S37.2: Digestive strategies of avian herbivores
Scott R. McWilliams
Department of Natural Resources Sciences, University of Rhode Island, Kingston, RI 02881, USA, e-mail
srmcwill@uriacc.uri.edu McWilliams, S.R. 1999. Digestive strategies of avian herbivores. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2198-2207. Johannesburg: BirdLife South Africa.Body size puts limits on the quality of foods that can be eaten by herbivores and influences the processes by which the food is digested. Recent work on avian herbivores demonstrates that selective retention of digesta and nutrient uptake rates may help minimise these allometric constraints. Like other foregut fermenting vertebrates, the Hoatzin selectively retains particulate digesta and passes the liquid fraction much more quickly. The opposite pattern, selective retention of fluid digesta, is typical of some avian cecum fermenters such as galliformes although other hindgut fermenting birds such as the Emu Dromaius novaehollandiae, Canada Goose Branta canadensis, and Snow Goose Anser caerulescens show no selective retention of digesta at the whole-animal scale. For geese, however, the lack of whole-animal differences in passage rate of fluid or particulate fractions of the digesta may be the result of an interaction between selective retention of fluid digesta in the hindgut and increased mechanical processing of particulate digesta in the foregut (i.e. gizzard). Patterns of specific uptake rates in the goose support the hypothesis that birds feeding on relatively bulky diets maintain high rates of ileal uptake and thereby increase their integrated uptake capacity (IUC) without the costs associated with increasing gut mass. The cecal contribution to IUC for glucose and amino acids was higher for geese (24% and 6-19%) compared to chickens (2% and 4%), but modest compared to those for grouse (49% and 25%) and is consistent with the hypothesis that galliformes rely more on caecal fermentation and absorption of the products than do geese. Relatively high rates of aminopeptidase-N activity in the goose caeca (accounting for 24% of total hydrolytic capacity) along with high nutrient uptake capacity supports the hypothesis that the avian caeca plays an important role in protein digestion and absorption.
INTRODUCTION
Herbivore diet selection is influenced by available food nutrients, the relative ability to extract nutrients from the food, and anti-nutrient features such as indigestible bulk and toxins. Body size is thought to put limits on the quality of foods that can be eaten by herbivores and to influence the processes by which the food is digested. But current ideas about how body size influences diet breadth, mode of digestion, and ability to tolerate possibly antinutritive plant secondary metabolites are either mainly theoretical, based on a limited number of studies, or are based on ruminant physiology that may not apply to hindgut fermenters.
Avian herbivores are particularly interesting in terms of the proposed allometric constraints because they have relatively high metabolic rates and are generally smaller in size compared to mammalian herbivores. The relative rarity of avian herbivores suggests these allometric constraints are difficult to circumvent. Only 3% of existing bird species eat leaves and most birds that eat leaves are poor flyers (e.g. grouse, quail, ptarmigan (all in the Order Galliformes), and the Hoatzin Opisthocomus hoazin). True geese (Order Anseriformes, Family Anatidae, Tribe Anserini ) are exceptional herbivorous birds in that they are strong flyers that range in adult body size from the dimunitive Ross’ Anser rossi and Red-breasted Goose Branta ruficollis (ca. 1000 g) to the 'Giant' subspecies of Canada Goose Branta canadensis maxima (ca. 7000 g).
In this review, I will primarily discuss recent work on the digestive strategies of certain species of true geese in relation to other avian herbivores, specifically, and to vertebrate herbivores, generally. I use the term 'digestive strategy' as shorthand for the interplay between energy and nutrients in ingested food, the nutritional requirements of the forager, and features of digestive physiology (retention time, extraction efficiency, gut morphometrics, hydrolysis rates of digestive enzymes, nutrient uptake rates) that influence the supply of usable energy and nutrients to the forager. Excellent recent reviews are available that outline how adjustments in digestive physiology influence the supply of energy and nutrients in primarily insectivorous, frugivorous, and nectarivorous birds (Martinez del Rio et al. 1994 , Karasov 1996, Karasov & Hume 1997). Here I will focus on what we know about selected aspects of the digestive strategies of herbivorous birds.
