S36.5: Immune function and sexual selection
Marlene Zuk
Department of Biology, University of California, Riverside, CA 92521, USA, fax 1 909 787 4286, e-mail mzuk@citrus.ucr.edu
Zuk, M. 1999. Immune function and sexual selection. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2173-2181. Johannesburg: BirdLife South Africa.Although many models of sexual selection predict that females choose mates of high quality to pass on superior fitness to offspring, definition of that quality remains elusive. Resistance to disease, or immune competence, have often been invoked as ideal criteria to be used in mate choice. 'Good genes' models for the evolution of female mating preferences and male ornaments assume that ornaments are costly to males, which suggests that energy devoted to these traits might be less likely to be allocated to other functions, such as defence against disease. Females in many bird species discriminate among males on the basis of these ornaments, and males with better-developed ornaments have also often been shown to have fewer parasites, which suggests that they have better immune systems. How might information about the immune system be conveyed to prospective mates? Few studies have directly examined the link between ornamentation and immune function, but these have often found that highly ornamented individuals appear to pay a price by reducing some aspect of immunity, such as decreasing lymphocyte numbers. Alternatively, one might expect males with elaborate ornaments to also have better immune systems. Males and females may show different relationships between morphological characters and immunity. We can employ several different measures of immunity in animals, but it is important not to ignore the environmental and social context in which the measures are taken.
INTRODUCTION
One of the most important components of sexual selection is obtaining information from prospective mates. Both sexes, but females in particular, are thought to evaluate potential mates and choose a mate based on behaviour, morphology and secondary sexual traits, or some combination of these (Andersson 1994). Mates may be selected because they are likely to be good parents (Hoelzer 1989), contribute resources to the female, or possess genes that increase the fitness of their offspring (Kirkpatrick & Ryan 1991). At least in some cases, females do choose those males most likely to confer higher fitness to their young. For example, Petrie (1994) has shown that the offspring of Peacocks Pavo cristatus with more eyespots in their tails are more likely to survive than the offspring of less ornamented fathers. But how does the female get such information? How can an ornamental trait indicate aspects of a male's fitness? Furthermore, what aspect of fitness is essential? Presumably, a trait cannot show every facet of a male's genotype, and several attributes might be argued to be the most important ones. In 1982, Hamilton & Zuk suggested that ornaments could reveal the health of their bearer, and that species with elaborate secondary sexual characters had been subject to extreme pressure from parasites and disease. Since that time, many researchers have demonstrated female preference for males without parasites, and parasites have been demonstrated to influence mate choice in many different species (Clayton 1991; Møller1990; Read 1990; Sullivan 1991; Zuk 1992; Hillgarth & Wingfield 1997), although making generalisations about their strength as a selective agent has sometimes been difficult. More recently, Folstad & Karter (1992) suggested that because testosterone, the sex hormone responsible for the production of many male secondary sexual characters in vertebrates, is often associated with a suppressed immune system, females might use testosterone-dependent traits as honest indicators of parasite resistance. According to their model, ornaments such as antlers and showy plumage are 'honest' signals of a male's ability to withstand the obligatory immunosuppressive effects of high testosterone titres. Cheating is unlikely because the cost of doing so, of presenting showy characters when viability or fitness is low, is an automatic loss of immune function that makes the male too vulnerable to pathogenic effects of parasites for it to be maintained (Folstad & Karter 1992; Wedekind & Folstad 1994). Males with highly-developed secondary sex characters are therefore indicating their capacity to resist the effects of prevalent parasites even on a compromised immune system. Again, recent work, particularly on birds, has examined endocrine-immune interactions in the context of sexual selection, in an attempt to determine if, for example, males with well-developed secondary sexual characters also have high testosterone levels but weakened immunity (Weatherhead et al. 1993; Zuk et al. 1995; Saino & Møller 1996; Saino et al. 1997a). The immune system is thus viewed as an important component of this process, which is logical given the role of the immune system in fighting parasite infections. In part because of this interest in parasites and the immune system and their effects on ornamental traits, sexual selection has recently seen a resurgence of interest in physiological mechanisms, especially immunological and endocrinological ones (Zuk 1994; Lochmiller 1995; Sheldon & Verhulst 1996, Saino & Møller this volume). In this paper I address the relationship between sexual selection and the immune system. First I outline the reasons for studying immune response in the context of sexual competition, and then give some examples of studies examining the relationship between immune parameters and sexually selected traits. By dissecting the immune response into several components, we can begin to understand what different tests of immunity can tell us about the evolution of secondary sexual characters. Does a male with well-developed secondary sexual characters show a less robust immune response to challenge than a drab male, because of the cost of ornament development and maintenance? Or are better males those with both better immune systems and showier ornaments? Do different aspects of the immune system tell us different things? Finally, I will suggest some caveats and potential problems for consideration as we begin to formulate hypotheses about the interaction of sexual selection and the immune response.
