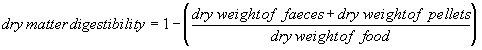 (1)
(1)S37.1: Digestion strategies of meat- and fish-eating birds
Geoff M. Hilton1, David C. Houston2, Nigel W.H. Barton3 & Robert W. Furness4
1,2,4Ornithology Group, Institute of Biomedical & Life Sciences, Graham Kerr Building, University of Glasgow, Glasgow, UK, e-mail 19406792h@ udcf.gla.ac.uk; 2 gbza80@udcf.gla.ac.uk; 4r.furness@bio.gla.ac.uk; 3Dubai Falcon Hospital, Box 23919 Dubai, United Arab Emirates
Hilton, G.M., Houston, D.C., Barton, N.W.H. & Furness, R.W. 1999. Digestion strategies of meat- and fish-eating birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2184-2197. Johannesburg: BirdLife South Africa.There is considerable variation among meat- and fish-eating bird species in how they digest food, despite the apparent simplicity of their digestion. Underpinning this variation is an interspecific trade-off between gut retention time and digestive efficiency. Some species adopt a strategy of rapid but inefficient digestion whilst others have slow and efficient digestion. Gut morphology and the response to digestive challenges appear to vary in association with these different strategies. The ecological factors that determine the favoured digestion strategy for a given species are considered. Digestion strategy affects several features of time and energy budgets. For instance the rate at which birds excrete a meal determines their mass load of digesta, and this in turn may affect cost of transport, or prey capture rate. Digestion strategy may also constrain the diet of raptor and seabird species: species that have rapid but inefficient and inflexible digestion tend to specialise on high quality food items, whereas slow and efficient digesters often include low quality prey in their diet. Intraspecific plasticity of gut morphology and gut retention time among seabird species is also demonstrated, in response to characteristics of the diet, notably energy density and ease of gastric breakdown.
INTRODUCTION
The way in which an animal digests its food can have considerable implications for its ecology. This is because the rate and efficiency with which digestion takes place will determine how much energy the animal can assimilate. It is well known that some foods are far more resistant to digestion than others. For example, plant leaf diets may require highly specialised gut adaptations such as those found in the Hoatzin Opisthocomus hoatzin, which are comparable to the ruminant system in mammals (Grajal et al. 1989). Animals are also known to be able to alter their method of digestion in response to changes in their diet. For example, when some small mammals change to a bulky or less digestible food, the small intestine may increase in length by 50% and the caecum by 100% within a few weeks (Lee & Houston 1993,1995). Similar changes have been noted in birds such as Red Knot Calidris canutus (Piersma et al. 1993) and Japanese Quail Coturnix coturnix japonica (Savory & Gentle 1976) when they change diet. However, relatively little attention has been paid to the digestion strategies shown by predatory animals. This is because meat and fish are both relatively easy foods to digest, being quickly broken down by proteolytic enzymes in the stomach, without the need for a specialised gut structure. We might therefore expect all predatory vertebrates to be rather similar in their digestive strategies. However, this does not seem to be the case. We started wondering about this while watching Egyptian Vultures Neophron percnopterus eating Lion Panthera leo faeces. These vultures can sometimes be seen waiting patiently at lion prides, although no kill has been made. Whenever a lion stands up to defaecate, the vultures rush in to consume the steaming pile. These unpleasant observations pose the question of why the lions leave sufficient energy and nutrients in their faeces to make it worth while for another species to eat them? They suggest that some predatory animals are not as efficient at digesting food as others. Cats are known to have rather short digestive tracts, and to digest their prey rather inefficiently compared to dogs (Houston 1988), and maybe predatory birds also show variation in digestion. This is indeed the case. If, for example, we compare different species of birds of prey we find that, after correcting for body size, some species have a small intestine length that is only half that of other species (Barton & Houston 1994), and as a consequence have reduced digestive efficiency (Barton & Houston 1993b). In this paper we report on digestion strategies in birds of prey and fish eating birds, and consider why species with different lifestyles might differ in their digestion strategies. We firstly examine two main aspects of digestion, the time food is retained in the gut and digestive efficiency, and whether these vary between species. We then consider how gut retention time and digestive efficiency are related to gut morphology. We consider how these variations in digestion might be adaptive responses to the predatory behaviour that a species uses, to its energy requirements and to the quality of diet it selects. Finally we consider the implications of these findings for prey selection by predators and how it might influence their ability to change diets and take alternative prey.
METHODS
The methods employed in these studies are described fully in Barton & Houston (1993a, 1993b, 1994) and Hilton (1998). We carried out feeding trials on seabirds caught from the wild and held in captivity for up to 30 days, and on birds of prey from falconry collections. Inert markers have been shown to be an inaccurate method to investigate digestive parameters in these birds (Barton & Houston 1991 for raptors; our unpublished data for seabirds), and we therefore relied on total excreta collection. Birds were housed in specially prepared cages, which allowed accurate recording of daily food intake, and the time and total collection of all excreta production for up to 24 hours after feeding. Chemical composition and energy content of the meat and fish diets used were determined by bomb calorimetry, lipid extraction and nitrogen analysis. Excreta samples were frozen, dried at 55 oC and energy and nitrogen content determined.
