S34.5: Mechanisms of prey-patch detection by foraging seabirds
Gabrielle Nevitt1 & Richard Veit2
1Section of Neurobiology, Physiology and Behaviour, University of California, Davis, California, 95616, USA, fax 530 752 5582, e-mail ganevitt@ucdavis.edu; 2Biology Department, The College of Staten Island, 2800 Victory Boulevard, Staten Island, NY 10314, USA, e-mail veit@postbox.csi.cuny.edu
Nevitt, G. & Veit, R.R. 1999. Mechanisms of prey-patch detection by foraging seabirds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2072-2082. Johannesburg: BirdLife South Africa.Foraging seabirds are met with a daunting behavioural challenge to find highly patchily distributed food resources in the ocean. Seabirds have adapted to this challenge in a variety of ways, from developing sophisticated olfactory abilities for detecting prey, to using visually mediated search strategies that focus activity in areas where prey is likely to be found. This paper discusses the variety of work that has been accomplished to date using behavioural analysis to understand how seabirds locate their prey at both large and small scales. The methods employed in these studies include experimental manipulation, behavioural observation and computer simulation. In the context of large-scale foraging, we first summarise experimental evidence showing that procellariiform seabirds are sensitive to a wide range of olfactory stimulants including aromatics associated with krill, fish and phytoplankton. From these results, we suggest that some species may be able to identify foraging hotspots in part by using an ‘olfactory landscape’ superimposed upon the ocean’s surface. We then explore two possible small-scale search tactics: Area-Restricted Search (ARS) and Local Enhancement (LE). ARS is a method of restricting one’s search so that the likelihood of prey encounter is high whereas LE uses other foraging seabirds, or other marine predators, as cues for locating prey. Finally, we suggest that a more thorough understanding of the mechanisms that seabirds use to find patchily distributed prey resources is needed to develop effective methods of management and conservation for the future.
INTRODUCTION
The study of pelagic birds at sea increased dramatically in the 1970’s and since then analysis of shipboard data has focused on drawing inferences from patterns of spatial variation in abundance. It is the goal of this paper to describe how birds at sea can be studied to relate their behaviour to the spatial variation in the abundance of their prey. There are few published studies that have tried to quantify seabird behaviour on the basis of data collected from ships; we hope to inspire further work of this sort with this paper.
We begin by suggesting two important reasons why behaviour at sea needs to be researched in greater detail. First, in a comparative framework, pelagic seabirds are physiologically exceptional - they drink salt water, dive to remarkable depths, remain at sea for years on end, and some species (the procellariiforms) have developed an extraordinary sense of smell. Pelagic birds search for patches of prey that are sparsely and unpredictably spread out (e.g. Furness & Camphuysen 1997), so it is likely that selection for improving the efficiency of foraging has been strong. Second, it is becoming more and more evident that populations of seabirds throughout the world are threatened by man’s activities. There is increasing need to forecast how each species’ predicament may worsen, given climate change or resource depletion resulting from over-fishing or other human actions. Clearly, the simple existence of a prey stock is not sufficient to assure that birds that depend on that stock will fare well; birds must also be able to detect and locate prey with great efficiency to insure survival and reproduction. We would like, for example, to be able to predict the fate of a population of seabirds, given differing assessments of future stock sizes. To make such predictions, we have to be able to quantifiably link feeding success to prey stock size. We cannot accomplish this goal without understanding the details of how birds forage at sea.
While studying the behaviour of pelagic birds at sea presents formidable logistic problems, there are also advantages that render this system well suited to the analysis of foraging behaviour. Seabirds can be viewed without obstruction from the deck of a ship, and the distribution of their prey can be mapped continuously using acoustics or continuous plankton recorders. The apparent simplicity of the ocean environment thus presents a rare opportunity to match behavioural decisions with the geometric arrangement of resources within that environment.