I begin with a brief introduction to the theory derived from studies of mostly ruminants that relates body size and fibre digestion. I then discuss how recent work on small hindgut-fermenting herbivores demonstrates that selective retention of more digestible fractions of the diet and perhaps increased specific nutrient uptake rates and hydrolysis rates of digestive enzymes can ease or eliminate these allometric constraints. Most of the review focuses on whether these mechanisms for reducing the proposed allometric constraints are important for small avian herbivores.
Body size, metabolism, and fiber digestion
One feature of diet quality is fibre, which is typically considered the most important factor determining intake and digestibility in herbivores. In general, as body size of herbivores declines so does the fibre level of the selected diet and the proportion of energy requirements satisfied by energy yield from fermentation (Illius & Gordan 1993). This empirically and theoretically-based pattern of decline in reliance on fibre with decreasing body size in vertebrate herbivores is believed to occur because of unequal allometries of energy requirements and gut capacity (Demment & VanSoest 1985).
If metabolic rate determines the energetic requirement and gut size determines the capacity to process food into nutrients, then smaller herbivores generally and avian herbivores especially must process food rapidly to satisfy their higher mass-specific metabolic rates. Reliance on fermentation is also predicted to become increasingly difficult with decreasing body mass because fast passage rates and smaller absolute size of fermentation chamber reduce the efficiency of fermentative use of fibrous material.
More recently, however, the applicability of these allometric relationships for explaining digestive strategies of smaller herbivores has been questioned (Foley & Cork 1992, Cork 1994). Two of these criticisms are particularly pertinent for understanding digestive strategies of avian herbivores. First, the digestive strategies of smaller mammalian herbivores may not maximise fermentation of fibre by maximising retention time as assumed for ruminants (Parra 1978, Demment & Van Soest 1985). Instead, smaller mammalian herbivores may increase intake and passage rates and reduce fibre digestibility when eating higher fibre diets so that energy or nutrient gain is maximised. Apparently, higher intake increases energy yield from digestion of cell contents and not cell wall which compensates for increased endogenous losses caused by higher fibre intake (Cork 1994). The prediction is that small herbivores eating higher fibre diets will increase their intake (within limits), and that fibre digestion should decrease with increased intake (also see Illius & Gordan 1992, Justice & Smith 1992, Illius & Gordan 1993).
This potentially important adjustment in intake and digestibility in response to changes in dietary fibre has rarely been adequately tested in avian herbivores (Savory & Gentle 1976a,b; Karasov 1990). Daily food intake of Japanese Quail (Coturnix japonica) increased with dietary fibre, as predicted; however, contrary to the predictions, digestibility (total dry matter and just the fibre fraction) did not change with increased dietary fibre (Savory & Gentle 1976a,b). More such tests need to be conducted before we can evaluate whether avian herbivores maximise energy or nutrient gain by increasing their intake and passage rates and by reducing their fibre digestibility when eating diets with higher fibre.
A second reason why the proposed allometric relationships derived from work on larger mammalian herbivores may not hold for smaller mammalian or avian herbivores is that gut capacity as estimated by maximum volume or mass of the fermentation chamber may provide a relatively poor estimate of the capacity to process food into nutrients. There are at least two good reasons why gut capacity may provide a poor estimate of the capacity to process food into nutrients. First, small herbivores can effectively reduce the predicted allometric constraints by selectively increasing retention of the more digestible fibre while maintaining relatively fast passage rates. The prediction is that large particle-size, refractory portions of ingested fibre will have shorter retention times than smaller particle-size, soluble portions of ingested fibre which are more rapidly digestible. Second, higher reaction rates (e.g. hydrolysis rates of digestive enzymes and nutrient absorption rates) can increase the capacity to process food into nutrients independent of gut size. The prediction is that reaction rates are relatively high especially in regions of the gut of particular importance for breakdown and absorption of plant fibre. In the remainder of this review, I will evaluate these two predictions and the general hypothesis that both selective retention of digesta and increased reaction rates reduce the proposed allometric constraints for avian herbivores.
Selective retention of digesta in avian herbivores
Relative rates of passage of fluid versus particulate fractions of digesta provide important insights into digestive strategy. In foregut fermenting ruminants and macropods, and in many mammalian colon fermenters, particulate digesta is typically retained longer than the fluid digesta (Stevens & Hume 1995). The opposite pattern, selective retention of fluid digesta, is typical of small mammalian cecum fermenters such as rabbits and the Koala (Type C caecum fermenters in Stevens & Hume 1995), although many mammalian cecum fermenters show little or no selective digesta retention (Robbins 1993, Stevens & Hume 1995).