WHY IMMUNITY?
Neglected for many years by ecologists and behavioural biologists, parasites have been enjoying renewed attention in the last decade and a half (Toft et al. 1991; Grenfell & Dobson 1995; Clayton & Moore 1997). Birds have been particularly well-studied as hosts, especially in examining the effects of parasites on sexual selection. In several species, parasites affect both mate choice and the development of secondary sexual characters, suggesting that the ability to deal with pathogens is a useful gauge of genetic quality. How can we assess an individual's ability to resist parasites? Measuring the parasite prevalence or intensity itself is confounded by variation in exposure to the pathogen; an animal may be parasite-free because it has never been exposed or because it has resisted infection. In addition, parasite levels fluctuate seasonally, with the stage of the reproductive cycle, and even with the time of day. Ideally, one should measure how an animal would respond to a variety of potential pathogens. While anti-parasite behaviour can be effective, the primary means of resisting parasites is physiological, through both the acquired and genetic parts of the immune system. Examining both immune response and the condition of the immune system is therefore a logical way to evaluate resistance (see Møller & Saino 1999 for an overview of the avian immune system). As a general gauge of fitness, the state of the immune system is also highly appropriate. Predation may or may not be important, food may be scarce or abundant, but parasites are ubiquitous, and an animal that resists disease should virtually always be favoured, all else being equal, over one that does not. Other measures of 'condition', such as wing chord divided by body mass, or levels of subcutaneous fat, all have drawbacks, and do not necessarily reflect characters that have fitness consequences (Zuk 1994).
Are hormones necessary?
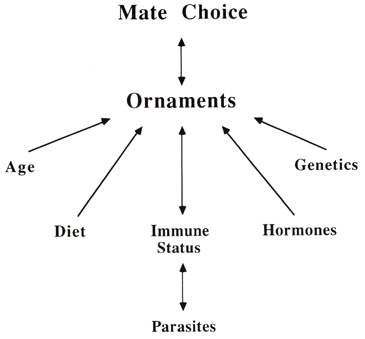
Although the immune system was originally implicated in parasite-mediated sexual selection because of the simultaneously immunosuppressive and ornament-enhancing effects of testosterone, it may not be necessary to use endocrine mechanisms to understand these interactions. What is required is an understanding of the general relationship between ornaments, mate choice, and immunity, with immunity as a surrogate of fitness (Fig. 1). Sexually selected traits are influenced by many factors, including testosterone as well as diet, age, time of year and the environment in which the traits developed. Testosterone and other hormones have an intriguing place among these influences, but their effects are not essential to understanding the role of parasites in sexual selection. The real question is how females can examine the secondary sexual characters of a male and gain information about his fitness, with fitness measured using immune function. In addition, focusing on testosterone is needlessly restrictive, because of course the majority of animals on earth, the invertebrates, lack steroid hormones. While the endocrinology of most invertebrates is not as well understood as that of mammals, male secondary sexual traits such as bright colours of male butterflies or the acoustic signals of insects such as crickets do not rely on testosterone. The basic principles of sexual selection, however, are the same regardless of the presence of testosterone, so in bypassing hormonal mechanisms we can see more generally how animals have responded to differential selection on the sexes.
