S35.5: The population dynamics of the Namaqua Sandgrouse: Implications for gamebird management in an arid, stochastic environment
Penn Lloyd, Robin M. Little & Timothy M. Crowe
Percy FitzPatrick Institute of African Ornithology, University of Cape Town, Rondebosch 7701, South Africa, e-mail plloyd@botzoo.uct.ac.za
Lloyd, P., Little, R.M. & Crowe, T.M. 1999. The population dynamics of the Namaqua Sandgrouse: Implications for gamebird management in an arid, stochastic environment. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2130-2143. Johannesburg: BirdLife South Africa.The Namaqua Sandgrouse Pterocles namaqua is an abundant, but highly nomadic, obligate granivore inhabiting the arid and semi-arid regions of southern Africa. As a successful arid-zone specialist, it is expected to have a relatively high reproductive potential by being opportunistic in its breeding strategy, with breeding timed to coincide with periods of food abundance following spatially and temporally patchy and unpredictable rainfall events. However, this species was found to have a relatively low reproductive potential due to high levels of nest predation, a relatively long dependency period (at least three months) and a clutch size fixed at 2-3 eggs due to the constraints of carrying water for the chicks in the belly feathers of the adult male. High nest predation resulting in low annual recruitment, which supports evidence of a population decline over the last 40 years, may have been exacerbated by meso-predator release following the anthropogenic alteration of the predator guild over large areas of the range of this species. The timing of breeding is unexpectedly variable between regions experiencing similar rainfall regimes, and this species does not always breed when food supplies are optimal. In some regions, the timing of breeding may be a trade-off between seasonally variable nest predation pressure and food availability. The possibility that mortality in Namaqua Sandgrouse populations may be largely density independent presents an added challenge to the determination of sustainable hunting quotas for this gamebird whose populations may already be declining.
INTRODUCTION
The Namaqua Sandgrouse Pterocles namaqua is a specialised, obligate granivore that feeds on the seeds of dicotyledonous annual plants within the arid and semi-arid zones of southern Africa (Lloyd et al. in review a). The chicks are precocial and feed themselves solely on seeds from the day they hatch (Lloyd et al. in review a). The advantage of a granivorous diet in an arid ecosystem is that annual plants produce superabundant quantities of seed in a predictable fashion following good rainfall (Brown et al. 1979). However, in such environments, rainfall is both highly variable in its quantity and unpredictable in its distribution in space and time. This particular suite of environmental features has favoured the development of the following ecological characteristics in the granivorous avifauna of arid environments:(1) wide-ranging regional (nomadic) and/or seasonal (migratory) shifts in distribution and abundance in response to fluctuating food supplies (Wiens & Johnston 1977; Andersson 1980; Dean 1997), and (2) a rapid, opportunistic and continuous breeding response when environmental conditions are suitable, and other traits, such as a flexible clutch size, that maximise reproductive output under fluctuating and relatively unpredictable conditions (Grant & Grant 1989; Lloyd in press).
The Namaqua Sandgrouse is a highly nomadic granivore that: (1) forms large feeding aggregations when food is abundant; (2) is opportunistic in its choice of foods; and (3) can locate and rapidly exploit locally concentrated food sources (Lloyd et al. in review a). These characteristics are associated with successful granivorous species that can maintain large populations (Wiens & Johnston 1977). The Namaqua Sandgrouse is a sought-after gamebird in southern Africa (Johnson & Wannenburg 1987), but possibly because of its widespread distribution and perceived abundance, there was little concern about its conservation status until evidence of a population decline over the last 40 years was detected recently (Little et al. 1996). This paper reviews our recent work that examines the reproductive potential and breeding strategy of this species in relation to rainfall, food availability and predation risk.
DISCUSSION
The cost of nomadism
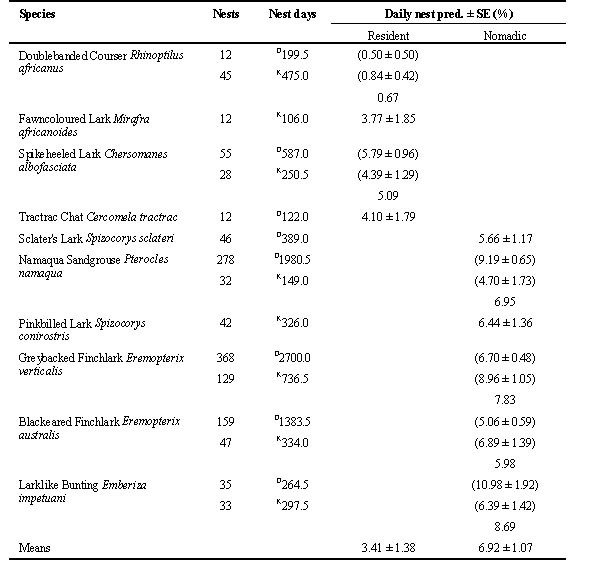
Nomadism allows birds to track patches of high productivity in a generally unproductive environment, but it could have drawbacks for the ability of nomadic birds to avoid nest predation. Lloyd (in review) found that daily nest predation rates of ground-nesting birds (Table 1) were significantly higher on nomads than on territorial residents (Mann-Whitney U-test: U(1),4,6 = 24.0, P < 0.02). The hypothesis that residents might incur lower nest predation rates than nomads due to their more detailed knowledge of predator behaviour and activity within the limited confines of their long-term territories requires more rigorous testing on a larger sample that caters for the effects of phylogeny.