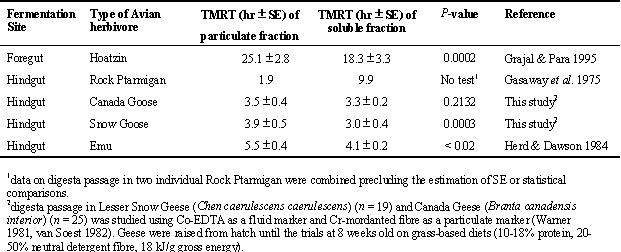
All avian herbivores so far described except the Hoatzin are hindgut fermenters and so are expected to retain smaller particle size, fluid fractions of digesta as long or longer than particulate fractions. Galliformes such as ptarmigan selectively retain fluid digesta in the caeca and rely on caecal fermentation of fibre (Gasaway et al. 1975). As a result, the fluid fraction of digesta has a longer total mean retention time than the particulate fraction in ptarmigan (Table 1). In contrast, the Emu (Herd & Dawson 1984) shows no selective digesta retention (Table 1) perhaps because the Emu is a large bird that relies primarily on fermentation of fibre in the colon. The Hoatzin like other foregut fermenting vertebrates selectively retains particles in the foregut and passes the liquid fraction much more quickly (Grajal & Parra 1995) (Table 1).
The few studies of selective retention of digesta in geese demonstrate that, like in galliformes, the caeca contains only small particles and there is likely retrograde movement of urinary by-products into the caeca (Clemens et al. 1975, Bjornhag & Sperber 1977). These studies as well as our own recent work on particle size distribution of digesta in the goose gut suggest that there is selective retention of small particle size, fluid digesta in the caeca. However, unlike in galliformes, total mean retention time of the fluid fraction of digesta in geese was similar or shorter than the particulate fraction (Table 1). If both geese and galliformes have similar selective retention mechanisms in the hindgut, perhaps differences in how food is processed in the foregut can explain why the fluid fraction of digesta had a longer total mean retention time than the particulate fraction in galliformes but not in geese.
Foregut processing of food in geese and galliformes involves storage in the proventriculus and crop, respectively, and then grinding of the food in the gizzard. The gizzard of geese is much larger than in galliformes for a bird of similar size (Hallsworth & Coates 1962, but see Sedinger 1997) which suggests more extensive foregut processing of food in geese. Clemens et al. (1975) showed that larger particles are retained for further degradation in the gizzard of geese until particle size is reduced. A pattern of selective retention of larger particles in the foregut of geese until food particles are reduced in size would be analogous to the particle-size reduction process in the ruminant fore stomach (van Soest 1982). If larger particles were selectively retained in the foregut as long as smaller particles were selectively retained in the hindgut, then total mean retention time of both fractions of digesta would be similar. Testing of this hypothesis requires more detailed studies of passage rates of particular fractions of ingested fibre in the foregut and hindgut of geese.
Hydrolysis rates of digestive enzymes
If hydrolysis rates of digestive enzymes or nutrient uptake rates per unit volume or mass of the small intestine or caeca are elevated in small herbivores then gut size may provide a poor estimate of the capacity to process food into nutrients. Increasing reaction rates per unit of gut has been proposed as an especially effective way to circumvent the proposed allometric constraints associated with being an avian herbivore (Obst 1991) because the alternative, increasing gut size, has definite limits for a bird that must fly (Dudley & Vermeij 1992). Thus, for avian herbivores in particular, the prediction is that reaction rates are relatively high especially in regions of the gut that are particularly important for breakdown and absorption of plant fibre.
Information on activities of digestive enzymes in birds is limited to studies of nectarivorous, insectivorous, frugivorous, granivorous, and omnivorous species (see Afik et al. 1995 for a review). Information on activities of digestive enzymes in herbivorous birds is limited to studies of domestic geese fed commercial diets (e.g. Nitsan et al. 1973). Herein I present for the first time information on activities of digestive enzymes for an herbivorous bird eating ad libitum a natural, grass-based diet. This study of digestive enzymes in geese was done in collaboration with Dr. William H. Karasov, University of Wisconsin, and both Eugenia Ciminari and Dr. Enrique Caviedes-Vidal, Universidad Nacional de San Luis, Argentina.