For raptors we measured digestive efficiency as dry matter digestibility:
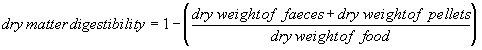 (1)
(1)
For seabirds we used Nitrogen corrected True Metabolisable Energy Coefficient (Miller & Reinecke 1984):
![]() (2)
(2)
where Qi is the dry mass of food eaten and Qo is the dry mass of excreta (g); GEi is the energy density of the food, and GEo is the energy density of excreta (kJ g-1 dry mass); EELN is Nitrogen corrected endogenous energy loss (kJ g body weight-1 day-1). Nc is a Nitrogen Correction Factor, standardising the birds to zero Nitrogen retention:
![]() (3)
(3)
where Ni is percent Nitrogen content of food, No is percent Nitrogen content of excreta, and 34.4 kJ g-1 is estimated energy density of excretory Nitrogen (Harris 1966).
Mass specific endogenous energy losses for fish eating birds were estimated from separate experiments in which birds were fed meals of different mass each day, and gross energy excreted per kg body mass was regressed on gross energy ingested. The x-intercept of this regression was taken as the mass-specific endogenous energy loss (Guglielmo & Karasov 1993).
For both raptors and seabirds, mean retention time (Blaxter, Graham & Wainman 1956) was used as the estimate of gut retention time of digesta:
![]() (4)
(4)
Gut morphology measurements
Dissections of birds were made to determine gross gut morphology. Wild adult birds from a variety of sources were used, e.g. birds handed in for pesticide analysis, scientific culls, pest control, collision victims etc.. All birds that were malnourished, or not in fresh condition, were discarded. Birds were stored double wrapped at –20 oC and thawed immediately prior to dissection: we have shown that storage conditions and freezing do not greatly affect gut morphology measurement (Barton & Houston 1992). For measurement of length and width, the stomach and small intestine were placed on a wet surface and straightened without tension. Mass of components of the gut and other body organs were determined after drying at 55 oC. For raptors, which are all members of the order Falconiformes and which are rather uniform in overall body shape, a skeletal body size measure was calculated for each species, using measurements of keel and diagonal sternum length. This method of recording body size for making interspecific comparisons has the advantage over using body mass that it does not vary with the body condition of the bird. We then regressed measures of gut size against the body size index. The seabirds used in our study fell into many different orders, and were far more variable in their body shape, and for this group we therefore used body mass as the independent variable with which to standardise digestive organ size.
RESULTS AND DISCUSSION
Digestive efficiency – digestion rate trade-off
Theory predicts that the longer food is retained in the digestive tract, the more thoroughly it will be digested (Sibly 1981). Thus digestive efficiency (the proportion of the ingested energy which becomes available for metabolism by the consumer) should increase as gut retention time of digesta increases. Clearly high digestive efficiencies are likely to be adaptive: the higher the efficiency the smaller the quantity of food that must be eaten in order to achieve a given metabolisable energy gain. Thus foraging time – which is energetically expensive and potentially risky for a predator - can be reduced by having high digestive efficiency. However, there may also be disadvantages to having a long retention time of digesta (Sibly 1981). Birds, with their relatively high metabolic rates, require high food intake, and carrying this considerable mass of digesta is energetically expensive for flying animals (Pennycuick 1989). Furthermore the mass load of digesta may reduce flight performance (Andersson & Norberg 1981), making birds less effective as predators, and more susceptible to predation themselves. Finally, long digesta retention times decrease the rate at which food can be processed, and thus potentially reduce the metabolisable energy intake of birds (Kersten & Visser 1996). If digestive constraints determine the rate of intake of metabolisable energy, then they ultimately set an upper limit to energy expenditure, which may be an important determinant of an animal’s fitness (Weiner 1992; Hammond & Diamond 1997).
Thus one can envisage a trade-off between conflicting benefits of rapid digestion and high digestive efficiency. We therefore tested the prediction that this trade-off would be apparent as a positive relationship among species between digestive efficiency and digesta retention time. The two digestion parameters were examined in separate experiments on seven raptor species and eight seabird species (Barton & Houston 1993a, 1993b; Hilton et al. in press a; Hilton et al. in press b). Within each experiment all species were fed equal amounts (as a proportion of body mass) of identical diets. Fig. 1 shows that there was a significant relationship between digesta retention time and digestive efficiency for raptor species, and there is a very similar relationship among seabirds (Hilton et al. in press b). We have found that birds can digest meat or fish with similar levels of efficiency, with values varying between approximately 75 – 85%.
The concept of a digestion rate – digestive efficiency trade-off, with conflicting benefits of rapid digestion and high digestive efficiency, implies that for any bird on any diet there is an optimum digestion strategy (retention time and associated digestive efficiency) which maximises overall fitness by balancing the opposing pressures. Our results show that both birds of prey and seabirds show considerable variation in both their gut retention time and their digestive efficiency. This presumably reflects variation between species in optimal digestion strategy, as a result of variation in lifestyles.
Gut morphology and retention time
Having established that a trade-off between digestion rate and digestive efficiency does seem to occur at an interspecific level among raptors and seabirds, we examined the differences in gut morphology that underlie this relationship. A short digesta retention time might arise through either more rapid movement of digesta through the gut, or by maintaining a constant rate of digesta flow and having a shorter gut.