Breeding success
The Nama Karoo biome is a core breeding area for the Namaqua Sandgrouse in South Africa. We examined the breeding success of this species over four consecutive breeding seasons on Droëgrond farm (29
°07' S 20°16' E) in the Kakamas district of the Nama Karoo (Lloyd et al. in review b).Despite substantial inter-annual variation in rainfall before and during the breeding season, and in nesting density, there was little variation in nest predation pressure, the primary cause of Namaqua Sandgrouse nest losses at Droëgrond (Table 2). Furthermore, the average nest predation rate for Namaqua Sandgrouse at Droëgrond (91%) is considerably higher than the 54% recorded for a stable, resident Yellowthroated Sandgrouse population (Tarboton et al. in press).
Nest failure rates greater than 70% have been implicated in population declines in shrubsteppe passerines on the Iberian Peninsula (Suárez et al. 1993; Yanes & Suárez 1995) and Neotropical migrants (Sherry & Holmes 1992; Böhning-Gaese et al. 1993; Donovan et al. 1995; Hoover et al. 1995). These declines are thought to be caused by unnaturally elevated nest predation rates due, in turn, to either edge effect resulting from habitat fragmentation (Wilcove 1985; Sherry & Holmes 1992; Paxton 1994; Donovan et al. 1995; Hoover et al. 1995), or increasing densities of small, generalist predators following the anthropogenic exclusion of top predators (Eisenberg et al. 1979; Glanz 1982; Emmons 1984; Soulé et al. 1988).
Habitat fragmentation is not an issue at Droëgrond, and the high predation rates were not the result of abnormal events in a stochastic environment, as there was little inter-annual variation in predation intensity despite considerable variation in rainfall, and therefore the productivity of the environment (Table 2). Daily nest predation rates were not significantly different between years at Droëgrond (z = 0.14-1.21, all P > 0.05). The study site at Droëgrond is, however, in a sheep farming region where top predators (Blackbacked Jackal Canis mesomelas, Caracal Caracal caracal and large raptors) have been subjected to intense control programmes that involve the use of poisons, traps and hunting. As a result, the Tawny Eagle Aquila rapax, an important predator on small mammals (Clutton-Brock et al. in press), has disappeared from the Bushmanland region within the last 100 years (Boshoff et al. 1983). Other top predators now occur at substantially reduced densities. Daily nest predation rates on Namaqua Sandgrouse were significantly lower in the Kalahari Gemsbok National Park, where an entirely natural complement of predators is present, than they were at Droëgrond (z = 2.53, P < 0.01; Lloyd et al. in review b). A case could, therefore, be made for meso-predator release (sensu Soulé et al. 1988) being responsible for the higher nest predation at Droëgrond.
Annual recruitment was low at Droëgrond and at several other sites in farming regions across southern Africa (Table 3). Minimum estimates were typically in the region of 3-10% and maximum estimates in the region of 5-20%. This is to be compared with 18-36% estimated annual recruitment for Yellowthroated Sandgrouse (Tarboton et al. in press), and 33-66% for Namaqua Sandgrouse in a protected area, the Kalahari Gemsbok National Park. Without data on average annual adult survival, it is difficult to evaluate whether annual recruitment of 3-20% is sufficient to maintain populations over the long term. Annual adult mortality among charadriiforms, to which sandgrouse are most closely related, is commonly 20-40% (Boyd 1962; Brooke & Birkhead 1991; Gill 1995). This suggests that, if the relatively low productivity of the Namaqua Sandgrouse is sustained in the long term within the core Nama Karoo region, a population decline is a likely result. Long-term records for a private game reserve in the Northern Cape show an apparent Namaqua Sandgrouse population decline between 1950 and 1992, but this may be an artifact of increased sandgrouse dispersion in response to an increased number of artificial watering points being constructed over this period (Little et al. 1996).