We studied hydrolytic activity of digestive enzymes in 12 week old Canada Geese Branta canadensis interior fed a grass-based diet (10-18% protein, 20-50% neutral detergent fibre, 18 kJ/g by dry wt) since hatch. We assayed aminopeptidase-N activities using the method developed by Maroux et al. (1973). We used L-alanine-p-nitroanilide as a substrate for aminopeptidase-N. We assayed disaccharide activities using the calorimetric method developed by Dahlqvist (1984) as modified by Martinez del Río (1990).
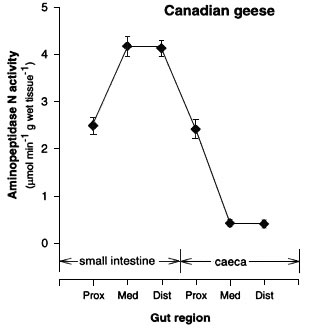
Activity of disaccharidases decreased distally in the small intestine and was relatively low in the caeca (Fig. 1). In contrast, activity of aminopeptidase-N increased distally in the small intestine, was similar in the proximal small intestine and caeca, and decreased distally in the caeca (Fig. 1). We used the specific activity of aminopeptidase-N and disaccharidases along with measurements of size of the gut regions to estimate the caecal contribution to a bird’s total hydrolytic capacity. The caecal contribution to total hydrolytic capacity was 24% for aminopeptidase-N and < 3% for both disaccharidases.
Patterns of decreasing disaccharidase activity or increased aminopeptidase-N activity with distance along the small intestine have been observed in nonherbivorous birds (Martinez del Río 1990, Caviedes-Vidal et al. 1994, Afik et al. 1995, Caviedes-Vidal & Karasov 1996) and so should not be interpreted as necessarily providing some adaptive benefit for birds with particular food habits. However, the impressively high activity of aminopeptidase-N in the goose caeca is unique and potentially nutritionally important. Active peptidases in the avian caeca would digest dietary, microbial, and recycled urinary protein which could then be absorbed if appropriate transporters were active in the caecal membrane (see below).
Nutrient uptake and absorption
Absorption of nutrients is an essential part of the digestion process. Understanding the absorptive capacity of particular gut regions may provide insights into the functional significance of particular portions of the gut. For example, Obst & Diamond (1989) demonstrated that glucose and amino acids are actively transported across the caecal membrane in some birds including geese and grouse. Such amino acid transport ability in the caeca is unknown for herbivorous mammals (Ilundain & Naftelin 1981, Hume et al. 1993) and so highlights a potentially fundamental difference between these groups with similar foraging habits. Transport of amino acids across the wall of the caeca may be nutritionally important because it provides a way for hindgut fermenters to use microbial protein as a source of essential amino acids (Sedinger 1997).
Obst & Diamond (1989) documented transport of amino acids in the caeca of five bird species (domestic chicken Gallus domesticus, Canada Goose, Red-necked Phalarope Phalaropus lobatus, Sage Grouse Centrocercus urophasianus, and Rock Dove Columba livia). However, their conclusions about phylogenetic and ecological correlates of the interspecific variation in cecal transport were limited because of small sample size (e.g. n = 1 for the Canada Goose) and because no attempt was made to control for diet effects.
We studied nutrient uptake in Cackling Canada Geese Branta canadensis minima fed either freshly cut alfalfa (32% protein, 22% neutral detergent fibre, 18 kJ/g by dry wt) or pasture grasses (23% protein, 35% neutral detergent fibre, 18 kJ/g by dry wt). We measured glucose, proline, and lysine uptake rates in specific locations of the gut using the everted sleeve technique (Karasov & Diamond 1983). Because we used (3H) L-glucose as our marker when measuring uptake of D-glucose (which allows correction for both extracellular space and passive diffusion), our estimates of nutrient uptake are for carrier-mediated or active glucose uptake. Because we used 14C PEG as our marker when measuring amino acid uptake our estimates of nutrient uptake include both active and passive components of amino acid uptake.
Uptake rates for glucose throughout the small intestine were similar for geese on both diets and uptake rates in the caeca were as high as those in the small intestine (Table 2). Uptake rates for proline and lysine like glucose were similar for geese on both diets, however, uptake rates for both amino acids differed by location in the gut (Table 2). Proline uptake increased distally in the small intestine but was much lower in the caeca. Lysine uptake decreased distally in the small intestine and was much lower in the caeca.
