quality, other concerns can be raised about the two tests of responses to altered food acquisition costs. During the waxwings' trials the birds were fed somewhat discontinuously in a manner that does not match exactly the assumptions of models based on continuous digestion in plug-flow reactors (Martinez del Rio & Karasov 1990) or completely discontinuous digestion in batch reactors (Cochran 1987). The Yellow-rumped Warblers, however, were permitted to ingest food continuously during their trials that followed a period of interval feeding, and the results were similar to those in the trials with Cedar Waxwings (Table 1). If one accepts the notion that the no food interval effectively increased the time cost of acquiring the food that was ingested and digested subsequently, then it appears that an increase in the cost of food acquisition had no significant effect on retention time or digestive efficiency, contrary to the prediction (Fig. 1). (It is worth pointing out that these experiments had sufficient power to detect increases in digestive efficiency of 5%; McWilliams & Karasov 1998a).
As regards optimization criteria, perhaps the assumption about energy maximisation is inappropriate for an animal in energy steady state (i.e. not producing). There may be little selective advantage in maximising the rate of absorption except during periods of extensive storage, growth or reproduction. Other factors in the wild, such as predation risk, may have selected for feeding time minimisation. For a bird with relatively fixed energy requirements, foraging time is minimised when extraction efficiency is maximised.
Interestingly, physiological studies of the control of digesta flow do appear to reflect an avian digestive system designed to achieve relatively constant high digestive efficiency. They indicate control mechanisms that result in longer retention and higher extraction than might be predicted by rate maximisation. In poultry (Mateos et al. 1982; Duke 1989) gastric emptying is stimulated by intragastric volume and inhibited by negative feedback arising from duodenal and ileal receptors stimulated by products of digestion of food such as monosaccharides and osmolytes. For example, high concentrations of amino acids and fats in the duodenum inhibit gastric motility in poultry through hormonal (possibly cholecystokinin and pancreatic polypeptide) and neural reflexes. These mechanisms may be present in wild birds because retention times of markers are longer in Yellow-rumped Warblers fed higher fat diets (Afik & Karasov 1995) and in American Robins fed insects vs. fruits (Levey & Karasov 1992). Longer retention times on seed diets compared to vegetation in the Common Canary Serinus canariu; (Malone 1965), Mallard Duck Anas platyrhynchos (Malone 1965), Spur-winged goose Plectropterus gambensis (Halse 1984) and Graylag Goose Anser anser (Storey & Allen 1982) may also result from the same mechanism, though an additional factor in these cases is that harder substances such as seeds take longer to clear the stomach (Karasov 1990).
Insights gained using the optimization framework
What is concluded, then, about the proposal of optimal design of the avian digestive system? Because of uncertainties about model features, constraints, and optimization criteria, one does not reject the premise of optimal design despite the repeated rejections of model predictions. Indeed, the value of the research framework is not a proof that the digestive responses of birds are optimal. The benefits lie elsewhere and are manifold, as illustrated below.
The exercise helped us conceptualise a functional model of digestion that can still be used without reference to the concept of optimality. The model (Martinez del Rio & Karasov 1990) indicates that digestive efficiency is a positive function of both retention time and the rate(s) of hydrolysis and absorption and is a negative function of both concentration of nutrients in the food and digesta volume. Retention time itself is longer with larger gut volume and shorter with increased rate of digesta flow caused, for example, by increased gut motility or higher food intake. This model has been very effective helping us understand chronic adjustments of birds to altered diet composition and feeding rate (Karasov 1996). For example, the primary adjustment to chronically increased feeding rate is an enlarged gut. The net effect is to maintain retention time and hence digestive efficiency constant with increased load. However, when the model is viewed in conjunction with the acute responses of birds reviewed in this paper, some interesting questions arise, which underscores a second utility of the optimization framework.
They have pointed the way to previously unappreciated but important physiological features. The model implies that any change in one parameter will cause a change in digestive efficiency unless a compensatory change occurs in another parameter; and hence there is a tradeoff between rate of digesta processing and digestive efficiency - faster processing results in reduced extraction efficiency. How, then, were Yellow-rumped Warblers and Cedar Waxwings able to increase their feeding rates 25-50% with no decline in either retention time or digestive efficiency? The model assumed that the intestinal volume was constant, but perhaps rapid modulation of gut volume occurred by adjustments in smooth muscle which is elastic. Clearly, the digestive system of these birds has some spare capacity that enables them to maintain digestive performance across a range of food intakes without appreciable changes in digesta flow. In contrast, nestling altricial birds (Lepczyk et al. 1998a) and Broad-tailed Hummingbirds Selasphorus platycercus (C. Martinez del Rio, pers. comm.) appear to have no spare digestive capacity for increasing food intake, and American Robins and Yellow-rumped Warblers appear to have very limited spare capacity for switching rapidly to foods with different primary nutrients (Karasov 1996). One of the challenges for the future is delineating how much spare capacity birds maintain, its exact nature, and in what situations if any the level of spare capacity is modulated. The five optimality studies reviewed here illustrate how acute challenge experiments can be utilised to make this determination.