The present status of the Namaqua Sandgrouse in South Africa is therefore uncertain. Whereas their annual productivity, within the Nama Karoo region at least, appears to be too low to maintain populations, there is no irrefutable evidence of a population decline. Future studies should determine: 1) annual adult survival, and 2) more accurate and longer-term estimates of breeding success in various regions of the Namaqua Sandgrouse's distribution. The hypothesis that anthropogenic effects at the landscape level are affecting breeding success should be tested. These studies should be coupled with an investigation of the degree of movement of breeding populations between these different regions, for such movement may mask poor reproductive output in certain regions.
Breeding activity
For most birds, the physiological demands of reproduction are the most rigorous and critical of any in their annual cycle. In response, most species have evolved the timing of breeding such that the most nutritionally demanding stage of the breeding cycle coincides with seasonal peaks in food supply, particularly in strongly seasonal environments (Lack 1954; Perrins 1970; Immelmann 1971; Daan et al. 1988; Grant & Grant 1989). In the arid to semi-arid subtropics, erratic and unpredictable rainfall is the key proximal determinant of the timing and duration of breeding in most species through its influence on food availability (Moreau 1950; Marchant 1960; Immelmann 1973; Boag and Grant 1984). Three of the four species of southern African sandgrouse, namely Doublebanded Sandgrouse P. bicinctus, Burchell's Sandgrouse P. burchelli and Yellowthroated Sandgrouse P. gutturalis nest primarily through the dry winter months, from April to August, when seed availability is high following late-summer (November-April) rains (Skinner 1996; Harrison et al. 1997; Tarboton et al. in press). Similarly, the Chestnutbellied Sandgrouse P. exustus and Blackfaced Sandgrouse P. decoratus in East Africa breed during the dry seasons when food is most abundant (Kalchreuter 1980; Njoroge et al. 1997).
The breeding seasons of Namaqua Sandgrouse, however, are unexpectedly variable and not consistently correlated with periods of peak food availability following seasonal rainfall (Fig. 1). In the northern Namib, peak rainfall is in February-March, and in the southern Namib a month later (March-April). In these arid regions, the annual plants germinate, grow and set seed rapidly after rain, and thus do not exhibit an extended growing season. The number of nest records in the period January-May in the northern Namib suggests that Namaqua Sandgrouse breed soon after the earlier rains in this region, and thus at a time when food is most abundant. The birds appear to exhibit a similar breeding response in the southern Namib, with a slightly later nesting peak in response to a later rainfall peak. The spread of nest records throughout the year may be due to the highly erratic nature of rainfall in this region. Dixon and Louw (1978), studying the thermal properties of Namaqua Sandgrouse nests in the Namib, found that when the soil surface temperature at one nest exceeded 50°C, the incubating bird was unable to prevent the nest temperature from rising above 45° C, which caused the death of the embryos. This observation lead them to believe that Namaqua Sandgrouse in this region nest mainly in winter to avoid high summer temperatures. Nonetheless, throughout the Nama Karoo and Kalahari, where temperatures are no less extreme, Namaqua Sandgrouse often nest through January, the hottest month of the year (Lloyd et al. in review c). Midday temperatures of 48°C in the shade and soil temperatures of 68°C have been recorded near incubating Spotted Sandgrouse P. senegallus (George 1970), and Crowned Sandgrouse P. coronatus have been observed incubating at air temperatures of 41-51°C with no apparent ill effects (Johnsgard 1991). These observations suggest that Namaqua Sandgrouse are capable of coping with high temperatures and nesting successfully through midsummer.
Winter rainfall in the Namaqualand and Western Cape is both predictable and more continuous through the winter rainfall season. The annual plants here thus only begin to set seed at the start of the summer dry season. Here Namaqua Sandgrouse breed strictly through the summer months when food supplies are optimal. Thus, in the western arid and semi-arid zones of southern Africa, Namaqua Sandgrouse appear to start breeding soon after food becomes abundant, as one would predict. The north-south trend for later breeding in the south corresponds to later rainfall and a more extended growing season at more southerly latitudes.
The situation in the eastern Nama Karoo and Kalahari regions of both Namibia and South Africa is somewhat different. In these late summer rainfall regions, the other sandgrouse species breed, as expected, between April and September when food availability is maximal (Skinner 1996; Harrison et al. 1997; Tarboton et al. in press). However, Namaqua Sandgrouse populations here generally exhibit a breeding season starting August-September and extending to January-March in the Nama Karoo, and from June-September to January-March in the Kalahari. Thus these birds often only start egg-laying as much as five months after food becomes abundant, and therefore at a time when the food supply is diminishing. Interestingly, the breeding season for Namaqua Sandgrouse throughout the Nama Karoo biome appears to be relatively fixed, populations in the southern Karoo breeding at the same time as populations in central Namibia. A puzzling feature of this breeding season is that these regions receive most of their rainfall as thundershowers between December and April. Namaqua Sandgrouse will therefore often have young, flightless chicks, the most nutritionally demanding stage of the annual cycle (Lloyd et al. in review a), during the period of lowest food availability.