Among raptors there was a strong relationship between the length of the small intestine and the total digesta retention time in the body (Hilton in press a) (Fig. 2). Clearly those raptor species which have long retention times have longer small intestines. However, among seabird species there was no such relationship. This is because, in this group of birds, the stomach is far more important in determining overall retention time (Hilton et al. in press b). It is possible to estimate the time food is retained in the stomach and small intestine separately by using chemical reactor theory (Penry & Jumars 1987). The seabird gut consists of an acid-proteolytic stomach, from which leads a tubular small intestine. This suggests that the gut acts as a continuous flow stirred tank reactor (the stomach) in series with a plug flow reactor (the intestine). Seabirds produce excretion curves which very closely approximate a negative exponential curve with the x-intercept offset from time zero, which is consistent with this model, and which permit intestine passage time and stomach retention time to be separated mathematically. Analysis of our data in this way shows that in seabird species intestine passage time was positively related to residual small intestine length. Similarly, estimated retention time of digesta in the stomach was positively correlated with residual stomach mass. Thus food was retained for longer in the intestines of species which have long small intestines, and longer in the stomachs of species with heavy stomachs. However, in seabirds food is retained in the stomach for far longer than the length of time it is retained in the small intestine, and this means that when we come to look at the total time digesta is retained in the whole digestive tract this is related to stomach mass, but not to intestine length (Hilton et al. in press b).
Ecological determinants of digestion strategy
We have shown that there is considerable variation between species in their digestion strategy and that this is associated with differences in their gut anatomy. These presumably reflect differing benefits of rapid digestion and high digestive efficiency between species. What ecological factors might explain these observed differences in digestion?
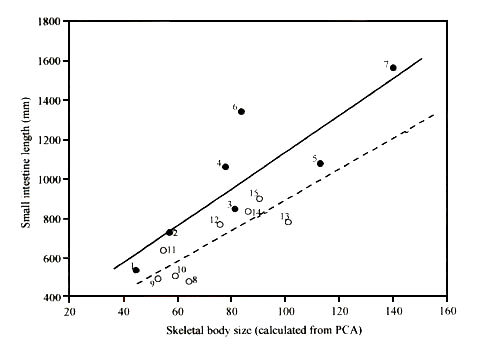
One major influence is likely to be the way in which the benefits of mass minimisation might vary between species. These would result in differences in the strength of selection pressure for rapid digestion. One obvious factor is the predatory behaviour used by a species. Raptors show a wide range of predatory strategies, but there are two clearly identifiable methods which differ in the demands that they place on flight performance. Some raptor species, such as sparrowhawks Accipiter spp and falcons Falco spp, capture small birds by means of a high-speed aerial pursuit. Such species rely on powered flight, and they use a very demanding form of hunting: capture success rates are typically low, with less than 10% of attacks resulting in prey capture (Temeles 1985). These species will experience strong selection pressure for flight performance, particularly acceleration, manoeuvrability and agility. All of these are strongly mass-dependent (Andersson & Norberg 1981). In contrast to these 'pursuit' predators, other raptor species can be classified as 'searchers' – these are species such as vultures (Cathartidae, Accipitridae), eagles (Accipitridae) and buzzards (Buteo spp.) which rely on soaring flight over large areas in search of carrion, or small mammals, which are caught by a surprise drop from the air. For these species, prey capture rates are far higher (Temeles 1985), and success at prey capture is not likely to be so strongly mass dependent. We might therefore predict that pursuit predators, which require rapid acceleration, would be under selection pressure to minimise the weight of all organ systems in the body other than the muscle systems involved in flight. Such species might develop short, light digestive tracts, which carry reduced weight of digesta because of smaller gut volume and rapid excretion rate, as well as reduced intestine tissue mass. This would result in a reduced digestive efficiency, but this would still be selected if it were compensated for by an increased rate of prey capture. Searching birds of prey, however, would not be under such strong selection for mass-minimisation, and would be predicted to develop longer digestive tracts, and correspondingly higher digestive efficiency. This is indeed found to be the case, and Fig. 3 shows that among raptor species, those with a pursuer lifestyle have significantly shorter small intestines than searchers, some species having an intestine length half that of other species. As we have shown earlier, these short-gut pursuit predators have digestive efficiencies up to 7% lower than those of searching species. This is ecologically important, because these species need to capture 7% more prey each day to compensate for poor digestion. Other organ systems are also involved (Barton & Houston 1996). Similar effects seem to operate in mammalian carnivores, where cat species, which largely rely on a brief chase with a rapid acceleration to catch prey, tend to have relatively short small intestines and low digestive efficiency, while dog species, which rely more on stamina over a long-distance chase, have longer small intestines and higher digestive efficiency (Houston 1988). This might, incidentally, explain why vultures find it worthwhile to eat lion dung, but have never been observed eating the faeces of African Hunting Dogs Lycaon pictus or Spotted Hyenas Crocuta crocuta.