Our objective here is to review the mechanisms of how pelagic seabirds find prey. The investigations we discuss combine controlled behavioural experimentation performed at sea with statistical analysis of seabird distribution patterns relative to prey abundance, as well as computer simulation. This paper is a preliminary analysis of the work that has been accomplished to date and suggests a need for future work in this area.
Large-scale foraging decisions: odour cues and landscapes
Work in this area has focused on experimental at-sea studies of procellariiform seabirds. Members of this group have among the largest olfactory bulbs of any bird, suggesting that olfaction plays a fundamental role in their life history and behaviour (Bang 1965; 1966; 1971; Minguez 1997). Results from several behavioural studies suggest that a pronounced sense of smell assists in foraging. For example, many species are attracted to fishy smelling odorants (e.g. cod liver oil, tuna oil or fish homogenate), suggesting that procellariiforms use their sense of smell to locate food sources (Grubb 1972; Hutchison & Wenzel 1980; Lequette et al. 1989; Verheyden & Jouventin 1994; Nevitt & Hunt 1996). However, it is still not known which natural aromatics are used to hunt for food under normal foraging conditions.
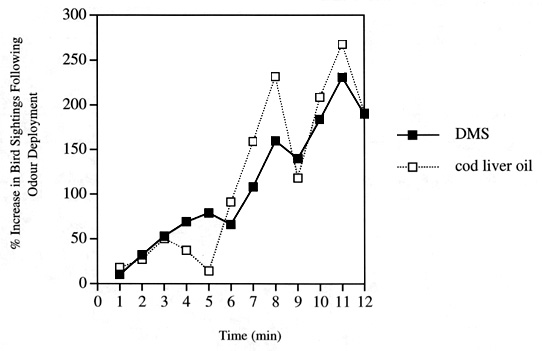
We have recently presented experimental evidence that many species within this group are highly attracted to a naturally occurring scented compound, dimethyl sulphide (DMS; Nevitt et al. 1995; Nevitt & Hunt 1996; Fig. 1). Marine DMS is a by-product of the metabolic decomposition of dimethyl sulphoniopropionate (DMSP) in marine phytoplankton (most notably, Phaeocystis pouchetii). Laboratory studies indicate that this process is dramatically accelerated during grazing by zooplankton (Dacey & Wakeham 1986; Kellor et al. 1989; Daly & DiTullio 1996). The ability to detect and recognise DMS as a feeding cue could thus be advantageous to a seabird trying to locate and exploit zooplankton-rich areas, especially as natural DMS emissions are not strictly ephemeral, but can persist for hours or even several days (Berressheim 1987). Since DMS tends to be high in areas where productivity is also high, this presents the possibility that birds may use natural odour landscapes to locate areas where food tends to be historically abundant (McTaggart & Burton 1992). Indeed, peak numbers of surface-foraging prions Pachyptila sp. have recently been reported to coincide with peaks in zooplankton abundance near Bird Island (Goss et al. 1997). These birds also respond to DMS in experimental trials (Nevitt et al. 1995). In addition our preliminary results correlating at-sea measurements of species densities with changing DMS concentrations suggest that members of this group are abundant in areas where DMS is naturally elevated (Nevitt, unpublished).
Investigations with DMS suggest that procellariiforms may use this odour to identify a general area where prey is likely to be found, but can birds smell prey such as Antarctic Krill Euphausia superba directly? If so, is there a species-specific attraction to krill aromatics as there appears to be for DMS? Finally, could a species-specific difference in olfactory responsiveness reflect differences in diet or foraging strategies? In terms of what we know, both whole krill homogenates and component aromatics derived from krill, including trimethylamine (TMA) and pyrazene (PYR), appear to attract Leach’s Storm-petrels Oceanodroma leucorhoa in breeding colonies on Kent Island, New Brunswick (Clark & Shah 1992), but little information is available for Antarctic species. Results from pilot experiments suggest that some Antarctic procellariiform species are highly attracted to surface oil slicks spiked with krill homogenate but other species respond indifferently to krill odours. Cape Petrels Daption capense, for example, responded five times as much to experimental vegetable oil slicks scented with krill homogenate as compared to plain vegetable oil slicks, whereas Wilson's and Black-bellied Storm-petrels (Oceanites oceanicus and Fregetta tropica) showed no such difference (Nevitt in press). These distinctive olfactory responses may indeed reflect differences in foraging strategies since storm-petrels showed highly significant responses to DMS-scented slicks whereas Cape Petrels did not (Nevitt et al. 1995). Pilot trials suggest that PYR and TMA also attract foraging Antarctic procellariiforms, but species-specific responses have not yet been addressed.