Several possible hypotheses to explain these unusual breeding seasons, including: 1) that Namaqua Sandgrouse breed twice in the year, 2) that moulting precludes an early start to breeding, and 3) that adults require several months to build up nutritional reserves for breeding in some regions, have been discounted (Lloyd et al. in review c). This suggests that their breeding response is modified by one or more additional selection factors, at least in certain regions. The unusual timing of breeding of Namaqua Sandgrouse in the Nama Karoo may be explained better as a trade-off between the risks associated with seasonal variation in nest predation pressure and food availability than on the basis of food availability alone. In 1994, daily nest predation rates on Namaqua Sandgrouse at Droëgrond (averaged weekly) decreased significantly from late winter to midsummer (Fig. 2). Similarly, nest predation rates on a variety of birds breeding at Droëgrond in 1996 decreased significantly from spring to midsummer (Fig. 2). Daily nest predation rates may, therefore, decrease by at least 50% between winter and summer. Although the small mammals that are the main nest predators are opportunistic foragers, they feed primarily on arthropods (Skinner & Smithers 1990). The higher nest predation in winter may stem from reduced arthropod availability in the cool, dry winter months. Because of the very high predation rates (Table 2), a reduction in predation will have a very significant effect on nesting success. For example, a 7% increase in nest predation on Namaqua Sandgrouse at Droëgrond between 1993 and 1994 resulted in a 44% reduction in nesting success (Table 2). The seasonal variation in nest predation pressure should, therefore, provide a strong selection pressure for delayed breeding by Namaqua Sandgrouse in this region. Nest predation is known to influence life history traits such as clutch size and developmental rates (Slagsvold 1982; Bosque & Bosque 1995; Martin 1995; Julliard et al. 1997), but a direct link between nest predation and the timing of breeding has not previously been demonstrated. Future studies should examine this hypothesis more fully by evaluating the extent to which variation in food availability and nest predation pressure in different regions within southern Africa can account for the observed variation in the timing of breeding of Namaqua Sandgrouse between regions.
Reproductive potential
The modal clutch size of Namaqua Sandgrouse is three eggs (range 2-3, mean = 2.9, n = 224) and there were no significant differences in mean clutch size between years that differed greatly in terms of food availability (Lloyd et al. in review b). The clutch size for sandgrouse is possibly restricted by the number of chicks that the adult male can provision with water carried over long distances in the feathers of his belly (Maclean 1976). Furthermore, the high rates of nest predation (Table 2), a one-month nesting period and a chick-dependency period lasting at least two months (for water carried by the male), and relatively fixed breeding seasons suggests that pairs will rarely have the potential to raise more than one successful brood per year (Lloyd et al. in review c). This combination of factors means that the Namaqua Sandgrouse has an unexpectedly low reproductive potential for an opportunistic, nomadic granivore.
Management concernsThe answer to the question of what constitutes a sustainable off-take for the Namaqua Sandgrouse will depend on which definition of sustainability is adopted. The classical definition holds that a sustainable off-take is a level of off-take that does not exceed the capacity of the population to replenish itself (McCullough 1996). Using this definition, one might, based on the results discussed above, conclude that there is no sustainable off-take level for Namaqua Sandgrouse, as recruitment may be too low to maintain populations over extensive areas. Only long-term monitoring studies will be able to resolve this issue more satisfactorily.
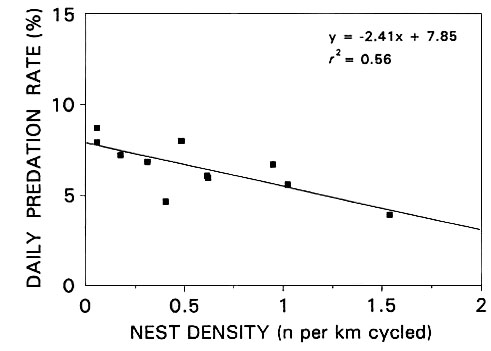
From a theoretical point of view, a sustainable off-take is reliant on natural density dependent factors that largely compensate for the off-take (Sinclair & Pech 1996; Callaghan et al. 1997). In other words, a sustainable hunting off-take removes the proportion of the population that would have died naturally due to density dependent factors (the 'doomed surplus') and/or stimulates breeding to replace the birds removed in the off-take. There is no evidence for density dependent mortality and/or natality in the Namaqua Sandgrouse. On the breeding grounds, nest predation did not increase as nesting density increased and, in fact, the opposite appeared to hold true (Fig. 3). During periods of widespread drought, food limitation might result in density dependent mortality, although such mortality could result more from chance events such as the failure of birds to find a very patchily distributed resource. The Namaqua Sandgrouse is a central-place forager in which the central place, the waterhole, is probably used as an information centre (Ward 1972). Their ability to find widely dispersed and cryptic food patches of high quality may therefore increase as the local population size sampling the area around the waterhole increases. Although group foraging may not increase daily energetic benefits for individuals, it can represent an evolutionary stable adaptation to foraging and survival in arid areas with patchy food resources by diluting foraging costs and reducing the risks of unproductive foraging (Spinks & Plagányi in press).