Among seabird species there is also a pursuer – searcher dichotomy. The pursuit foraging auks (Alcidae), which catch prey by swimming using the wings underwater, have shorter digesta retention times than species such as Herring Gull Larus argentatus and Northern Fulmar Fulmarus glacialis, which are soaring, marine searchers and scavengers (Hilton et al. in press b). However, the distinction for seabirds is far more complex than in birds of prey. Firstly, the diversity of predatory strategies shown by marine birds is greater than among birds of prey. Some species use soaring flight and pluck floating prey from the sea surface, some use powered flight and capture submerged prey by plunge diving, some surface swim and locate food on the surface, others surface swim and make active foraging dives after fast moving prey (Cramp & Simmons 1977, 1983; Cramp 1985). We know very little about how these various methods of predation compare in terms of the degree to which they are influenced by body mass, and so we cannot confidently make simple predictions. The situation is complicated by buoyancy effects, which may reduce the benefits of mass minimisation in underwater pursuit of fish. Secondly, during the breeding season most seabirds need to make long foraging journeys between the colony and their feeding areas. This is in marked contrast to birds of prey, almost all of which are territorial and where prey is caught within a short distance of the nest site. For seabirds, therefore, the energetic cost of transport of the prey back to the nest is a major consideration. Finally, many seabird species are subject to heavy piracy of food, both at sea and at the breeding colony, from specialist species such as skuas (Stercorariidae) and frigatebirds Fregata spp. This is rarely a factor in raptor biology. The risk of piracy may have imposed selection pressures on seabirds to carry food in the stomach, rather than the bill or feet as do most raptor species. It may also have imposed other selection pressures involved with the need to maintain flight speed and manoeuvrability in the air while laden with food, to avoid piracy attacks.
Because so many factors may interact in determining an optimum strategy for a seabird, a modelling approach can help to understand which foraging patterns are likely to result in selection for rapid but inefficient digestion, and which might lead to slow and efficient digestion. We have used this approach to consider the influence of time and energy costs in commuting to feeding areas in seabirds.
Modelling optimal digestion strategies for seabirds
We developed a model which describes the time - energy budgets of seabirds during the chick-rearing period (Hilton 1998). We used estimates of the energy cost of flight from Pennycuick (1989), and took data from the literature for likely distances travelled to reach feeding areas. The model birds were allowed to vary the frequency and duration of their daily foraging trips, and those combinations of frequency and duration that allowed the bird to maintain energy balance while provisioning the chick were derived. From this set, the optimal trip frequency and duration was determined according to two optimisation criteria. The first of these was 'time minimisation' – minimising the total time the bird spent away from the nest. The second was 'energy minimisation' – minimising the daily energy expenditure.
Within this framework, the effect of digestion strategy on time and energy minimisation was determined. We used the trade-off curve between digestion rate and digestive efficiency that we obtained from our feeding trials to develop the model. The minimum daily foraging time and minimum daily energy expenditure of a hypothetical slow digester (with concomitant high digestive efficiency) and a rapid digester (with low digestive efficiency) were calculated for a range of different foraging conditions. Digestion strategy affects not only digestion rate and digestive efficiency, but also the rate at which energy is gained during a feeding session. This is because once a bird reaches the point where the gut is full of food, it is only able to ingest additional food at the same rate as the rate of excretion. A fast digester will, once this ingestion bottleneck has been reached, be thereafter able to consume food at a greater rate than a slow digester because of its faster excretion rate. It might then become worthwhile to wait in the feeding area and prolong the length of each feeding bout. Because seabirds need to obtain all of their energy in long distance foraging trips, these considerations of the cost of carrying food back to the colony and decisions on how long to stay in a feeding area once they have reached it become important factors. Considerations of the optimum number of feeding trips to make per day involve a compromise between two opposing factors. Firstly, birds should take as few foraging trips as possible, because for each foraging trip there is a fixed time and energy cost in making the journey from the colony to the feeding area and back. Secondly birds should terminate feeding periods before the ingestion bottleneck sets in. To continue feeding after the onset of the bottleneck is extremely time inefficient, because the rate of food ingestion decreases dramatically. The first factor favours prolonged foraging trips, whilst the second factor means that briefer foraging trips tend to be favoured.
The foraging trip frequency which gives the minimum time or energy expenditure is that which strikes the optimal balance between these two factors. Our model shows that the critical factor influencing this balance is the rate of excretion. The slow digester can avoid the ingestion bottleneck by making more and briefer foraging trips than the rapid digester, in which case it pays a time and energy penalty of making extra commuting flights. If the flight is brief and energetically cheap (such as in birds with low wing loadings, or which employ soaring flight) then the cost might be less than the advantage gained by having higher digestive efficiency. Rapid digestion however is favoured by birds with large foraging ranges and/or where the energetic cost of flight is high. This is broadly in accordance with the foraging strategies found in different seabird species. Thus auks, which often have large foraging ranges (Bradstreet & Brown 1985; Phillips et al. in press), and extremely high power output in flight (Pennycuick 1987) tend to have rapid and rather inefficient digestion. By contrast gulls and skuas which typically have shorter foraging ranges (Cramp & Simmons 1983; Phillips et al. in press) and lower flight power output (Pennycuick 1987), have slower but more efficient digestion.
We can conclude therefore, that for birds of prey the method of predation has probably been a strong influence on the digestive strategy a species adopts. This may also have been a factor in seabirds, but perhaps more important are effects of digestion strategy on time and energy budgets.