The poor fit of results to the original models' predictions has led to reexamination of both the assumptions of the models and the methods used to test the predictions. Incorporation of new osmotic and assimilation efficiency constraints alter the model's predictions in a way that conforms better with experimental results (Levey & Martinez del Rio 1998).Thus, future research needs to test the osmotic constraint model. Tests will require an improvement in ability to partition mouth-to-cloaca retention time into times in specific regions of the gut. This is because researchers have repeatedly pointed out how the distribution patterns of markers are not consistent with the assumed reactor designs (Karasov & Cork 1996; McWilliams & Karasov 1998; Levey & Martinez del Rio 1998). For example, statistical analyses of the marker distributions (Levey & Martinez del Rio 1998) and direct visualisation of digesta flow (Levey & Duke 1992) indicate considerable mixing in the small intestine, which contravenes the assumption that the intestine operates as a simple plug-flow reactor with little longitudinal mixing.
Optimal digestion studies have led us to useful empirical results. The following consistent patterns emerged from the tests of the model conducted to date: (1) extraction efficiency does not change in situations where either sugar concentrations or intermeal-intervals are manipulated; (2) when intermeal-intervals are manipulated, retention time does not change; (3) when sugar concentrations are manipulated, retention time either does not change or increases with increasing sugar concentrations. From an ecological perspective, the findings on effects on retention time can improve predictions about relative dispersal distances for seeds of fruits with differing nutrient contents. Also, the lack of an effect of food quality or costs of feeding on extraction efficiency suggests that estimates of digestive efficiency are robust. However, this conclusion is relevant to situations where food type is constant (e.g. diets with high vs. low glucose concentrations). In situations where birds switch between diets that differ in primary nutrients (e.g. between diets high in lipid (e.g. seeds) to diets high in sugar (e.g. fruits) changes in extraction efficiency can be significant (Afik & Karasov 1995). Also, several studies have provided evidence that birds in different physiological states (e.g. migratory status) can exhibit different extraction efficiencies on the same diet (Bairlein 1985; Hume & Biebach 1996).
In summary, even if current reactor-based models of gut function prove too simplistic, their application in a research program is neither inappropriate nor misleading. They force us to sharpen our thinking and our tests. Research on optimised control of digestive processing is converging on long-standing research on control of food intake. Careful thinking and experimental design will be necessary to distinguish competing hypotheses, such as control of feeding rate according to models of regulation of energy balance vs. a digestive optimization model with an osmotic constraint (Levey & Martinez del Rio 1998). We probably progress fastest when forced to acknowledge assumptions and make explicit predictions.
ACKNOWLEDGMENTS
I thank Doug Levey and Carlos Martinez del Rio for sharing their unpublished manuscripts, and they and Scott McWilliams for years of productive interaction including helpful criticisms of earlier drafts of the manuscript. The work was supported by NSF IBN-9318675 and IBN-9723793.
REFERENCES
Afik D. & Karasov, W.H. 1995. The trade-offs between digestion rate and efficiency in warblers and their ecological implications. Ecology 76:2247-2257.
Afik, D., McWilliams, S.R. & Karasov, W.H. 1997. Test for passive absorption of glucose in yellow-rumped warblers, and its ecological implications. Physiological Zoology 70:370-377.
Alexander, R.M. 1991. Optimization of gut structure and diet for higher vertebrate herbivores. Philosophical Transactions of the Royal Society of London B 333:249-255.
Bairlein, F. 1985. Efficiency of food utilization during fat deposition in the long-distance migratory garden warbler, Sylvia borin. Oecologia 68:118-125.
Cochran, P.A. 1987. Optimal digestion in a batch-reactor gut: The analogy to partial prey consumption. Oikos 50:268-230.
Downs, C.T. 1997. Sugar digestion efficiencies of Gurney's sugarbirds, malachite sunbirds, and black sunbirds. Physiological Zoology 70:93-99.
Duke, G.E. 1989. Gastrointestinal motility and its regulation. Poultry Science 61:1245-1256.
Halse, S.A. 1984. Food intake, digestive efficiency, and retention time in spur-winged geese (Plectropterus gambensis). South African Journal of Wildlife Research 14:106-110.
Hume, I.D. & Biebach, H. 1996. Digestive tract function in the long-distance garden warbler, Sylvia borin. Journal of Comparative Physiology B 166:388-395.
Karasov, W. H. 1990. Digestion in birds: Chemical and physiological determinants and ecological implications. In: Morrison, M.L., Ralph, C.J., Verner, J. & Jehl, J.R. (eds) Avian foraging: Theory, methodology, and applications. Cooper Ornithological Society, Kansas; Studies in Avian Biology No. 13:391-415.
Karasov, W.H. 1996. Digestive plasticity in avian energetics and feeding ecology. In: Carey, C. (ed) Avian energetics and nutritional ecology. New York; Chapman and Hall: 61-84.