In highly stochastic environments, where plant-herbivore dynamics do not reach or closely approach equilibrium levels, the concept of a carrying capacity implicit in maximum sustainable yield models (Caughley 1976), is viewed as more a mathematical abstraction than from the perspective of sustainable utilisation (Macnab 1985; McLeod 1997). The amplitude of population fluctuations is determined by both the strength of density dependence and the size of density independent mortalities (Sinclair & Pech 1996). If density independent mortalities are high (e.g. high reproductive failure in Namaqua Sandgrouse), and if environmental conditions fluctuate markedly (as they do in arid zones), density dependent mortality may be relatively unimportant. The Budgerigar Melopsittacus undulatus, an abundant, nomadic granivore inhabiting arid and semi-arid regions of Australia, is thought to incur greater density independent mortality due to its unpredictable environment (Ford 1989, p. 175). Since density dependence is a central assumption of most sustainable off-take models, further research is required to determine the relative importance of density dependent and density independent factors to the population dynamics of the Namaqua Sandgrouse.
Martin (1997) adopted a rather different approach to the definition of sustainability, viewing the ultimate criterion of sustainability as the persistence of the species. In other words, as long as a species' populations are not reduced to the level that extinction is a real threat, then use can be regarded as sustainable. The attraction of sandgrouse to hunters is, to a large extent, dependent on the numbers of birds that congregate at a waterhole (Malan et al. 1993), i.e. it is density dependent. The corollary is that, should hunting lead to substantial reductions in populations, the species would approach commercial extinction well before it reached biological extinction. The very nature of the arid environment, the widespread distribution of the Namaqua Sandgrouse and its extensive nomadism suggest that this species is likely to be resilient to over-exploitation. However, its low reproductive output, and the possibility that natural mortality and natality rates may be largely density independent, would ensure that population recoveries following over-exploitation would be slow.
ACKNOWLEDGEMENTS
We thank Dekker and Sikkie Stadler and Hendrik and Lientjie Maritz for their generous hospitality and field assistance to PL. The study was supported by the African Gamebird Research, Education and Development Trust, De Beers Consolidated Mining Company Ltd, and grants from the Foundation for Research Development.
REFERENCES
Andersson, M. 1980. Nomadism and site tenacity as alternative reproductive tactics in birds. J. Anim. Ecol. 49: 175-184.
Boag, P.T. & Grant, P.R. 1984. Darwin's Finches (Geospiza) on Isla Daphne Major, Galápagos: breeding and feeding ecology in a climatically variable environment. Ecol. Monogr. 54: 463-489.
Böhning-Gaese, K., Taper, M.L. & Brown, J.H. 1993. Are declines in North American insectivorous songbirds due to causes on the breeding range? Cons. Biol. 7: 76-86.
Boshoff, A.F., Vernon, C.J. & Brooke, R.K. 1983. Historical atlas of the diurnal raptors of the Cape Province (Aves: Falconiformes). Ann. Cape Prov. Mus. (Nat. Hist.) 14: 173-297.
Bosque, C. & Bosque, M.T. 1995. Nest predation as a selective factor in the evolution of developmental rates in altricial birds. Amer. Natur. 145: 234-260.
Boyd, H. 1962. Mortality and fertility of European Charadrii. Ibis 104: 368-387.
Brooke, M. & Birkhead, T. (eds) 1991. The Cambridge encyclopedia of ornithology. Cambridge: Cambridge University Press.
Brown, J.H., Reichman, O.J. & Davidson, D.W. 1979. Granivory in desert ecosystems. - Ann. Rev. Ecol. Syst. 10: 201-227.
Callaghan, D.A., Kirby, J.S. & Hughes, B. 1997. The effects of recreational waterfowl hunting on biodiversity. In: Freese, C.H. (ed.) Harvesting wild species: implications for biodiversity conservation: 507-574. Baltimore: Johns Hopkins University Press.
Caughley, G. 1976. Wildlife management and the dydamics of ungulate populations. In: Coaker, T.H. (ed.) Applied biology, vol. 1: 183-246. London: Academic Press.
Clutton-Brock, T.H., Gaynor, D., MacIlrath, G.M., MacColl, A.D.C., Kansky, R., Chadwick, P., Manser, M. & Skinner, J.D. in press. Predator density and population dynamics in a co-operative mongoose, Suricata suricatta. J. Anim. Ecol.