Sexual selection and immunity: examples
Comparative work has examined the relative development of immune organs such as the spleen or bursa of Fabricius in birds in relation to the degree of ornamentation or sexual dichromatism (Møller & Saino 1994; Møller 1997). Sexually dichromatic species had larger spleens, after accounting for body mass, than monochromatic species (Møller 1997), and also had higher concentrations of white blood cells (Saino & Møller 1994). Such studies, while intriguing, are somewhat difficult to interpret because the exposure of different species to disease is uncontrolled. Saino, Møller and colleagues have examined the response of Barn Swallows Hirundo rustica to injections of sheep red blood cells (Saino & Møller 1996; Saino et al. 1997a, 1997b). The sheep cells act as a foreign protein that induces an immune response not tied to a particular pathogen, and a stronger response to these cells indicates a more robust immune system. Males with longer tails, a sexually selected trait, had stronger responses to the cells, but males with artificially elongated tails had a weaker response than those with naturally long tails. Song rate was also reflected in immune response; males that sang less had higher lymphocyte concentrations and gamma globulin titres (Saino et al. 1997b). Work on House Sparrows Passer domesticus has also examined the relative size of the bursa of Fabricius in males with different badge sizes; males with larger badges had smaller bursae than males with smaller ornaments (Møller et al. 1996). Males with relatively few fault bars on the feathers, an indicator of general condition, had smaller bursae, consistent with the idea that the size of the organ is inversely proportional to the ability of the immune system to fight disease (Møller et al. 1996). Other research has examined the role of immunity in sexual selection in Gallus gallus, the Red Jungle Fowl (Zuk et al. 1990a, 1990b, 1995). This bird is the ancestor of domestic chickens, and is highly sexually dimorphic, with the male possessing a red fleshy comb and wattles as well as bright plumage, while the female is relatively drab and cryptic. Mate choice experiments have shown a consistent preference by females for what might be called the 'soft parts' on a male: comb size and colour, eye colour, and spur length (Zuk et al. 1990a, 1995). Comb length in particular appears to be reliably correlated with mating success. An intestinal parasite, the nematode Ascaridia galli, significantly reduces the development of these secondary sexual characters, and disproportionately affects ornaments rather than growth characteristics (Zuk 1990b). To determine if immune system parameters are correlated with morphology, we have performed canonical correlations of a set of blood measurements, including white blood cell differentials, hematocrit, and white:red blood cell ratios with a set of the morphological traits we know females prefer. This type of analysis allows the use of a group of intercorrelated variables simultaneously without the problems of running multiple univariate tests. Of the traits measured, the soft parts showed the most significant relationship between blood parameters and morphology (Zuk et al. 1995). Individually, the most striking single correlation was between differential lymphocyte count and comb length, so that males with longer combs had fewer lymphocytes, which suggests they have a weaker immune response (Zuk et al. 1995). To assess cell-mediated immunity, which is the part of the acquired immune system responsible for generalised reactions to wounding, allergens, and graft vs. host response, that, we examined the cutaneous hypersensitivity response (Roitt et al. 1989). This test evaluates the short-term inflammatory response to a foreign protein, before specific antibodies to it are produced (Roitt et al. 1989). In birds, this response is measured after injection of a protein such as phytohemagglutinin into the web of the wing between the radius and humerus. Because these are generalised antigens, rather than a disease organism, response in the birds can be evaluated in the short term without waiting for a disease process to get underway and without the confounding effects of specific responses to the pathogen; in other words, the birds do not get sick, but their immune system is challenged. By measuring the thickness of the wing web before the injection and at regular intervals afterwards, we can determine the amount of swelling, which indicates the degree of the immune response(Parmentier et al. 1993, 1994). Birds with larger swelling responses have better T-cell reactivity. Examination of the birds' response 6 hours after they got their injection into the wing web reveals that males with longer combs had significantly larger swellings (Zuk & Johnsen in press). This is the opposite relationship to the one found when we examined the proportion of lymphocytes, where males with longer combs had a seemingly less robust response. To attempt to resolve this paradox, we decided to place the results in the context of the birds' natural history. Both of the measures described above were made during the breeding season, when the males are reproductively competitive and presumably at their maximum stress levels. What happens outside this time? We looked at the same two measures before the breeding season, when the males are presumably not as stressed and not engaged in reproductive competition. Before the breeding season, males with larger combs have more pre-injection lymphocytes than do males with smaller combs, the opposite relationship to the one we found during the breeding season (Zuk & Johnsen in press). However, the wing web swelling relationship remains the same, with more ornamented males having stronger responses. This finding is mirrored by changes in the relative numbers of heterophils, white blood cells which are essential in cell-mediated immune response, particularly at wound sites. Heterophil numbers increased significantly after the injection was administered. In addition, males with larger combs showed a significantly larger increase in heterophil number (Zuk & Johnsen in press). Apparently, one facet of immunity, the cell-mediated response reflected in the cutaneous hypersensitivity reaction, is adaptively maintained during the breeding season, while another facet, the nonspecific immunity reflected in lymphocyte numbers, is not. The ability of more ornamented males to show this difference to a greater degree suggests that high quality individuals