It is likely that procellariiforms use odour cues in at least two distinct types of foraging activity. As has been commonly noted, birds may zigzag upwind to focus activity near the source of an odour plume (e.g. Hutchison & Wenzel 1980; see below). Alternatively, tubenoses may use ‘odour landscapes’ to identify general regions where prey is likely to be found (Nevitt et al.1995). This behaviour does not imply that birds follow odour gradients to locate a likely feeding target, but that potentially rich feeding grounds share an olfactory signature that the long distance forager recognises upon arrival. Elevated sea and atmospheric levels of DMS are known to be associated with shelf breaks, seamounts and areas of general upwelling (McTaggart & Burton 1992). These localities represent areas of high primary productivity where many species of procellariiform seabirds are known to forage. Odour cues associated with these regions would logically provide birds with a direct and instantaneous means of assessing the potential productivity of such historically rich areas. Such site-specific olfactory cues may also trigger a behavioural bias or ‘switch’ in long distance foragers to engage upon arrival in visual search strategies aimed at pinpointing feeding activity by other seabirds and marine mammals.
Small-scale foraging decisions: visual location of prey
For visual searches, we have explored two possible search tactics: ‘Area-Restricted Searches’ (ARS) and ‘Local Enhancement’ (LE). ARS is a method of restricting one’s search to areas where the likelihood of prey encounter is high (e.g. Kareiva & Odell 1987). LE uses other foraging seabirds or marine predators as cues for locating prey (Bayer 1983; Clark & Mangel 1984; Brown 1988; Buckley 1996).
Area-restricted search
ARS is one of the simplest search modes. It only requires that a bird can distinguish between areas of high and low prey density within a local region. The basic hypothesis is that once a bird detects prey, it increases its turning rate (Fig. 2). This action, changing from a straight-line flight to a series of course changes, has the consequence of keeping the forager within the vicinity of the prey. The sensory cues that trigger this switch from a straight-line flight to ARS need to be determined but probably involve a combination of olfactory, visual and probably other inputs. We have not yet explored ARS in the context of olfaction so the discussion here will focus on visual ARS.
Despite its simplicity, ARS can be effective, in the sense that it can result in aggregations of foragers around patches of prey. Most demonstrations of ARS in the wild have been for insects (e.g. Kareiva & Odell 1987). However, the use of ARS has also been described in thrushes (Smith 1974) and some species of pelagic birds (Veit et al. 1994; Nevitt et al. 1995; Veit & Prince 1997). ARS is relatively simple to monitor at sea. Observers, blind to the amount of prey in the area, target a bird with binoculars and record each time a bird turns by ~90 degrees or more over a given period of time. Each turning event is entered into a laptop computer and thus receives a time-stamp for when it occurred. The length of time between turns is called a ‘step-length’ and serves as a reflection of how tightly a bird mills around a given area (Fig. 2).
Evidence for the use of ARS by seabirds has been accumulating from the Antarctic and Subantarctic. Cape Petrels at South Georgia change their flight directions five times as often when near a krill swarm than when over krill-less water (Veit et al. 1994; Veit & Prince 1997; Fig. 3). Similarly, giant-petrels increase their turning rate by a factor of five over krill swarms, and White-chinned Petrels turn significantly more when presented with DMS aerosols (Nevitt et al. 1995). However, ARS by albatrosses occurs at a much larger spatial scale: with these birds, long periods of straight-line flight are interspersed with periods of milling (Prince et al. 1992; Veit & Prince 1997). These large-scale ARS patterns may be of particular value for using seabirds as monitors of krill or other prey: as a consequence, birds travel farther when prey stocks are scarce than when they are plentiful, resulting in lowered densities of birds at sea.