Daan, S., Dijkstra, C., Drent, R. H. & Meijer, T. 1988. Food supply and the annual timing of avian reproduction. Proc. 19th Int. Ornithol. Congr., vol. 1: 392-407.
Dean, W.R.J. 1997. The distribution and biology of nomadic birds in the Karoo, South Africa. J. Biogeogr. 24: 769-779.
Dixon, J.E.W. & Louw, G.N. 1978. Seasonal effects of nutrition, reproduction and aspects of thermoregulation in the Namaqua sandgrouse (Pterocles namaqua). Madoqua 11: 19-29.
Donovan, T.M., Thompson, F.R. III, Faaborg, J. & Probst, J.R. 1995. Reproductive success of migratory birds in habitat sources and sinks. Cons. Biol. 9: 1380-1395.
Eisenberg, J., O'Connell, M. & August, P.V. 1979. Density, productivity, and distribution of mammals in two Venezuelan habitats. In: Eisenberg, J.F. (ed.) Vertebrate ecology in the northern Neotropics: 187-207. Washington D.C.: Smithsonian Institute Press.
Emmons, L.E. 1984. Geographic variation in densities and diversities of non-flying mammals in Amazonia. Biotropica 16: 210-222.
Gill, F.B. 1995. Ornithology. New York: Freeman and Company.
Glanz, W.E. 1982. The terrestrial mammal fauna of Barro Colorado Island: censuses and long-term changes. In: Leigh, E.G., Rand, A.S. & Windsor, D.M. (eds) The ecology of a tropical forest: 239-251. Washington D.C.: Smithsonian Institute Press.
Grant, B.R. & Grant, P.R. 1989. Evolutionary dynamics of a natural population. Chicago: University of Chicago Press.
Ford, H.A. 1989. Ecology of birds: an Australian perspective. Sydney: Surrey Beatty & Sons.
George, U. 1970. Beobachtungen an Pterocles senegallus und Pterocles coronatus in der Nordwest-Sahara. J. Ornithol., Lpz. 111: 175-188.
Harrison, J.A., Allan, D.G., Underhill, L.G., Herremans, M., Tree, A.J., Parker, V. & Brown, C.J. (eds) 1997. The atlas of southern African birds, vol. 1. Johannesburg: Birdlife South Africa.
Hoover, J.P., Brittingham, M.C. & Goodrich, L.J. 1995. Effects of forest patch size on nesting success of Wood Thrushes. Auk 112: 146-155
Immelmann, K. 1971. Ecological aspects of periodic reproduction. In: Farner, D.S. and King, J.R. (eds) Avian biology, vol. 1: 341-389. New York: Academic Press.
Immelmann, K. 1973. Role of the environment in reproduction as source of 'predictive' information. In: Farner, D.S. (ed.) Breeding biology of birds: 121-147. Washington, D. C.: National Academy of Sciences.
Johnsgard, P.A. 1991. Bustards, hemipodes, and sandgrouse: birds of dry places. Oxford: Oxford University Press.
Johnson, P.G. & Wannenburgh, A.J. 1987. The world of shooting. Lausanne: The World of Shooting.
Julliard, R., McCleery, R.H., Clobert, J. & Perrins, C.M. 1997. Phenotypic adjustment of clutch size due to nest predation in the Great Tit. Ecology 78: 394-404.
Kalchreuter, H. 1980. The breeding season of the Chestnutbellied Sandgrouse Pterocles exustus and the Blackfaced Sandgrouse P. decoratus in northern Tanzania and its relation to rainfall. Proc. 4th Pan-Afr. Ornithol. Congr.: 277-282.
Lack, D. 1954. The Natural Regulation of Animal Numbers. Oxford: Oxford University Press.
Little, R.M., Crowe, T.M. & Villacastin-Herrero, C.A. 1996. Conservation implications of long-term population trends, environmental correlates and predictive models for Namaqua Sandgrouse Pterocles namaqua. Biol. Cons. 75: 93-101.
Lloyd, P. in press. Rainfall as a breeding stimulus and clutch size determinator in South African arid-zone birds. Ibis.
Lloyd, P. in review a. The influence of nesting habits and the nomadic/residency dichotomy on nest predation rates in arid-zone birds. Ibis.
Lloyd, P., Little, R.M. and Crowe, T.M. in review a. The diet and nutrition of the Namaqua Sandgrouse, an arid zone granivore. J. Arid Environ.
Lloyd, P., Little, R.M. and Crowe, T.M. in review b. The breeding biology of the Namaqua Sandgrouse. Ostrich.
Lloyd, P., Little, R.M. and Crowe, T.M. in review c. Rainfall and food availability as factors influencing the migration and breeding activity of Namaqua Sandgrouse in southern Africa. Ostrich.