Digestion strategy and diet
We have demonstrated an association between foraging behaviour and digestion strategy. The fact that some types of raptors and seabirds have lower digestive efficiencies than do other species has important implications for the prey that they should catch. Among raptors, it is notable that only certain species feed on carrion from large ungulates. These tend to be long-gut species with high digestive efficiency, such as vultures, eagles and buzzards. Carrion is a low quality food, because it has low fat content, has to be heated to body temperature before digestion can begin, and may contain microbial toxins which impede digestion. Possibly only species with high digestive efficiencies can successfully utilise such poor quality foods. Short gut species, with low digestive efficiency, must catch prey of high quality such as small passerines, which have high fat content. Indeed, short gut species such as falcons appear to ignore carrion even when it is readily available and abundant (Cramp & Simmons 1980). In experiments on captive birds, ‘short gut’ Peregrines Falco peregrinus lost body mass when fed a diet of Rabbit Oryctolagus cuniculus meat (which is low in fat) in quantities calculated from Kirkwood (1981) to be sufficient to meet energy requirements. Conversely, ‘long gut’ Eurasian Buzzards Buteo buteo gained mass on the same diet. Both species maintained body mass when fed the same quantity of a diet of Pigeon Columba livia meat which is lipid-rich (Barton & Houston 1993a). American Kestrels Falco sparverius fed on small birds which had unusually low fat reserves because they were close to starvation, were unable to satisfy their energetic demands (Taylor et al 1991). Digestive strategy thus limits some raptors to a narrow range of potential prey species.
Among seabirds there is also a relationship between diet and digestion strategy. Species can be divided into two groups. 'Generalists', such as gulls, eat a wide variety of prey, including low quality items such as intertidal invertebrates and carrion; 'specialists', such as auks, eat mainly energy dense and readily digestible fish species which have a high lipid content. Marine prey shows far greater variation in ‘quality’ than the prey taken by raptors. Fish differ markedly in their energy density, both between species and temporally within species (Steimle & Terranova 1985; Hislop et al. 1991). This is primarily due to the large lipid deposits produced by some fish species prior to spawning, which can double their energy density. Furthermore there is also considerable variation in the ease with which fish are broken down. Commonly eaten fish species differed by a factor of two in the rate of breakdown in an in vitro acid proteolytic solution which mimics gastric digestion (Jackson et al. 1987; Hilton et al. 1998). In our feeding trials we found that digestive efficiency was greater and retention time was longer in generalist species than in specialist species (Hilton et al. in press b). Furthermore, residual stomach mass was also greater in generalists than specialists, but residual intestine length did not differ between specialists and generalists. It may be that species such as auks, which have low digestive efficiency can only maintain energy balance if they catch fish species which have a high oil content. In captivity Common Guillemots (Uria aalge) cannot maintain body mass when fed on an ad. lib. diet of fish species with low fat content, but can when fed on oily fish species (H. Brugge pers. comm.).
What are the consequences for a bird when it changes its diet?
We have shown that among seabird species there is a range of digestion strategies, from slow and efficient digestion to rapid but inefficient digestion. Are there also differences between species in their ability to deal with 'digestive challenges' – situations in which the digestion of the diet might be expected to present problems? Specifically, does a strategy of rapidly digesting food with rather low efficiency result also in less flexible and adaptable digestive function?
We examined the responses of two contrasting seabird species, the Common Guillemot, which we selected as a typical low efficiency digester, and the Lesser Black-backed Gull Larus fuscus, as an example of a high efficiency digester, to digestive challenges. We investigated how they were able to cope when they had been accustomed to a low quality fish diet (Whiting Merlangius merlangus, with an energy density of 4.4 kJ g-1 fresh weight) and were then switched onto a high quality diet (Sprat Sprattus sprattus, of energy density of 7.9 kJ g-1 fresh weight), and vice versa. Diet switching might result in reduced digestive performance because there is ample evidence that many aspects of digestion can change over a relatively short time scale, in order to optimise digestion and assimilation of the current diet. These adaptations include up- and down-regulation of enzyme and transport systems (Karasov 1996), micro-morphological and gross anatomical changes to the gut (Savory & Gentle 1976; Brugger 1991; Lee & Houston 1995), and adjustments in gut motility (Levey & Karasov 1989; Afik & Karasov 1995; Hume & Biebach 1996). Therefore when switched to a new diet, gut function might be expected to be non-optimal at first, and switched birds might show reduced digestive performance compared to birds which are acclimated to the novel diet. Similarly, gut function cannot be 'fine-tuned' to achieve optimal digestion on two different diets simultaneously. Therefore digestive performance when eating two food types in the same meal should be reduced compared to eating the same food types separately, and we investigated whether this was true.
Birds were acclimated to either the Whiting or the Sprat diet for three weeks. They were then abruptly switched onto the other diet, and the changes in their retention time and digestive efficiency were recorded for the following six days. Lesser Black-backed Gulls, when switched from low to high quality diets or vice-versa, were able to maintain the same digestive efficiency on the new diet as birds that had been acclimated to it. Indeed, when moved from a low to a high quality fish diet they actually showed significantly higher digestive efficiency than birds which were acclimated to the diet. Gulls increased their digestive efficiency over the five days following a switch onto a novel diet, whether the switch was from Sprat to Whiting or from Whiting to Sprat. These birds therefore demonstrate that fine-tuning of digestion was occurring.
Common Guillemots, however, showed a very different and dramatic response when switched from a low quality to high quality diet. Retention times became extremely short, and digestive efficiency decreased progressively after the diet switch. Individual birds differed markedly in the severity of the response, and the variance of digestive efficiency increased dramatically. Some of the birds appeared to suffer a diarrhoeic reaction to the novel diet, even though they were otherwise healthy. They did not, unlike the gulls, improve in digestive efficiency over the few days they were kept on the trial.
When given a mixed diet of Sprat and Whiting in the same meal, Common Guillemots showed a reduction in digestive efficiency compared to the same individuals’ performance on the two diets separately. However Lesser Black-backed Gulls showed no detectable reduction in digestive efficiency when challenged with a mixed diet.