may indeed be better able to pay the price of maintaining elaborate secondary sexual characters. Sexual competition among male jungle fowl is extreme; males routinely engage in exhausting battles with other males. Although we prevent injury to males during our own experiments, observations of free-ranging birds as well as the practice of cockfighting in many cultures suggests that the risk of wounding is extremely high. The ability to resist infection and promote healing should therefore be advantageous to breeding males (Møller et al. in press). Because comb length is positively correlated with both humoral and cell-mediated immune responses at times other than the breeding season, however, females should still benefit by choosing males with well-developed combs, even if those males temporarily pay a price in reduced immunocompetence. Males with large combs may be more likely to allocate resources to cell-mediated immunity because of their greater predilection for engaging in fights. Similar seasonality in the strength of the immune response is common among mammals, particularly those that must overwinter in temperate or boreal regions of the world (Nelson & Demas 1996). Usually, immune function improves after the breeding season and before the onset of cold winter temperatures; short day length has been postulated as the cue for such changes (Nelson & Demas 1996; Nelson et al. 1998). Nelson et al. (1998) suggest that immune function is upregulated from a baseline level before winter, but it is also plausible that the converse is true: immune response is depressed during the stressful breeding season and can return to baseline after reproduction ceases. This latter interpretation is consistent with the findings reported above for male Red Jungle Fowl. Distinguishing between these two alternatives will require further work on the costs of immune function. At least in the jungle fowl, and potentially in other species as well, females are under different constraints than males and may exhibit different trade-offs. Male jungle fowl infected with the intestinal nematode A. galli show the effects of the parasite in secondary sexual traits, such as comb size, but not in mass or tarsus length at sexual maturity (Zuk et al. 1990b). In contrast, females with the same parasite infection show reduced growth of both body size characters and comb size (Zuk et al. in press). Comb size in female jungle fowl appears to be relatively unimportant in social interactions, unlike the situation for males (Zuk et al. in press), and thus the trade-off appears to differ in the two sexes: males in good condition may be able to maintain investment in both comb and body size, while males in poor condition cannot do so and truthfully indicate such to females. This differential allocation is unnecessary for the females, because females maintaining a large comb obtain no benefits.
THE ROLE OF TRADEOFFS
Central to the immunocompetence handicap idea, and to the studies discussed above, is the idea of trade-offs, a familiar concept from life history theory (Roff 1992; Stearns 1992). The idea that resources allocated to one function, such as offspring number, limit the ability to invest in offspring size is well-understood. In the case of investment in the immune system, however, it is not entirely clear what exactly is being traded off, and what currency is used in doing so (Zuk et al. 1996). One might suggest that ornament development is traded off against investment in somatic growth or immune defence, because the ornaments are costly, either due to their dependence on testosterone or for some other reason. Alternatively or along with that idea is the one just presented in the jungle fowl study, that certain aspects of immunity are important at different times, and those may be traded off against each other. In either case, we need to consider how the trade-offs are being paid. In other words, do animals have some aliquot of 'energy' or 'resource', as if in a figurative measuring cup, which they can pour into T-cells, tail length, or fat reserves? Is their growth rate, either somatic or that of the ornamental characters, dependent on something left over from the body's need for immune defence or other physiological processes? The answer is unclear. Very little information is available on the cost, in the sense that evolutionary biologists use the term, of immune responses (Hillgarth & Wingfield 1997; Demas et al. 1997). Certainly animals with insufficient resources, suffering from physiological stress, probably have reduced immune response. How different stressors translate into day-to-day maintenance of disease resistance, however, is less than obvious. Fever, or increased body temperature in response to a pathogen, places increased energy demands by increasing metabolic rate (Maier et al. 1994), but the only quantification of the energetic costs of immunity comes from a recent study of adult house mice (Demas et al. 1997). Animals injected with a protein, keyhole limpet hemocyanin, showed increased oxygen consumption as well as metabolic heat production, suggesting that the production of antibodies has an energetic cost. The cost of other immune parameters, such as maintenance of populations of lymphocytes, is not understood. Furthermore, as in other studies of life history, it is important to beware of what has sometimes been called the car-house paradox (van Noordwijk & de Jong 1986; Zuk et al. 1996). If the amount of resource, or money, invested in ones house means less is available for ones car, which makes sense, then a trade-off should occur and we should expect a negative correlation between the two. But of course that is not the case; people with expensive cars also have expensive houses, because the starting point is different for different individuals and some people have more resources at the outset. Hence the paradox, which is composed of negative trade-offs along a positive trajectory. Similarly, males with well-developed ornaments may also have other facets of immunity that allow them to compensate for testosterone-induced immunosuppression of one facet of immunity. For example, the interaction between Major Histocompatibility Complex (MHC) genes and aspects of the acquired immune response is not well understood; perhaps individuals with particular MHC genotypes can compensate for less robust antibody-mediated responses, thus reducing the immediate cost of mounting an immune response (Zuk 1994). The relationship between energy, immunity and growth may not be linear.
