S34.1: Areas and scales of interactions between albatrosses and the marine environment: Species, populations and sexes
1
Prince, P.A., 2Weimerskirch, H., 1Wood, A.G. & 1Croxall, J.P. 1British Antarctic Survey, Natural Environment Research Council, High Cross, Madingley Road, Cambridge CB3 0ET, UK, fax 44 1223 221259, email j.croxall@bas.ac.uk; 2Centre National de la Recherche Scientifique, Centre d’Etudes Biologiques de Chizé, 79360 Beauvoir-sur-Niort, France.Prince, P.A., Weimerskirch, H., Wood, A.G. & Croxall, J.P. 1999. Areas and scales of interactions between albatrosses and the marine environment: Species, populations and sexes. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2001-2020. Johannesburg: BirdLife South Africa.
Until recently, little has been known about how albatrosses organise their time at sea. Satellite tracking studies of albatrosses, coupled with the use of activity recorders, are providing unique data on the scales of interactions between albatrosses and the marine habitat, ranging from the total realised annual home range to favoured locations for feeding. Home ranges may differ between species by orders-of-magnitude, as exemplified by data for Black-browed, Grey-headed and Wandering Albatrosses, Diomedea melanophrys, D. chrysostoma and D. exulans, breeding at South Georgia (South Atlantic Ocean), Iles Crozet and Kerguelen (South Indian Ocean). For Wandering Albatrosses, we also examine seasonal variation in the overall ranges and restricted foraging areas for birds of different sexes and populations. The data for the three species illustrate the diversity of spatial scales of interaction and also the variation in the nature of features of the marine environment targeted by a single seabird species. These range from shelf and shelf-slope, to frontal systems to basin-wide searches using quasi-random movements. The resulting patterns are reviewed in terms of the foraging strategies of albatrosses, and in relation to their conservation implications, particularly in respect of interactions with fisheries.
INTRODUCTION
Seabirds are typical central place foragers, breeding on land and foraging for marine resources, often at great distances. Among seabirds albatrosses are extreme, with the traditional view of a group of large, long-lived seabirds of rather homogeneous biology and ecology, foraging by covering vast distances and ranging widely over large marine ecosystems. Direct at-sea observations from surveys and ships of opportunities has allowed some characterisation of the distribution of albatross at sea but generally only at the broader scales. The rarity of observing albatrosses actually feeding and the propensity of many species for following ships has hindered better understanding of foraging patterns and habitat requirements. Indeed, land-based studies of provisioning of chicks probably provided as much insight into at-sea activities, by showing that sympatric albatross species often had quite different diets and distinctive patterns of foraging trip duration (Croxall & Prince 1980, Weimerskirch et al. 1986). However the main problem of all such studies is that the origin and status of birds observed at sea was unknown. This is fundamental when trying to relate life-history traits to the marine environment exploited by breeding seabirds because reproduction imposes extensive constraints on seabirds, especially the continuous return trips between breeding grounds and feeding zones at sea. The availability and distribution of prey species as well as the distance and foraging costs, together with the ability to transport energy, are of prime importance.
The ability to carry out individual-based tracking studies (using satellite platform terminal transmitters (PTTs)) has revolutionised knowledge of the distribution and foraging ecology of albatrosses since 1989 when the first PTTs were fitted on wandering albatrosses (Jouventin & Weimerskirch 1990). All aspects of interactions between albatrosses and their marine environment can be investigated. While important data on distribution (inferentially highly relevant to feeding) can be obtained using PTTs on their own, linking such information to studies of diet and provisioning, including of various types of activity recorders, has provided some of the most detailed data on the foraging patterns of any group of pelagic seabirds.
In this paper we illustrate aspects of the diversity of foraging patterns of albatrosses, principally by comparing two of the most widely distributed and distinctive species, Black-browed Albatross Diomedea melanophris and Wandering Albatross D. exulans chionoptera (with additional data mainly from Grey-headed Albatross D. chrysostoma). We also use these species, especially Wandering Albatross, to examine variation in foraging patterns in relation to site, season and sex, mainly using data from studies at South Georgia in the South Atlantic Ocean and Iles Crozet and Kerguelen in the South Indian Ocean.