Although confined to only one species-pair, this comparative study provides some evidence that species which specialise on high quality fish prey, and have rapid digestion, are less able to deal with digestive challenges such as diet switching and diet mixing than are species which typically eat a varied and low quality diet. The difference between the diets we used was relatively subtle: two different types of small marine fish, which differed greatly in lipid content, but were otherwise very similar in composition. The effects of diet switching and diet mixing on digestive parameters may therefore be widespread in nature.
Intraspecific digestive responses to diet quality
Among the changes that birds might make when adapting in this way to new diets are changes in gross gut anatomy and gut retention times (Karasov 1996). Since the prey of seabirds differs considerably in quality, we would predict that optimal retention times would differ within seabird species, in association with intraspecific diet variation.
This is found to be the case. In digestion trials, retention times of different fish diets differed in a consistent way across several seabird species (Table 1). Retention times of Lesser Sandeel Ammodytes marinus were less than those of Whiting in all species except Northern Fulmar (ANOVA with diet and species as factors and meal size as covariate: diet F1,94 = 9.0, P = 0.003, species F8,94 = 21.4, P < 0.001; meal size F1,94 = 5.2, P = 0.025, all interaction terms n.s.). Sprat and Whiting, had similar in vivo retention times. These differences partly reflect the in vitro digestion times of these fish species: mean digestion time of Lesser Sandeel, small Whiting and Sprat were 3.18 S.E. 0.02 hours, 4.99 S.E. 0.10 hours and 5.93 S.E. 0.06 hours respectively (ANOVA with fish species as factor (also including Capelin Mallotus villosus and large Whiting): F4,31 = 40.2; P < 0.0001; Tukey's HSD all pairs significantly different, P < 0.05) (Hilton et al. 1998).
CONCLUSIONS
We can therefore conclude that there is a considerable degree of variation in digestive function among members of the raptor and seabird guilds. This variation is underpinned by a trade-off between digestion rate and digestive efficiency. Some species have rapid but inefficient digestion, whereas others adopt the opposite strategy. A range of digestive and ecological factors are associated with these different strategies. Disentangling cause and effect among these factors is probably not productive; rather they should be seen as co-evolved suites of characters. Thus species with rapid digestion tend to have smaller guts than do those with slow digestion. They also seem to be less able to deal with digestive challenges, having a less flexible digestive function. They tend to be active pursuit foragers, with high flight costs. Rapid digesters have restricted diets, specialising on a narrow range of high quality food items. By contrast, slow digesters tend to have large guts, and an adaptable digestive system that is able to respond to digestive challenges. They tend to be less active foragers, and to have lower flight costs. They tend to be opportunist feeders, eating a broad range of prey, including low quality items.
ACKNOWLEDGEMENTS
We are extremely grateful to a large number of people who kindly gave us birds for the post mortem aspects of this study, especially Ian Wyllie and Ian Newton, Clive Craik, Rob Barrett and Fridjtof Mehlum, Colin Pennycuick, John Croxall and Peter Prince, and Mick Marquiss. Thanks are also due to Kristjan Lilliendahl, Jon Solmundsson and David Thompson for assistance with the study of morphology in Icelandic seabirds. We are indebted to Rob Barrett, Rob Holbourn, and Bill Makim for assistance with fieldwork, and to Geir Gabrielsen and Henk Brugge for advice on seabird husbandry.
REFERENCES
Afik, D. & Karasov, W.H. 1995. The trade-offs between digestion rate and efficiency in warblers and their ecological implications. Ecology 76: 2247-2257.
Andersson, M. & Norberg, R.A. 1981. Evolution of sexual size dimorphism and role partitioning among predatory birds, with a size scaling of flight performance. Biological Journal of the Linnean Society 15: 105-130.
Barton, N.W.H. & Houston, D.C. 1991. The use of titanium dioxide as an inert marker for digestion studies in raptors. Comparative Biochemistry and Physiology 100A: 1025-1029.
Barton, N.W.H. & Houston, D.C. 1992. Post-mortem changes in avian gross intestinal morphology. Canadian Journal of Zoology. 71: 273-279.
Barton, N.W.H. & Houston, D.C. 1993a. The influence of gut morphology on digestion time in raptors. Comparative Biochemistry & Physiology 105A: 571 578.
Barton, N.W.H. & Houston, D.C. 1993b A comparison of digestive efficiency in birds of prey. Ibis 135: 363-372.Karasov
Barton, N.W.H. & Houston, D.C. 1994. Morphological adaptations of the digestive tract in relation to feeding ecology in raptors. Journal of Zoology: 232:133-150.
Barton, N.W.H. & Houston, D.C. 1996. Factors influencing the size of some internal organ systems in raptors. Journal of Raptor Research. 30: 219-223.
Blaxter, K.L., Graham, N.M. & Wainman, F.W. 1956. Some observations on the digestibility of food by sheep, and on related problems. British Journal of Nutrition 10: 69-91.
Bradstreet, M.S.W. & Brown, R.G.B. 1985. Feeding ecology of the Atlantic Alcidae. In: Nettleship, D.N. & Birkhead, T.R. (eds) The Atlantic Alcidae; The Evolution, Distribution and Biology of the Auks Inhabiting the Atlantic Ocean and Adjacent Water Areas. London; Academic Press: 264-318.