MEASURING IMMUNE RESPONSE
Immunologists have an enormous range of tools for evaluating immune response in clinical and laboratory situations. How can these be operationalised for use in the field, or even with captive wild animals? First, one can take cross-sectional or longitudinal samples from unmanipulated individuals and try to rank their immunocompetence. Such a technique is probably what many scientists envisioned when studying the immune system in an ecological context became popular; one could add 'immunocompetence' to a list of measurements that already include variables such as tarsus length or amount of body fat. Unfortunately, most aspects of the immune response are evaluated in the context of challenge with a specific foreign protein or disease agent. No one measure is unequivocally linked to a better immune system, and many measures, such as total or differential counts of lymphocytes, spleen size, and total immunoglobulin levels, are difficult to interpret when the history of exposure to disease is unknown (Lochmiller 1995). The advantage to this type of sampling is that it does not require repeated recaptures of animals at precise time intervals, an advantage that may make continued efforts to find a simple measure of immune system robustness worthwhile. In addition, general measures of immunity are, by definition, not linked to response to any one antigen. A second method provides a way out of this difficulty by using challenges to the immune system, such as the injections of phytohemagglutinin or sheep red blood cells in the studies described above. These tests are widely accepted among immunologists, and have at least two advantages. First, they give a clear-cut directional result; better immunity means a stronger response, whether in the form of higher immunoglobulin titres or larger swelling of the wing web. Second, they allow examination of a very general immune response to a protein the animal has never experienced before, rather than response to a specific disease organism. This generality means that the results presumably are not limited to resistance to only one pathogen, when others might be more important under natural circumstances. On the other hand, although the assumption that a strong immune response to a plant protein is equivalent to better resistance to real diseases, such may not be the case; indeed, selection for resistance to one disease is at least sometimes accompanied by the evolution of susceptibility to another (Roitt et al. 1989). Another approach takes a somewhat different view of selection on immune response. Artificially selected lines have long been used by evolutionary biologists to examine genetic variation in traits and their capability of responding to selection. Rather than looking at changes in an individual's lifetime, such experiments examine how selection on one trait changes other correlated characters. As workers in life history theory know, this can be an excellent way to examine trade-offs in parameters such as offspring size and number. In the case of immune response, we can also look at trade-offs - between various aspects of immunity, between ornaments and immune response - by examining traits in these selected lines. The most promising candidates for these techniques are invertebrates, particularly insects, which makes the suggestion that we ignore hormones more appealing. It should be feasible to use a similar approach in birds, either by using domesticated species such as pigeons or chickens, or by taking advantage of long-term field studies of passerines such as great tits or barn swallows. One might also compare lines with differing degrees of investment in sexual competition, and see if increased intensity of sexual selection is accompanied by a concomitant decrease in investment in immunity. Finally, it is important to remember that social factors can influence immune function. Recent work on arvicoline rodents in the genus Microtus has found that the manner in which animals are housed affects their response a mitogen, concanavalin A, as well as splenocyte proliferation values (Klein et al. 1997). When animals are housed individually, males and females do not differ in their response to the mitogen, regardless of species. But when they are housed either in pairs or groups, meadow vole Microtus pennsylvanicus males had higher responses than females, while prairie voles M. ochrogaster housed in same sex pairs showed higher responses in females than males (Klein et al. 1997; Nelson et al. 1998). Sex and species differences thus become apparent only in the context of the social environment similar to that in which reproduction would occur in the wild. Although this study is limited, there is no reason to expect that the general conclusion would be confined to rodents, or indeed to mammals, and therefore the immune system should ideally be examined under a variety of social circumstances.