We used the specific uptake rates along with measurements of size of the gut regions to estimate the caecal contribution to a bird's Integrated Uptake Capacity (IUC). Geese on both the alfalfa and pasture diets had similar mass-specific gut morphometrics and similar specific uptake rates so that there were no differences related to diet in IUC for either glucose (F
1,4 = 0.756, P = 0.43) or proline (F1,4 = 3.98, P = 0.12) or lysine (F1,4 = 0.4, P = 0.55). In general, the cecal contribution to IUC for glucose was 24.2% ± 2.9%, for proline was 6.1% ± 1.9%, and for lysine was 8-19% (higher in alfalfa-fed geese). These estimates of caecal contribution are modest compared to those for grouse (49% and 25%, respectively) but relatively large compared to chickens (2% and 4%, respectively) which are the only other birds for which similar evidence is available (Obst & Diamond 1989).CONCLUSIONS
Avian herbivores such as the Hoatzin are apparently like many other foregut-fermenting herbivores in that particulate, fibrous digesta is selectively retained and microbially digested in the foregut. Digesta flow in the hindgut of the goose is apparently similar to that of many small mammalian hindgut fermenters as well as to other avian herbivores like the galliformes. Large particle size, fibrous fractions move through the hindgut relatively rapidly while small particle size, more soluble fractions are routed through the caeca. This selective retention of certain portions of digesta can effectively reduce the predicted allometric constraints by selectively increasing retention of the more digestible fibre in the caeca while maintaining relatively fast passage rates.
Comparisons of total mean retention time of particulate and soluble fractions of digesta in geese and galliformes suggests these two groups of avian herbivores may have very different selective retention mechanisms in the foregut. TMRT of soluble fractions of digesta are longer than particulate fractions of digesta in galliformes apparently because soluble fractions of digesta are selectively retained in the hindgut. In contrast, TMRT of particulate and soluble fractions of digesta are similar in geese even though geese like galliformes selectively retain soluble fractions of digesta in the hindgut. We suggest that in geese there is an interaction between selective retention of soluble digesta in the hindgut and selective retention of particulate digesta for grinding in the foregut that results in little whole-animal differences in passage rate of fluid or particulate fractions of the digesta. This hypothesis has not been adequately tested.
Our results on nutrient transport and hydrolysis rates of enzymes in geese have three important implications. First, patterns of specific uptake rates in various gut regions support the hypothesis that birds feeding on relatively bulky diets have relatively constant or increasing uptake rates along the intestine (Obst 1991). Maintenance of high rates of ileal uptake is relatively unusual in mammals and may represent a key avian adaptation that increases IUC without the costs associated with increasing gut mass. The increased activity of aminopeptidase-N along the intestine suggest that this hypothesis could be extended to also include activity of digestive enzymes, although this pattern of activity is also evident in nonherbivorous birds. Second, the uptake rates for glucose and proline in the caeca demonstrate that the avian caeca is a potentially important site for nutrient absorption. The relative importance of caecal uptake to the bird's nutrition depends on how caecal uptake rates are affected by competition for the same nutrients from microbes and the degree to which uptake is active or passive. The relatively high activity of peptidase enzymes in the ceaca strongly supports the hypothesis that the caeca may be important in protein nutrition in avian herbivores and especially in geese. Third, interspecific differences between galliformes and geese in the relative contribution of caecal uptake to IUC are consistent with the hypothesis that galliformes rely more on caecal fermentation and absorption of the products than do geese.
ACKNOWLEDGMENTS
I thank Jim Sedinger, Jim Leafloor, and Jon Dunn for may helpful discussions about geese and their physiology. Particular thanks to Eugenia Ciminari for her excellent thesis work on the digestive enzymes of geese, and to Bryan Obst for his help with the uptake experiments on Cackling Geese. I especially thank Bill Karasov and Enrique Caviedes-Vidal, my two primary collaborators on much of the work on geese described here. The work was supported by a University of California at Davis Fellowship, and grants from the California Dept. of Fish and Game, Wildlife Management Institute, and Ministry of Ontario.