Brugger, K.E. 1991. Anatomical adaptation of the gut to diet in Red-winged Blackbirds (Agelaius phoeniceus). Auk 108: 562-567
Cramp, S. 1985. Handbook of the Birds of Europe, the Middle East and North Africa. The Birds of the Western Palearctic; Volume 4: Terns to Woodpeckers. Oxford, UK; Oxford University Press: 960pp.
Cramp, S. & Simmons, K.E.L. 1977. Handbook of the birds of Europe the Middle East and North Africa: The birds of the Western Palearctic; Volume 1: Ostrich to Ducks. Oxford, UK; Oxford University Press.
Cramp, S. & Simmons, K.E.L. 1980. Handbook of the Birds of Europe, the Middle East and North Africa. The Birds of the Western Palearctic; Volume 2: Hawks to Bustards. Oxford, UK; Oxford University Press: 695pp.
Cramp, S. & Simmons, K.E.L. 1983. Handbook of the Birds of Europe, the Middle East and North Africa. The Birds of the Western Palearctic; Volume 3: Waders to Gulls. Oxford, UK; Oxford University Press: 913pp.
Grajal, A., Strahl, S.D., Parra, R., Dominguez, M.G. & Neher, A. 1989. Foregut fermentation in the Hoatzin, a neotropical leaf-eating bird. Science 245: 1236-1238.
Guglielmo, C.G. & Karasov, W.H. 1993. Endogenous mass and energy-losses in Ruffed Grouse. Auk 110: 386-390.
Hammond, K.A. & Diamond, J. 1997. Maximal sustained energy budgets in humans and animals. Nature 386: 457-462.
Harris, L.E. 1966. Biological energy interrelationships and glossary of energy terms. Washington D.C.; National Academy of Science Publications No. 1411.
Hilton, G.M. 1998. Digestion strategies of North Atlantic seabirds. PhD Thesis, University of Glasgow, Glasgow, UK.
Hilton, G.M., Houston, D.C. & Furness, R.W. 1998. Which components of diet quality affect retention time of digesta in seabirds? Functional Ecology 12: 929-939.
Hilton, G.M., Houston, D.C., Barton, N.W.H. & Furness, R.W. in press a. Ecological constraints on digestive physiology in carnivorous and piscivorous birds. Journal of Experimental Zoology.
Hilton, G.M., Furness, R.W. & Houston, D.C. in press b. A comparative study of digestion in North Atlantic seabirds. Journal of Avian Biology.
Hislop, J.R.G., Harris, M.P. & Smith, J.G.M. 1991. Variation in the calorific value and total energy content of the Lesser Sandeel (Ammodytes-marinus) and other fish preyed on by seabirds. Journal Of Zoology 224: 501-517.
Houston, D.C, 1988. Digestive efficiency and hunting behaviour in cats, dogs and vultures. Journal of Zoology. 216:603-605.
Hume, I.D. & Biebach, H. 1996. Digestive-tract function in the long-distance migratory Garden Warbler, Sylvia borin. Journal Of Comparative Physiology 166B: 388-395.
Jackson, S., Duffy, D.C. & Jenkins, J.F.G. 1987. Gastric digestion in marine vertebrate predators: in vitro standards. Functional Ecology 1: 287-291.
Karasov, W.H. 1996. Digestive plasticity in avian energetics and feeding ecology. In: Carey, C. (ed) Avian Energetics and Nutritional Ecology. New York; Chapman and Hall: 61-84.
Kenward, R.E. & Sibly, R.M. 1977. A Woodpigeon (Columba palumbas) feeding preference explained by a digestive bottleneck. Journal of Applied Ecology 14: 815-826.
Kersten M. & Visser, W. 1996. The rate of food-processing in the Oystercatcher - food-intake and energy-expenditure constrained by a digestive bottleneck. Functional Ecology 10: 440-448.
Kirkwood, J.K. 1981. Maintenance energy requirements and rate of weight loss during starvation in birds of prey. In: Cooper, J.E. & Greenwood, A.G. (eds) Recent Advances in the Study of Raptor Diseases. Keighley, U.K; Chiron. Publications: 153-157.
Lee, W.B. & Houston, D.C. 1993. The effect of diet quality on gut anatomy in British voles (microtinae). Journal of Comparative Physiology B 163: 337-339.
Lee, W.B. & Houston, D.C. 1995. The rate of change of gut anatomy in voles in relation to diet quality. Journal of Zoology 236: 341 – 345.
Levey, D.J. & Karasov, W.H. 1989. Digestive responses of temperate birds switched to fruit or insect diets. Auk 106: 675-686.
Miller, M.J. & Reinecke, K.J. 1984. Proper expression of metabolizable energy in avian energetics. Condor 86: 396-400.
Pennycuick, C.J. 1987. Flight of auks (Alcidae) and other northern seabirds compared with southern Procellariiformes: ornithodolite observations. Journal of Experimental Biology 128: 335 - 347.
Pennycuick, C.J. 1989. Bird flight performance: a practical calculation manual. Oxford, U.K; Oxford University Press: 153pp.
Penry, D.L. & Jumars, P.A. 1987. Modelling animal guts as chemical reactors. American Naturalist 129: 69-96.
Phillips, R.A., Ratcliffe, N. & Riley, H.T. in press. Factors influencing the foraging range and marine distribution of U.K. seabirds with an evaluation of possible marine extensions to Special Protection Areas in Scotland. Edinburgh, U.K; Scottish Natural Heritage Research and Advisory Series.