Local enhancement
It has been evident for years (Collins 1884; Murphy 1936) that seabirds use each other as cues for locating food. However, pelagic seabird biologists need to work hard to dispel the folklore (birds of a feather..) that pelagic birds search for food as co-ordinated groups. Most seabirds search individually, while carefully monitoring the actions of others. There have been many detailed descriptions of this process (e.g. Hoffman et al. 1980; Porter & Sealy 1982; Bayer 1983; Grover & Olla 1983; Chilton & Sealy 1987; Harrison et al. 1991, Mahon et al. 1992, Silverman & Nevitt 1995), but more effort is needed to quantify the potential benefits (Fraser et al. 1989). We do not wish to imply that hunting in flocks never occurs and carries no advantages to foraging seabirds. Gotmark et al. (1986) have shown in a laboratory setting how capture success of individual gulls is higher when those individuals are part of a flock. Individuals in flocks benefit from prey (fish) being simultaneously startled by many predators at once. This kind of benefit may well be important to seabirds, but it is different from a benefit arising from the combined searches of numerous individuals.
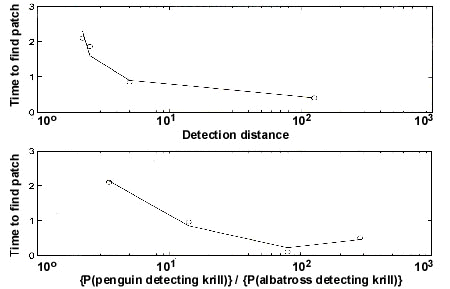
The quantitative evidence suggesting a benefit of LE is mostly theoretical (Haney et al. 1992; Bretagnolle 1993). We have recently simulated foraging trajectories of seabirds over computer-generated krill swarms (Veit & Barnes, unpublished; Meir 1997). Using simulated birds that each have their own flight speed, radius of prey detection and probability of detecting a patch within the radius, we found that LE would be beneficial under a particular set of circumstances (Fig. 4). We hypothesised that one reason LE should be beneficial is that other feeding birds should be visible from a much greater horizontal distance than are swarms of krill, or whatever prey the seabird seeks. So we simulated albatrosses searching for krill patches, and varied in each simulation the farthest distance at which an albatross could detect a flock of foraging birds. This distance was expressed in terms of a multiple of the distance at which an albatross could detect a krill patch directly. Our simulation showed that, for albatrosses to benefit from LE, flocks of foraging birds ought to be visible from at least 10-100 times farther away than are krill patches (Fig. 4A). We also have begun to identify conditions that would benefit an albatross if it searched for flocks of penguins that have located krill, rather than searching for krill directly. Our simulations suggest that albatrosses will benefit by searching for penguins only if penguins are 5-10 times more likely to detect a krill patch than they are (Fig. 4B). This may be true, given the much greater depth-range exploited by penguins (Ancel et al. 1992), but more work needs to address the underlying sensory capabilities of these seabirds before any firm conclusions can be drawn.
Perhaps more importantly, if birds do gain by monitoring the foraging success of other birds, fish, and mammals, this suggests a mutualistic relationship that is likely to have consequences at a population level. That is, it stands to reason that if individual seabirds catch more food when watching the actions of others, then reproductive success is likely, within a range of values, to be positively related to population size. Thus it is possible that, below some threshold, reproductive success will be lowered due to the difficulty that lone birds have in locating prey swarms. There is evidence that spatial distributions of species at sea reflect a mutual association: in the South Atlantic, abundance relationships among species that share the same habitats are generally positive (Veit 1995). This pattern would be expected if species ‘used’ each other to locate prey, to mutual benefit (e.g. Hunt et al. 1992; Silverman & Nevitt 1995). Clearly these and the other behavioural tactics reviewed here need to be evaluated when devising conservation recommendations for marine birds. This paper thus stands as a plea that more attention be focused in this area to insure effective management and conservation for the future.