REFERENCES
Afik, D., Caviedes-Vidal, E., Martinez del Rio, C. & Karasov W.H. 1995. Dietary modulation of intestinal hydrolytic enzymes in yellow-rumped warblers. American Journal of Physiology 269:R413-R420.
Bjornhag, G. & Sperber G. 1977. The transport of various food components through the digestive tract of turkeys, geese and guinea fowl. Swedish Journal of Agricultural Research 7:57-66.
Caviedes-Vidal, E., Afik, D., Martínez del Río, C. & Karasov W.H. 1994. Omnivory and dietary plasticity are not necessarily correlated: dietary modulation of intestinal enzymes in four bird species. The Physiologist. 37(5):A81.
Caviedes-Vidal, E. & Karasov W.H. 1996. Glucose and amino acid absorption in house sparrow intestine and its dietary modulation. American Journal Physiology 271(40): R561-R568.
Clemens, E. T., Stevens, C. E. & Southworth M. 1975. Sites of organic acid production and pattern of digesta movement in the gastrointestinal tract of geese. Journal of Nutrition 105:1341-1350.
Cork, S. J. 1994. Digestive constraints on dietary scope in small and moderately-small mammals: how much do we really understand? In: Chivers, D.J. & Langer, P. (eds) The digestive system in mammals: food, form and function; Cambridge; Cambridge University Press: 337-369.
Dahlqvist, A. 1984. Assay of intestinal disaccharidases. Scandinavian Journal of Clinical Laboratory Investigations 44:69-172.
Demment, M. W. & Van Soest P.J. 1985. A nutritional explanation for body-size patterns of ruminant and nonruminant herbivores. American Naturalist 125:641-672.
Dudley, R. & Vermeij G.J. 1992. Do the power requirements of flapping flight constrain folivory in flying animals? Functional Ecology 6:101-104.
Foley, W. J. & Cork S.J. 1992. Use of fibrous diets by small herbivores: how far can the rules be 'bent'? Trends in Ecology and Evolution 7:159-162.
Gasaway, W.C., Holleman, D.F. & White R.G. 1975. Flow of digesta in the intestine and cecum of the rock ptarmigan. Condor 77:467-474.
Grajal, A. & Parra O. 1995. Passage rates of digesta markers in the gut of the hoatzin, a folivorous bird with foregut fermentation. Condor 97:675-683.
Hallsworth, E.G. & Coates J. L. 1962. The growth of the alimentary tract of the fowl and the goose. Journal of Agricultural Sciences 58:153-163.
Herd, R.M. & Dawson T.J. 1984. Fibre digestion in the emu, Dromaius novaehollandiae, a large bird with a simple gut and high rates of passage. Physiological Zoologist 57:70-84.
Hume, I.D., Morgan, K.R. & Kenagy G.J. 1993. Digesta retention and digestive performance in sciurid and microtine rodents: effects of hindgut morphology and body size. Physiological Zoologist 66:396-411.
Illius, A.W. & Gordan I.J. 1992. Modelling the nutritional ecology of ungulate herbiovres - evolution of body size and competitive interactions. Oecologia 89:428-434.
Illius, A.W. & Gordan I.J. 1993. Diet selection in mammalian herbivores: constraints and tactics. In: Hughes, R.N. (ed) Diet selection: an interdisciplinary approach to foraging behavior; Oxford; Blackwell Scientific Publishers: 157-181.
Ilundain, A. & Naftelin R.J. 1981. Na+-dependent co-transport of alpha-methyl-D-glucose across the mucosal border of rabbit descending colon. Biochem. Biophys. Acta 644:316-322.
Justice, K.E. & Smith F.A. 1992. A model of dietary fibre utilization by small mammalian herbiovres, with empirical results for Neotoma. American Naturalist 139:398-416.
Karasov, W.H. 1990. Digestion in birds: chemical and physiological determinants and ecological implications. Studies in Avian Biology 13:391-415.
Karasov, W.H. 1996. Digestive plasticity in avian energetics and feeding ecology. In: Carey, C. (ed) Avian energetics and nutritional ecology; New York; Chapman and Hall: 61-84.
Karasov, W.H. & Diamond J.M. 1983. A simple method for measuring solute uptake by intestine in vitro. Journal of Comparative Physiology B 152:105-116.