Piersma, T., Koolhaas, A. & Dekinga, A. 1993. Interactions between stomach structure and diet choice in shorebirds. Auk 110: 552-564.
Savory, C.J. & Gentle, M.J. 1976. Changes in food intake and gut size in Japanese Quail in response to manipulation of dietary fibre content. British Poultry Science 17: 571-580.
Sibly, R.M. 1981. Strategies of digestion and defaecation. In: Townsend, C.R. & Calow, P. (eds) Physiological Ecology: an evolutionary approach to resource use. Oxford, UK, Blackwell Scientific Publications: pp 109-139.
Steimle, F.W. & Terrranova, R.J. 1985. Energy equivalents of marine organisms from the continental shelf of the temperate northwest Atlantic. Journal of Northwest Atlantic Fisheries Science 6: 117-124.
Taylor, R.L., Temple, S.A. & Bird, D.M. 1991. Nutritional and energetic implications for raptors consuming starving prey. Auk 108:716-719
Temeles, E.J. 1985. Sexual size dimorphism of bird-eating hawks: the effect of prey vulnerability. American Naturalist 125: 485-499.
Weiner, J. 1992. Physiological limits to sustainable energy budgets in birds and mammals - ecological implications. Trends In Ecology & Evolution 7: 384-388.
Table 1. Mean retention time (hours) of three fish species fed to nine seabird species.
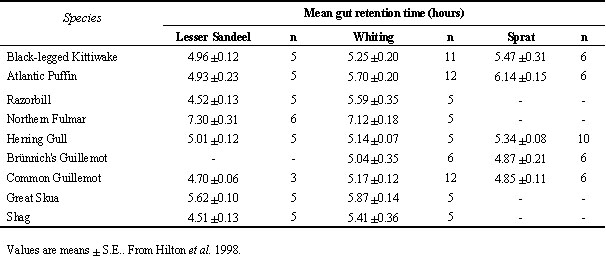
Fig. 1. The relationship between dry matter digestibility and gut retention time in raptor species. (Spearman-rank correlation: rs = 0.82, n = 7, P = 0.023). Western Honey Buzzard Pernis apivorous n = 1; Peregrine Falco peregrinus n = 3; Eurasian Sparrowhawk Accipiter nisus n = 2; Common Kestrel Falco tinnunculus n = 5; Eurasian Hobby Falco subbuteo n = 2; Eurasian Buzzard Buteo buteo n = 4; Red Kite Milvus milvus n = 2. From Hilton et al. in press a.
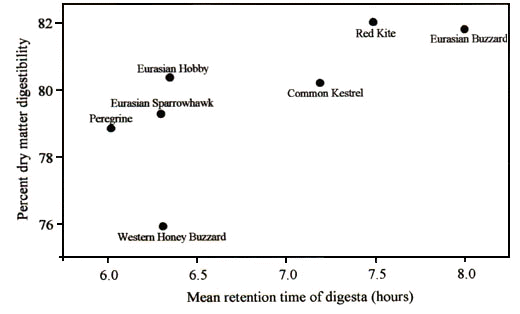
Fig. 2. The relationship between gut retention time and small intestine length among raptor species. (Spearman-rank correlation: rs = 0.81, n = 7, P = 0.04). For small intestine length: Western Honey Buzzard n = 1; Peregrine n = 16; Eurasian Sparrowhawk n =89; Common Kestrel n =24; Eurasian Hobby n = 1; Eurasian Buzzard n = 53; Red Kite n = 9. For gut retention time sample sizes are as in Fig. 1. Modified from Barton & Houston 1993b.
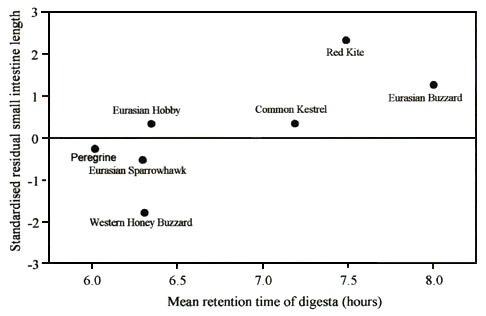
Fig 3. The relationship between foraging mode and small intestine length in raptor species. (ANCOVA, F1,11 slopes = 0.19, n.s; F1,12 elevation = 8.94, P = 0.01). Open circles and dotted line: 'pursuers'; closed circles and solid line: 'searchers'. 1 = Common Kestrel (Falco tinnunculus) (n=24); 2 = Hen Harrier (Circus cyaneus) (n=4); 3 = Rough-legged Buzzard (Buteo lagopus) (n=1); 4 = Eurasian Buzzard (Buteo buteo) (n=53); 5 = Tawny Eagle (Aquila rapax) (n=1); 6 = Red Kite (Milvus milvus) (n=9); 7 = Golden Eagle (Aquila chrysaetos) (n=6); 8 = Eleonora's Falcon (Falco eleonorae) (n=1); 9 = Merlin (Falco columbarius) (n=3); 10 = Eurasian Sparrowhawk (Accipiter nisus) (n=89); 11 = Eurasian Hobby (Falco subbuteo) (n=1); 12 = Lanner Falcon (Falco biarmicus) (n=2); 13 = Northern Goshawk (Accipiter gentilis) (n=49); 14 = Peregrine (Falco peregrinus) (n=16); 15 = Saker Falcon (Falco cherrug) (n=1). Modified from Barton & Houston 1994.