REFERENCES
Ancel, A., Kooyman, G.L., Ponganis, P.J., Gendner, J.P., Lignon, J., Mestre, X., Huin, N., Thorson, P.H., Robisson P. & LeMaho, Y. 1992. Foraging behaviour of emperor penguins as a resource detector in winter and summer. Nature 360: 336-338.
Bang, G.B. 1965. Anatomical adaptations for olfaction in the Snow Petrel. Nature 205: 513-515.
Bang, G.B. 1966. The olfactory apparatus of the tube-nosed birds (Procellariiforms). Acta Anatomica 65: 391-415.
Bang, G.B. 1971. Functional anatomy of the olfactory system in 23 orders of birds. Acta Anatomica Suppl. 58: 1-76.
Bayer, R.D. 1983. Black-legged Kittiwake feeding flocks in Alaska: Selfish/Reciprocal-altruistic flocks? Journal of Field Ornithology 54: 196-199.
Beressheim, H. 1987. Biogenic sulfur emissions from the Subantarctic and Antarctic oceans. Journal ofcGeophysical Research. 92: 13, 245-13,262.
Bretagnolle, V. 1993. Adaptive significance of seabird coloration: the case of Procellariiforms. American Naturalist 142: 141-173.
Brown, C.R. 1988. Social foraging in cliff swallows: local enhancement, risk sensitivity, competition and avoidance of predators. Animal Behaviour 36: 780-792.
Buckley, N.J. 1996. Food finding and the influence of information, local enhancement, and communal roosting on foraging success of North American Vultures. Auk 113: 473-488.
Chilton, G. & Sealy, S.G. 1987. Species roles in mixed-species feeding flocks of seabirds. Journal of Field Ornithology 58: 456-463.
Clark, C.W. & Mangel. M. 1984. Foraging and flocking strategies: information in an uncertain environment. American Naturalist 123: 626-641.
Clark, L. & Shah, P. S. 1992. Information content of odour plumes: What do Leach’s Storm-petrels know? In: Doty, R.L. & Muller-Schwarze, D. (eds.) Chemical Signals in Vertebrates VI., New York; Plenum Press. pp. 421-427.
Chilton, G. & Sealy, S.G. 1987. Species roles in mixed-species feeding flocks of seabirds. Journal of Field Ornithology 58: 456-463.
Collins, J.W. 1884. Notes on the habits and methods of capture of various species of seabirds that occur on the fishing banks off the eastern coast of North America, and which are used as bait for catching codfish by New England fishermen. Annual Report to the Commissioner of Fish and Fisheries for 1882. Government Printing Office; Washington, D.C. pp 311-335.
Dacey, J.W.H. & Wakeham, S.G. 1986. Oceanic dimethyl sulfide: Production during zooplankton grazing on phytoplankton. Science 233: 1314-1316.
Daly, K.L. & DiTullio, G.R. 1996. Particulate dimethylsulfoniopropionate removal and dimethyl sulfide production by zooplankton in the Southern Ocean. In: Kiene, R.P., Visscher, P.T., Kellor, M.D. & Kirst. G.O. (eds.) Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds. New York: Plenum Press
Fraser, W.R., Pitman, R.L. & Ainley, D.G. 1989. Seabird and fur seal responses to vertically migrating winter krill swarms in Antarctica. Polar Biology 10: 37-41.
Furness, RW. & Camphuysen, C.J. 1997. Seabirds as monitors of the marine environment. ICES Journal of Marine Science 54: 726-737.
Goss, C., Bone, D.G., Peck, P.M. Everson, I., Hunt, G.L. & Murray, A.W.A. 1997. Small-scale interactions between prions Pachyptila spp. and their zooplankton prey at an inshore site near Bird Island, South Georgia. Marine Ecology Progress Series 154: 41-51.