Lloyd, P., Little, R.M. & Crowe, T.M. in review d. Does food availability or nest predation pressure control the timing of breeding in the Namaqua Sandgrouse? J. Avian Biol.
Ludwig, D., Hilborn, R. & Walters, C. 1993. Uncertainty, resource exploitation, and conservation: lessons from history. Science 260:17-36.
Maclean, G.L. 1968. Field studies on the sandgrouse of the Kalahari Desert. Living Bird 7: 209-235.
Maclean, G.L. 1976. Adaptations of sandgrouse for life in arid lands. Proc. 16th Int. Ornithol. Congr.: 502-516.
Malan, G., Little, R.M. & Crowe, T.M. 1994. Temporal and spatial patterns of abundance and breeding activity of Namaqua Sandgrouse in South Africa. S. Afr. J. Zool. 29: 162-167.
Marchant, S. 1960. The breeding of some S.W. Ecuadorean birds. Ibis 102: 349-381.
Martin, T.E. 1995. Avian life history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 65: 101-127.
Macnab, J. 1985. Carrying capacity and related slippery shibboleths. Wildl. Soc. Bull. 13: 403-410.
Martin, R. 1997. Sustainable use of wildlife. In: Borrini-Feyerabend, G. (ed.) Beyond fences: seeking social sustainability in conservation, vol. 2: 79-81. Gland (Switzerland): IUCN.
McCullough, D.R. 1996. Spatially structured populations and harvest theory. J. Wildl. Manage. 60:1-9.
McLeod, S.R. 1997. Is the concept of carrying capacity useful in variable environments? Oikos 79: 529-542.
Moreau, R.E. 1950. The breeding seasons of African birds. Ibis 92: 223-267.
Njoroge, P., Lens, L., Sutton, J. & Bennun, L.A. 1997. The validity of open seasons for sandgrouse shooting: analysis of an 11-year data set from Kenya. Afr. J. Ecol. 35: 186-193.
Paxton, P.W. 1994. The effect of edge on avian nest success: how strong is the evidence? Cons. Biol. 8: 17-26.
Perrins, C.M. 1970. The timing of bird's breeding seasons. Ibis 112: 242-255.
Sherry, T.W. & Holmes, R.T. 1992. Population fluctuations in a long-distance Neotropical migrant: demographic evidence for the importance of breeding season events in the American Redstart. In: Hagan III, J.M. & Johnston, D.W. (eds) Ecology and conservation of Neotropical migrant landbirds: 431-442. Washington D.C.: Smithsonian Institute Press.
Sinclair, A.R.E. & Pech, R.P. 1996. Density dependence, stochasticity, compensation and predator regulation. Oikos 75: 164-173.
Skinner, J.D. & Smithers, R.H.N. 1990. The Mammals of the southern African subregion. Pretoria: University of Pretoria.
Skinner, N.J. 1996. The breeding seasons of birds in Botswana. II. Non-passerine families (sandgrouse to woodpeckers). Babbler 31: 6-16.
Slagsvold, T. 1982. Clutch size variation in passerine birds: the nest predation hypothesis. Oecologia 54: 159-169.
Soulé, M.E., Bolger, D.T., Alberts, A.C., Wright, J., Sorice, M. & Hill, S. 1988. Reconstructed dynamics of rapid extinctions of chaparral-requiring birds in urban habitat islands. Cons. Biol. 2: 75-92.
Spinks, A.C. & Plagányi, É.E. in press. Habitat constraints and sociality in the Common Mole-rat, Cryptomys hottentotus hottentotus (Bathyergidae): a computer simulated foraging model. Oikos.
Suárez, F., Yanes, M. & Herranz, J. 1993. Nature reserves and the conservation of Iberian shrubsteppe passerines: the paradox of nest predation. Biol. Cons. 64: 77-81.
Tarboton, W.R., Blane, S. & Lloyd, P. in press. The biology of the Yellowthroated Sandgrouse Pterocles gutturalis in a South African agricultural landscape. Ostrich.
Thomas, D.H. 1984. Adaptations of desert birds: sandgrouse (Pteroclidae) as highly successful inhabitants of Afro-Asian arid lands. J. Arid Environments 7: 157-181.
Ward, P. 1972. The functional significance of mass drinking flights by sandgrouse: Pteroclidae. Ibis 114: 533-536.
Wiens, J.A. & Johnston, R.F. 1977. Adaptive correlates of granivory in birds. In: Pinowski, J. and Kendeigh, S.C. (eds) Granivorous Birds in ecosystems: 301-340. Cambridge: Cambridge University Press.
Wilcove, D.S. 1985. Nest predation in forest tracts and the decline of migratory songbirds. Ecology 66: 1211-1214.