Karasov, W.H. & Hume I.D. 1997. Vertebrate gastrointestinal system. In: Dantzler, W.H. (ed) Handbook of physiology. Section 13: Comparative physiology, Vol. 1; New York ; Oxford University Press: 409-480.
Maroux, S., Louvard, D. & Baratti J. 1973. The aminopeptidases from hog intestinal brush-border. Biochem. Biophys. Acta 321:282-295.
Martinez del Rio, C. 1990. Dietary, phylogenetic, and ecological correlates of intestinal sucrase and maltase activity in birds. Physiological Zoology 63:987-1011.
Martinez del Rio, C., Cork, S.J. & Karasov W.H. 1994. Modelling gut function: an introduction. In: Chivers, D.J. & Langer, P. (eds) The digestive system in mammals: food, form and function; Cambridge; Cambridge University Press: 25-53
Nitsan, Z., Nir, I., Dror, Y. & Bruckental I. 1973. The effect of forced feeding and of dietary protein levels on enzymes associated with digestion, protein and carbohydrate metabolism in geese. Poultry Science 52:474-481.
Obst, B.S. 1991. The avian feeding system: intestinal nutrient absorption in birds. Proceedings of 20th International Ornithological Congress: 920-926.
Obst, B.S. & Diamond J.M. 1989. Interspecific variation in sugar and amino acid transport by the avian cecum. Journal of Experimental Zoology Supplement 3:117-126.
Parra, R. 1978. Comparison of foregut and hingut fermentation in herbivores. In: Montgomery, G.G. (ed) The ecology of arboreal folivores; Washington D.C.; Smithsonian Institution Press: 205-230.
Robbins, C.T. 1993. Wildlife feeding and nutrition. 2nd Edition. Orlando, Florida; Academic Press.
Savory, C.J. & Gentle M.J. 1976a. Effects of dietary dilution with fibre on the food intake and gut dimensions of Japanese quail. British Poultry Science 17:561-570.
Savory, C.J. & Gentle M.J. 1976b. Changes in food intake and gut size in Japanese quail in response to manipulation of dietary fire content. British Poultry Science 17:571-580.
Sedinger, J.S. 1997. Adaptations to and consequences of an herbivorous diet in grouse and waterfowl. Condor 99:314-326.
Stevens, C.E & Hume I.D. 1995. Comparative physiology of the vertebrate digestive system (2nd ed.). New York; Cambridge University Press.
Van Soest, P. 1982. Nutritional ecology of the ruminant. Ithaca, NY; O & B Books, Inc.
Warner, A.C.I. 1981. Rate of passage of digesta through the gut of mammals and birds. Nutrition Abstracts and Reviews Series B 51:789-820.
Table 1. Total mean retention time (hour ± SE) of particulate and soluble digesta in avian herbivores.

Table 2. Specific nutrient uptake rates for glucose (uM/cm min) and two amino acids (nM/cm min) in Cackling Canada Geese (Branta canadensis minima) fed either fresh-cut alfalfa (n = 3) or pasture grass (n = 3). Uptake rates were measured using the everted sleeve technique (Karasov & Diamond 1983).
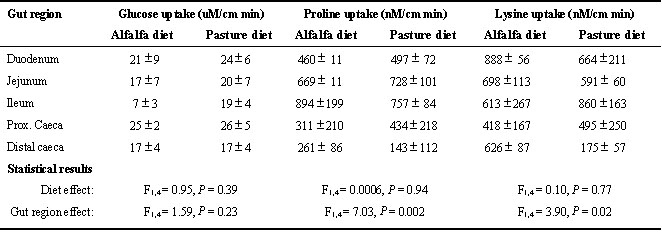
Fig. 1. Activities (m m ml-1 g wet tissue-1) ± SE of two disaccharidase enzymes (maltase, sucrase) in three regions of the small intestine and caeca of the Canada Goose.
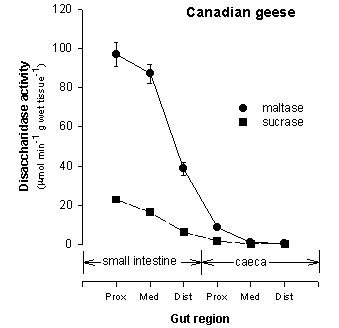
Fig. 2. Activity (m m ml-1 g wet tissue-1) ± SE of aminopeptidase-N in three regions of the small intestine and caeca of the Canada Goose
.