CONCLUSIONS
Examining immune function in the context of sexual selection - and vice versa - hold great promise for future studies. Although elucidating the mechanisms behind the relationship between ornamentation, fitness, and immune response can be interesting, it may not be necessary to include hormone analysis in such research. We can employ several different measures of immunity in animals, but it is important not to ignore the environmental and social context in which the measures are taken. Males and females may show different relationships between morphological characters and immunity. Finally, while trade-offs provide a useful analogy for examining our ideas about immune function in the context of sexual selection, it is important to understand the currency involved in the trade-off as well as the fitness consequences of differential investment in reproduction or sexually-selected traits and immune response.
ACKNOWLEDGMENTS
My research is supported by grants from the U.S. National Science Foundation and the University of California, Riverside, Academic Senate. Torgeir Johnsen and Kurt McKean provided useful discussion, and Anders Møller gave helpful comments on the manuscript.
REFERENCES
Andersson, M. 1994. Sexual selection. Princeton University Press, Princeton NJ. Brown, M.E. 1996. Assessing body condition in birds. Current Ornithology 13: 67-135.
Clayton, D. H. 1991. The influence of parasites on host sexual selection. Parasitology Today 7: 329-334.
Clayton, D.H. & Moore, J. (eds.) 1997. Host-parasite evolution: general principles and avian models. Oxford University Press, Oxford UK
Demas, G.E., Chefer, V., Talan, M.I., & Nelson, R.J. 1997. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. American Journal of Physiology 273: R1631-R1637.
Folstad I. & Karter, A.J. 1992 Parasites, bright males and the immunocompetence handicap. American Naturalist 139: 603-622.
Grenfell, B.T. & Dobson, A.P. (eds) 1995. Ecology of infectious diseases in natural populations. Cambridge University Press.
Hamilton, W.D. & Zuk, M. 1982 Heritable true fitness and bright birds: a role for parasites? Science 213: 384-387.
Hillgarth, N. & Wingfield, J.C. 1997. Parasite-mediated sexual selection: endocrine aspects. In Clayton, D.H. & Moore, J. (eds) Host-parasite evolution: general principles and avian models. Pp. 78-104. Oxford University Press, Oxford UK
Hoelzer, G. 1989. The good parent process of sexual selection. Animal Behaviour 38: 1067-1078.
Kirkpatrick, M. & Ryan, M.J. 1991. The evolution of mating preferences and the paradox of the lek. Nature 350: 33-38.
Klein, S.L., Hairston, J.E., DeVries, A.C., & Nelson, R.J. 1997. Social environment and steroid hormones affect species and sex differences in immune function among voles. Hormones and Behavior 32: 30-39.
Lochmiller, R.L. 1995. Testing the immunocompetence handicap theory. Trends in Ecology and Evolution 10: 372-373.
Maier, S.F., Washout, L.R., & Fleshner, M. 1994. Psychoneuroimmunology: the interface between behavior, brain, and immunity. American Psychologist 49: 1004-1017.
Møller, A.P. 1990. Parasites and sexual selection: current status of the Hamilton and Zuk hypothesis. Journal of Evolutionary Biology 31: 319-328.
Møller, A.P. 1997. Immune defence, extra-pair paternity, and sexual selection in birds. Proceedings of the Royal Society of London B 264: 561-566.
Møller, A.P. & Saino, N. 1994. Parasites, immunology of hosts, and host sexual selection. Journal of Parasitology 80: 850-858.
Møller, A.P. & Saino, N. 1999. The avian immune system: A jack-of-all-trades. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2147-2157. Johannesburg: BirdLife South Africa.
Møller, A.P., Kimball, R.T. & Errritzøe, J. 1996 Sexual ornamentation, condition, and immune defence in the house sparrow Passer domesticus. Behavioral Ecology and Sociobiology 39: 317-322.
Møller, A.P., Sorci, G. & Errritzøe, J. in press. Sexual dimorphism in immune defence. American Naturalist.
Nelson, R.J. & Demas, G.E. 1996 Seasonal changes in immune function. Quarterly Review of Biology 71: 511-548.
Nelson, R.J., Demas, G.E. & Klein, S.L. 1998. Photoperiodic mediation of seasonal breeding and immune function in rodents: a multi-factorial approach. American Zoologist 38: 226-237.