Yanes, M. & Suárez, F. 1995. Nest predation patterns in ground-nesting passerines on the Iberian Peninsula. Ecography 18: 423-428.
Table 1. Daily nest predation rates compared among resident and nomadic ground-nesting species. D = Droëgrond, K = Kalahari Gemsbok National Park. Where daily nest predation rates on a species were calculated separately for the two sites, the mean is given on the third line. Table reprinted from Lloyd (in review).

Table 2. Summary of nesting data for Namaqua Sandgrouse at Droëgrond and the Kalahari Gemsbok National Park (KGNP). Whole nest losses include losses due to predation and losses due to other causes (in brackets). Rainfall for the 12-month period (July-June) before the breeding season and for the six-month period (July-Dec) during the breeding season, is also indicated. Table reprinted from Lloyd et al. (in review b).
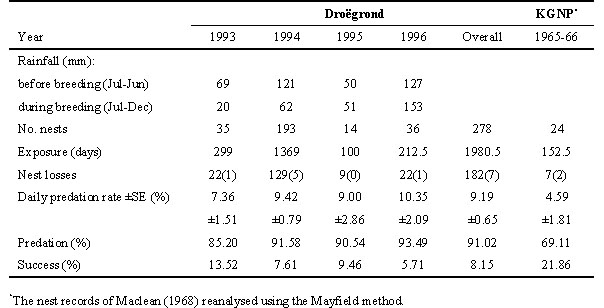
Table 3. Estimated annual sandgrouse recruitment from monthly adult:juvenile ratios in waterhole counts (Yellowthroated Sandgrouse = YTS; from Tarboton et al. in press) or from monthly belly-soaking frequencies (Namaqua Sandgrouse = NS; see methods) using the assumption that juveniles are distinguishable from adults for either one month (Recruitment 1) or two months (Recruitment 2). KGNP = Kalahari Gemsbok National Park. Table reprinted from Lloyd et al. (in review b).
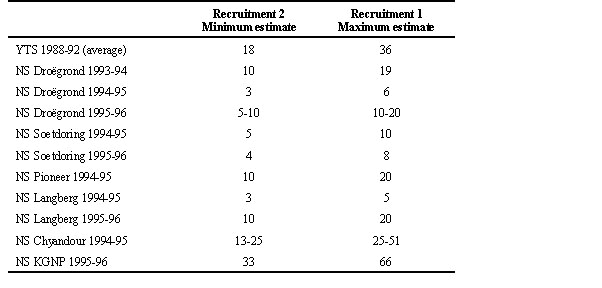
Fig. 1. Monthly nest records for Namaqua Sandgrouse (bars) and an approximation of relative mean monthly rainfall (line; derived from Fig. 15 in Harrison et al. 1997), to indicate rainfall seasonality, in different regions in southern Africa.
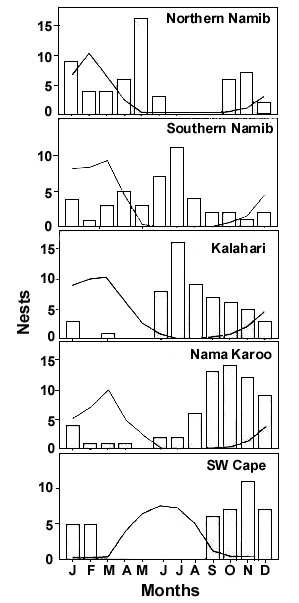
Fig. 2. Seasonal changes in the daily nest predation rate (averaged per week) on a) Namaqua Sandgrouse at Droëgrond farm in 1994 (n = 129 predation events during 1369 nest days; Lloyd et al. in review b), and b) a variety of bird species at Droëgrond farm in 1996 (n = 331 predation events during 5081 nest days; Lloyd in review). Regression a) F1,12 = 19.57, P < 0.001; b) F1,9 = 11.53, P < 0.01. Figure reprinted from Lloyd et al. (in review d).
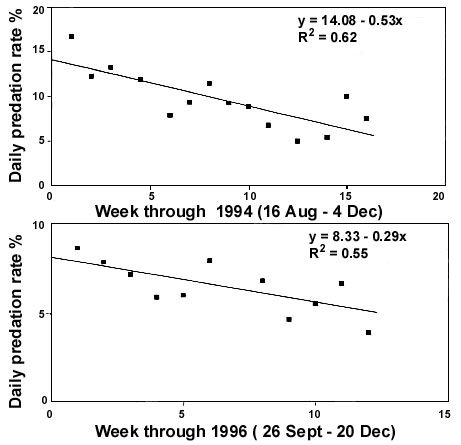
Fig. 3. The relationship between nest density and daily predation rate (for all species combined) through the 1996 breeding season at Droëgrond. Regression F9 = 11.53, P < 0.01. Figure reprinted from Lloyd (in review).