METHODS AND BACKGROUND
Sites
The location and surrounding marine environment of South Georgia, Kerguelen and Crozet indicates their similarities in respect of being isolated oceanic islands with small continental shelves. At a very general level the physical oceanography of the South Atlantic and South Indian Ocean sectors of the Southern Ocean is fairly similar. Thus a north-south transect across these regions shows in sequence the Subtropical Front (with sea surface temperatures of 14-18 °C), the Subantarctic Front (sea surface temperatures 7-9 °C) and Polar Front (sea surface temperatures 4-5 °C), separated by a Transitional Frontal Zone and the Antarctic Polar Frontal Zone respectively. The location, width and definition of these features, however, varies considerably between the two sectors. Thus South Georgia lie south of the Antarctic Polar Frontal Zone (APFZ), which is rather strongly delimited in the South Georgia region while Kerguelen lies just on the APFZ. In contrast Crozet lies to the north of the APFZ and in a region where it is less well developed. Furthermore, the waters around South Georgia are characteristically rich in Antarctic krill Euphausia superba, whereas euphausiids generally (and E. superba in particular) are less dominant around the Indian Ocean islands, being replaced by a greater diversity of crustacean species. In both areas, however, a characteristic fauna of lanternfish (Myctophidae) and squid are associated with the APFZ.
Species
Some important features of the breeding biology and diet of the main study species are summarised in Table 1. Wandering Albatrosses breed biennially if successful, the annual cycle starting in December with chicks fledging one year later, having been reared throughout the subantarctic winter. The species is markedly sexually dimorphic with males some 20% larger than females.
Grey-headed and Black-browed Albatrosses are both of similar size (half the mass of Wandering Albatross), sexually monomorphic and complete their breeding season in the austral summer. Laying takes place in October with Black-browed Albatross chicks fledging in April, Grey-headed Albatross chicks not until May. The one month difference is an important influence on breeding frequency, as Black-browed Albatrosses breed essentially annually, Grey-headed Albatrosses biennially if successful (Croxall et al. 1998).
Diet
Some of the main details of diet are summarised in Table 2. The diet of the Wandering Albatross is characterised by a very varied selection of squid (with cranchids, onychoteuthids and histioteuthids predominating), broadly consistent between sites (though more important to albatrosses at Crozet than fish), a selection of fish species which seem quite different between sites (and more important at South Georgia than Crozet), an absence of crustaceans and a noticeable amount of scavenged offal and carrion. Wandering Albatrosses are inveterate ship followers and some of their fish diet may also have been scavenged from behind fishing vessels. (More detailed analyses of their diet can be found in Rodhouse et al. 1987, Croxall et al. 1988a, Cooper et al. 1992, Imber 1992, Ridoux 1994, Weimerskirch et al. 1997b). They are also often observed at sea feeding on jellyfish (G.L. Hunt pers. comm.) but these are probably digested very rapidly and are rarely found in the stomach contents of adult or chicks in the colonies.
At South Georgia the diet of Black-browed Albatross is fairly evenly divided between krill, squid and fish whereas at Indian Ocean sites squid and fish predominate. Grey-headed Albatross depend mainly on squid, though fish may rival it in importance in some years. Both species have a much less varied squid diet than Wandering Albatross with the ommastrephid Martialia hyadesi predominating. Their fish diet is also quite distinct from Wandering Albatrosses with various species of Antarctic cods (Notothenidae), icefish (Channichthydae) and lanternfish (Myctophidae) predominating (further details of their diet can be found in Prince 1980, Croxall et al. 1988b, Rodhouse et al. 1990, Rodhouse & Prince 1993, Ridoux 1994, Cherel & Weimerskirch 1996, and Reid et al. 1996). Black-browed Albatrosses are persistent ship followers and may obtain substantial portions of their fish prey as offal or discards; Grey-headed Albatrosses are less commonly ship-associated.
Feeding
All three species feed by seizing prey while sitting on the water surface. Black-browed and Grey-headed Albatrosses, but not Wandering Albatross, also plunge-dive to obtain prey (Harrison et al. 1991) from depths up to 5m (Prince et al. 1994, Huin & Prince 1997). Although albatrosses have good visual acuity in fairly low light levels (Martin 1998) they probably feed mainly during daylight (including dawn and dusk). Wandering Albatrosses obtain most prey during daylight hours (Weimerskirch & Wilson 1992) probably mainly by scavenging. Black-browed and especially Grey-headed Albatrosses probably mainly take live-caught prey, particularly where squid are involved (Croxall & Prince 1994). These two species have been observed joining mixed species flocks or using subsurface foragers to force prey to the surface (Harrison et al. 1991). The distribution of foraging penguins, seals or cetacean could therefore influence the distribution of these seabirds. However the presence of fisheries discharging offal and waste within foraging ranges of the mollymawk species will undoubtedly influence their feeding patterns and diet.