Gotmark, F., Winkler, D.W. & Andersson, M. 1986. Flock-feeding on fish schools increases individual success in gulls. Nature 319: 589-591.
Grover, J.J. & Olla, B.L. 1983. The role of Rhinoceros Auklets (Cerorhinca monocerata) in mixed species feeding assemblages of seabirds in the Strait of Juan de Fuca, Washington Auk 100: 979-982.
Grubb, T.C. 1972. Smelling and foraging in petrels and shearwaters. Nature 237: 404-405.
Haney, J. C., Fristrup, K. M. & Lee, D. S. 1992. Geometry of visual recruitment by seabirds to ephemeral foraging flocks. Ornis Scandinavica 23: 49-62.
Harrison, N.M., Whitehouse, M.J., Heinemann, D., Prince, P.A. Hunt, G.L. Jr., &. Veit, R.R. 1991. Observations of multispecies seabird flocks around South Georgia. Auk 108: 801-810.
Hoffman, W., Heinemann, D. & Wiens, J.A. 1981. The ecology of seabird feeding flocks in Alaska. Auk 98: 437-456.
Hunt, G.L. , Heinemann, D. & Everson, I. 1992. Distributions and predator-prey interactions of Macaroni Penguins, Antarctic fur seals, and Antarctic krill near Bird Island, South Georgia. Marine Ecology Progress Series 86: 15-30.
Hutchison, L.V. & Wenzel, B.M. 1980. Olfactory guidance in foraging by Procellariiforms. Condor 82: 314-319.
Kareiva, P. & Odell, G. 1987. Swarms of predators exhibit ‘preytaxis’ if individual predators use area-restricted search. American Naturalist 130: 233-270.
Kellor, M.D., Bellows, W.K. & Guillard, R.R. 1989. Dimethyl sulfide production in marine phytoplankton. In: Saltzman, E.S. & Cooper, W.J. (eds) Biogenic Sulfur in the Environment. Washington, D.C.: American Chemical Society: 167-182
Lequette, B., Verheyden, C. & Jouventin, P. 1989. Olfaction in Subantarctic seabirds: Its phylogenetic and ecological significance. Condor 91: 732-735.
Mahon, T.E., Kaiser, G.W. & Burger, A.E. 1992. The role of Marbled Murrelets in mixed-species feeding flocks in British Columbia. Wilson Bulletin 104: 738-743.
McTaggart, A.R. & Burton, H. 1992. Dimethyl sulfide concentrations in the surface waters of the Australian Antarctic and Subantarctic oceans during an Austral summer. J. Geophys. Res. 97 (C9): 14407-14412.
Meir, E. 1997. Ecobeaker: Software for ecological modelling. Sinauer, New York.
Minguez, E. 1997. Olfactory nest recognition by British Storm-petrel chicks. Animal Behaviour 5(4): 701-707.
Murphy, R.C. 1936. The Oceanic Birds of South America. New York; American Museum of Natural History
Nevitt, G.A. Olfactory foraging in Antarctic procellariiform seabirds: A species-specific attraction to krill odours. Marine Ecology Progress Serices: in press.
Nevitt, G.A., Veit, R.R. & Kareiva, P.M. 1995. Dimethyl sulphide as a foraging cue for Antarctic Procellariiform seabirds. Nature 376: 680-682.
Nevitt, G.A. & Hunt, G.L. 1996. Olfactory sensitivities of foraging procellariid seabirds in the Aleutian Islands. Chem. Senses, 21 (5): 649-650.
Porter, J.M. & Sealy, S.G. 1982. Dynamics of seabird multispecies feeding flocks: age-related feeding behaviour. Behaviour 81: 91-109.
Prince, P.A., Wood, A.G., Barton, T. & Croxall, J.P. 1992. Satellite tracking of Wandering Albatrosses (Diomedea exulans) in the South Atlantic. Antarctic Science 4: 31-36.