Karasov, W.H. & Cork, S.J. 1994. Glucose absorption by a nectarivorous bird: The passive pathway is paramount. American Journal of Physiology 267:G18-G26.
Karasov, W.H. & Cork, S.J. 1996. Test of a reactor-based digestion optimization model for nectar-eating rainbow lorikeets. Physiological Zoology 69:117-138.
Karasov, W.H. & Levey, D.J. 1990. Digestive system trade-offs and adaptations of frugivorous passerine birds. Physiological Zoology 63:1248-1270.
Lepczyk, C.M., Caviedes-Vidal, E. & Karasov, W.H. 1998a. Digestive responses during food restriction and realimentation in nestling house sparrows (Passer domesticus). Physiological Zoology 71 (in press).
Lepczyk, C.M., Murray, A.K. & Winnett-Murray, K. 1998b. Seasonal fruit selection and digestive correlates in American robins. Bulletin of the Ecological Society of America (in press).
Levey, D.J. & Duke, G.E. 1992. How do frugivores process fruit? Gastrointestinal transit and glucose absorption in cedar waxwings (Bombycilla cedrorum). Auk 109:722-730.
Levey D.J. & Karasov, W.H. 1992. Digestive modulation in a seasonal frugivore, the American robin (Turdus migratorius). American Journal of Physiology 262:G711-G718.
Levey, D.J. & Martinez del Rio, C. 1998. Test, rejection, and reformulation of a chemical reactor-based model of gut function in a fruit-eating bird. Physiological Zoology 71 (in press).
Lopez-Calleja, M., Bozinovic, F., & Martinez del Rio, C. 1998. Effects of sugar concentration on hummingbird feeding and energy use. Comparative Biochemistry and Physiology 118A:1291-1299.
Malone, C.R. 1965. Dispersal of plankton: Rate of food passage in mallard ducks. Journal of Wildlife Management 29:529-533.
Martinez del Rio, C. & Karasov, W.H. 1990. Digestion strategies in nectar- and fruit-eating birds and the sugar composition of plant rewards. American Naturalist 136:618-637.
Martinez del Rio, C., Cork, S.J. & Karasov, W.H. 1994. Modelling gut function: An introduction. In: Chivers, D.J. & Langer, P. (eds) The digestive system in mammals: Food, form and function. Cambridge; Cambridge University Press:25-53.
Mateos, G.G., Sell, J.L. & Eastwood, J.A. 1982. Rate of food passage (transit time) as influenced by level of supplemental fat. Poultry Science 61:94-100.
McWilliams, S.R. & Karasov, W.H. 1998. Test of a digestion optimization model: Effects of costs of feeding on digestive parameters. Physiological Zoology 71:168-178
McWilliams, S.R. & Karasov, W.H. 1998b. Do variable-reward feeding schedules affect digestive performance of migratory birds? Oecologia (in press).
Penry D.L. & Jumars P.A. 1987. Modelling animal guts as chemical reactors. American Naturalist 129:69-96.
Place, A.R. & Stiles, E.W. 1992. Living off the wax of the land: Bayberries and yellow-rumped warblers. Auk 109:334-345.
Schoener T.W. 1971. Theory of feeding strategies. Annual Review of Ecology and Systematics 2:369-404.
Sibly, R.M. 1981. Strategies of digestion and defecation. In: Townsend, C.R. & Calow, P. (eds) Sunderland, MA; Physiological Ecology:109-139.
Storey, M.L. & Allen, N.K. 1982. Apparent and true metabolizable energy of feedstuffs for mature, nonlaying female embden geese. Poultry Science 59:1275-1279.
Warner A.C.I. 1981. Rate of passage of digesta through the gut of mammals and birds. Nutrition Abstracts Reviews 51:789-820.
Witmer, M.C. 1998. Do seeds hinder digestive processing of fruit pulp? Implications for plant/frugivore mutualisms. Auk 115:319-326.
Table 1. Primary experimental results in tests of optimal responses by birds to altered diet composition or increased time costs of food acquisition.

Fig. 1. Effect of (a) cost of food acquisition and (b) energy concentration on the optimal retention time. Increasing the cost of food acquisition (e.g., by increasing the distance between patches) has the effect of shifting the cost-benefit curve downwards and increasing optimal retention time (T*) and thoroughness of digestion (note tangent closer to the asymptote). Increased food concentration has the effect of increasing the steepness of the cost-benefit curve and lowering optimal T*. Note that these predictions are strongly dependent on the shape of the cost-benefit curve. The shape shown here, a curve decelerating towards the maximum, is for absorption of substrates that is largely passive, in which case absorption is fastest at initial luminal concentration and slows down as substrate is absorbed and luminal concentration falls. For a hummingbird digesting sucrose at enzyme-saturating concentrations, however, the rate of digestion is relatively constant at all but the lowest luminal concentrations and so the gain curve would be linear to the asymptote. Adapted from Martinez del Rio et al. (1994).