Foraging
For all species, foraging trips are of long duration during incubation, typically lasting 8-12 days with extremes of up to 20 days in mollymawks and 35 days in the Wandering Albatross. During the brooding period trips are at their shortest, averaging 1-2 days in mollymawks and 3 days in Wandering Albatross. During the main chick-rearing period, the overall average duration of foraging trips by mollymawks is about 2 days whereas it is about 7-8 days in Wandering Albatross. After chicks fledge, breeding birds of all species leave the colony and are seldom seen there until the start of the next breeding season. In the case of biennial breeding species, many individuals do not return to the colony in their ‘year off’ and are therefore away for a complete year (Wandering Albatross) or 16 months (Grey-headed Albatross).
RESULTS
Black-browed Albatross
At Kerguelen, in studies during the chick-rearing period of 2 years (1994 and 1995) most Black-browed Albatross (87% of trips) commuted from the breeding colony to areas of the northern and eastern edges of the Kerguelen Shelf, on average 250km distant (Fig. 1) (Weimerskirch et al. 1997a). The travel to and from foraging areas accounted for about one-half of the duration of the foraging trip, and about two-thirds of the overall distance travelled on each trip. In 1994 (which was characterised by heavier, faster growing chicks and higher breeding success than in 1995) the northern shelf edge of a prominent bank to the north east of Heard Island, 500 km from Kerguelen, was used by some birds. In all other respects, however, there were no important differences between the foraging patterns and areas in the 2 years. The diet in both years was a mixture of squid (mainly ommastrephids) and fish, although the proportions differed between years (Cherel & Weimerskirch 1995, Weimerskirch et al. 1997a).
At South Georgia, uplinks during the chick-rearing periods of 1992 and 1993 might suggest a rather different picture from that at Kerguelen, with foraging of South Georgia birds distributed over a region stretching from the APFZ (to the north and north-east of South Georgia at about 50°S), to the South Orkney Islands shelf at 60 °S (Fig. 2). However when these data are processed to indicate those areas where most time is spent (i.e. the foraging areas, rather than the commuting tracks), then four main foraging areas are revealed (Fig. 3; Wood et al.; in press). These are:
(1) Shelf and shelf-slopes close to (within 100km) of the breeding colony. (This will also include birds which spend time in rafts on the water within sight of the colony before and after feeding chicks);
(2) Shelf and shelf-slope areas to west and north of Shag Rocks, some 200km west of Bird Island;
(3) Shelf-slope of the north-west corner (<100km north-west of Coronation Island) of the South Orkney Islands shelf, some 600km from Bird Island;
(4) Discrete areas within the APFZ c.200km to the north and north-east of Bird Island.
This general pattern was evident in both years but in 1992, when breeding success was substantially lower than in 1993, there was appreciably less foraging over the shelf, particularly that of the South Orkney Islands, and more activity in the APFZ (P.A. Prince unpublished data).
In both years fish and squid (chiefly Martialia) were the predominant prey, together with krill in 1993, though this was rare in 1992. In recent years diet sampling of satellite-tracked individuals has revealed that krill and icefish are mainly obtained from trips over the shelf-break whereas squid (and lanternfish) only appear after trips which include foraging in the APFZ (P.A. Prince, N. Huin; unpublished data). No difference between sexes in diet or foraging patterns were evident. The few data from incubating birds indicate essentially similar foraging areas.