Parmentier, H.K., Schrama, J.W., Meijer, F. & Nieuwland, M.J.B. 1993. Cutaneous hypersensitivity responses in chickens divergently selected for antibody responses to sheep red blood cells. Poultry Science 72: 1679-1692.
Parmentier, H.K., Siemonsma, R. & Nieuwland, M.J.B. 1994. Immune responses to bovine serum albumin in chicken lines divergently selected for antibody responses to sheep red blood cells. Poultry Science 73: 825-835.
Petrie, M. 1994. Improved growth and survival of offspring of peacocks with more elaborate trains. Nature 371: 598-599.
Read, A.F. 1990. Parasites and the evolution of host sexual behaviour. In Parasitism and host behaviour, C.J. Barnard & J.M. Behnke eds. Pp. 78-99. Taylor & Francis, London.
Roff, D.A. 1992. The evolution of life histories: theory and analysis. Chapman & Hall, NY
Roitt, I., Brostoff, J. & Male, D. 1989. Immunology (2nd edition), New York, NY: Harper & Row
Saino, N. & Møller, A.P. 1996 Sexual ornamentation and immunocompetence in the barn swallow. Behavioral Ecology 7: 227-232.
Saino, N., Galeotti, P., Sacchi, R. & Møller, A.P. 1997a. Song and immunological condition in male barn swallows (Hirundo rustica). Behavioral Ecology 8: 364-371.
Saino, N., Bolzern, A.M. & Møller, A.P. 1997b Immunocompetence, ornamentation, and viability of male barn swallows (Hirundo rustica). Proceedings of the. National Academy of Science USA 94: 549-552.
Sheldon, B.C. & Verhulst, S. 1996 Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology and Evolution 11: 317-321.
Stearns, S.C. 1992. The evolution of life histories. Oxford University Press, Oxford UK
Sullivan, B.K. 1991. Parasites and sexual selection: separating causes and effects. Herpetologica 47: 250-264.
Toft, C.A., Aeschlimann, A., & Bolis, L. (eds). 1991. Parasite-host associations: coexistence or conflict? Oxford University Press, Oxford UK
van Noordwijk, A.J. & de Jong, G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. American Naturalist 128: 137-142.
Weatherhead, P.J., Metz, K., Bennett, G.F., & Irwin, R.E.1993. Parasite faunas, testosterone and secondary sexual traits in male red-winged blackbirds Agelaius phoeniceus. Behavioral Ecology and Sociobiology 33: 12-23.
Wedekind, C. & Folstad, I. 1994. Adaptive or non-adaptive immunosuppression by hormones? American Naturalist 143: 936-938.
Zuk, M., Thornhill, R., Ligon, J.D., Johnson, K., Austad, S., Ligon, S., Thornhill, N. & Costin, C. 1990a. The role of male ornaments and courtship behavior in female choice of red jungle fowl. American Naturalist 136: 459-473.
Zuk, M., Thornhill, R., Johnson, K. & Ligon, J.D. 1990b Parasites and mate choice in red jungle fowl. American Zoologist 30: 235-244.
Zuk, M. 1992. The role of parasites in sexual selection: current evidence and future directions. Advances in the Study of Behavior 21: 39-68.
Zuk, M. 1994. Immunology and the evolution of behavior. In: Behavioral Mechanisms in Ecology, (ed. L. Real), pp. 354-368. Chicago IL: University of Chicago Press.
Zuk, M., Johnsen, T.S. & MacLarty, T. 1995. Endocrine-immune interactions, ornaments and mate choice in red jungle fowl. Proceedings of the Royal Society of London B. 260: 205-210.
Zuk, M. Bryant, M.J., Kolluru, G.R., & Mirmovitch, V. 1996. Trade-offs in parasitology, evolution and behaviour. Parasitology Today 12: 46-47.
Zuk, M., Kim, T., Robinson, S., & Johnsen, T.S. in press. Parasites influence social rank and morphology, but not mate choice, in female red jungle fowl (Gallus gallus). Animal Behaviour.
Zuk, M. & Johnsen, T.S. in press. Seasonal changes in the relationship between ornamentation and immune response in red jungle fowl. Proc. R. Soc. Lond. B.
Fig. 1. Schematic diagram of influences on ornaments and mate choice via the immune system.