Silverman E. & Nevitt, G.A. 1995. Evidence for network foraging by Antarctic seabirds. Antarctic Journal of the United States 30: 186-187.
Smith, J.N.M. 1974. The food searching behaviour of two European thrushes. II. The adaptiveness of the search patterns. Behaviour 49: 1-61.
Veit, R.R., Silverman, E.D. & Everson, I. 1993. Aggregation patterns of pelagic predators and their principle prey, Antarctic Krill, near South Georgia. Journal of Animal Ecology 62: 551-564.
Veit, R.R., Silverman, E.D., Hewitt, R.P. & Demer, D.A. 1994. Spatial and behavioural responses by foraging seabirds to Antarctic krill swarms. Antarctic Journal of the United States.S. 29: 164-166.
Veit, R.R. 1995. Pelagic communities of seabirds in the South Atlantic Ocean. Ibis 137: 1-10.
Veit, R.R. & Prince, P.A. 1997. Individual and population level dispersal of Black-browed and Grey-headed Albatrosses in response to Antarctic krill. Ardea 85: 129-134.
Verheyden, C. & Jouventin, P. 1994. Olfactory behaviour of foraging Procellariiforms. Auk 111: 285-291.
Fig. 1. Experimental data from olfactory trials performed at sea near South Georgia. This graph illustrates the remarkably similar olfactory responses of Wilson’s Storm-petrels to DMS (black squares) and cod liver oil (white squares). While the ship was stationed, either a control, DMS, or cod liver oil scented slick was deployed off the stern. A team of two observers blind to the treatments being tested entered numbers, behaviours and species of birds into handheld computers. Observations were made every minute starting at two minutes before deployment. Data are combined from 10 paired trials for DMS and 6 paired trials for cod liver oil and are normalised to the numbers of birds present in the visual area before slicks were deployed. Responses to control slicks have been subtracted (from Nevitt et al. 1995).

Fig. 2. Step-length as an indicator of prey distribution. We define 'step-length' as the time between successive direction changes. Thus, long step-lengths reflect few turns and short step-lengths indicate many turns. Our prediction is that step-length should decrease over prey-patches (depicted as 'P'). Details about data collection are included in the text.
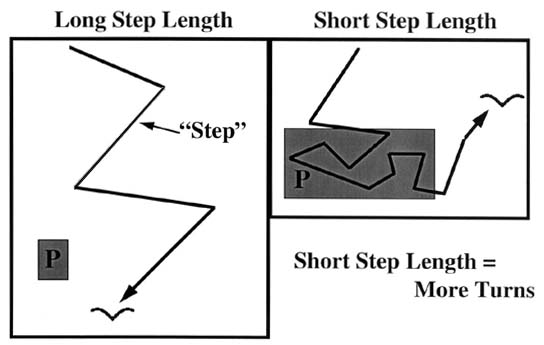
Fig. 3. Step-length of Cape Petrels in relation to krill abundance. Cape Petrels turned more frequently when close to krill patches. Data were collected off South Georgia in June 1993. Details about data collection are included in the text.
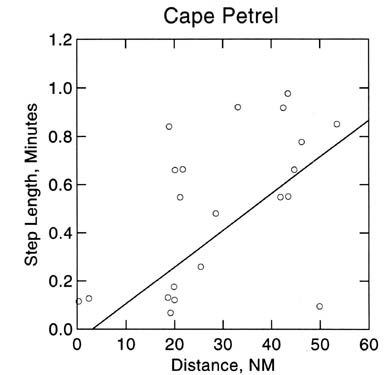
Fig. 4. Results from simulations. The y-axis on each graph represents the time required for an albatross to find a krill patch. (A) This simulation plots the detection distance relative to the time needed to find a patch of krill. The simulation involved only albatrosses. The units on the axis are arbitrary. (B) This simulation explores the interaction between albatrosses and penguins. The plot implies that albatrosses should search for penguins instead of directly searching for krill patches only if penguins are better than albatrosses at finding krill. The units on the axes are arbitrary.