Grey-headed Albatross
At South Georgia, Grey-headed Albatrosses were also tracked in 1992 and 1993, the same years as the main study of Black-browed Albatrosses (Fig. 4). Superficially the foraging range of Grey-headed Albatrosses (and pattern of uplinks within this) might appear very similar to that of Black-browed Albatrosses. However more detailed analysis of habitat use (Wood et al.; in press) reveals a very different situation (Fig. 5). The main differences from Black-browed Albatrosses are:
(1) very limited foraging over the shelf and shelf-break;
(2) intensive foraging in small discrete areas within the APFZ, usually at its closest proximity (c. 200-250km) to Bird Island;
(3) very intensive activity in one area of little more than 250km2, lying about 300km to the south-west
of Bird Island. This area lacks any obviously oceanographic or hydrological features.Like Black-browed Albatrosses, most trips showed strong directionality and clear separation into commuting and foraging elements, much of the latter being conducted in relatively small areas. Grey-headed Albatross were less affected by the scarcity of krill in 1992 and foraging patterns between years were very similar. In 1994, when krill was again very scarce around South Georgia (being reduced by about 90% in the diet of all predators, including the two mollymawk species; see Croxall et al. in press), Grey-headed Albatrosses foraged almost exclusively in the APFZ (see Rodhouse et al. 1996), where they took more fish (especially lanternfish) than usual and less squid (but still mainly Martialia). In no season has there been any evident difference in diet of foraging pattern between the sexes. The limited data on foraging trips during incubation indicate use of essentially similar foraging areas to those during chick-rearing.
Wandering Albatross
Although foraging in Wandering Albatross has been studied more intensively, particularly at Iles Crozet, than any other albatross species, there can be little doubt that this species shows a greater complexity and variation in foraging than the other albatross species hitherto studied using satellite tracking. The main emphasis of this section, therefore, is the variation in foraging pattern within and between different stages of the breeding cycle and, secondarily, indications of any obvious sex and site-specific differences.
Incubation
At Crozet during the first 60 days (three-quarters) of the incubation period, foraging trips are long (averaging 14 days), with birds making lengthy journeys (up to 15,000km) to areas up to 3500 km distant (Weimerskirch et al. 1993, Weimerskirch unpublished). Longer trips had significantly greater range but particularly longer distances covered. The overall foraging range is vast, lying between 32-70 °S, encompassing some 12 million km2. However, at least one bird foraged mainly over the Crozet Shelf and adjacent waters during this period. The main foraging pattern was either of quasi-continuous looping movements, which in some birds was interrupted for periods of 3-6 days while foraging was confined to a restricted area. These areas had no obvious oceanographic/hydrological features associated with them. In late incubation, foraging trip duration reduces rapidly and foraging areas and range also decrease concomitantly, until trip duration and foraging patterns resemble those of the brooding period.
Foraging ranges and patterns at South Georgia were generally similar, birds ranging between 34-68 °S, although relatively more of the foraging range of South Georgia birds was adjacent to a continental land mass, Wandering Albatross foraging never took place in neritic waters and rarely in water depths shallower than 1000m (Prince et al. 1998 and unpublished).
Brooding
At Crozet during the 3-day foraging trips at this time birds travelled only about 900km to a mean maximum range of 250km, with overall ranges encompassing some 45.000 km2. Males were essentially restricted to the neritic shelf brake around Crozet, though one bird travelled further, 1000km distant to a shelf (Weimerskirch et al. 1993). Female foraging ranges were similarly restricted, though they also included trips into nearby pelagic waters. At South Georgia overall average foraging ranges of both sexes were slightly larger than for Crozet birds but still confined to the continental shelf and nearby pelagic waters (Fig. 6). However some females, but no males, travelled nearly 1000km west to the Falkland Islands (Croxall & Prince 1996, Prince et al. 1998). In more detailed studies at South Georgia, Arnould et al. (1996) distinguished two main types of foraging trip: short ones (with total distances 400-750km) to the edge of the continental shelf (some 100km from Bird Island) and longer trips (total distances of 1300-2000km) to the APFZ (some 250km from Bird Island) (see Arnould et al. 1996). Neither at Crozet nor Bird Island were there significant relationships between foraging trip duration and range or distance travelled, in contrast to the situation during the incubation period.
Post-brooding chick-rearing
Weimerskirch et al. (1993) discovered that Crozet birds showed two distinct foraging strategies. Long trips (lasting on average 12 days) involved long distance travelling (mean 6100km, max. 15,000km) and large maximum ranges (mean 1500km, max 2600km). These trips were similar in these regards to foraging trips during the incubation period but very different in that the range was more northerly (30-52 °S) with no foraging in waters south of the APFZ and few interruptions to the looping nature of the trip. Apart from 3 males foraging over the Kerguelen Shelf, these long trips were over oceanic subantarctic and subtropical waters. Short trips lasted 2 days on average, covering distances of 500km (maximum 1300km), with ranges of only 120km (maximum 300km) (Weimerskirch et al. 1993). These trips were mainly over the shelf to the south of Possession and East Islands in the Crozet group and often involved individuals returning to the same area on several consecutive trips.
At South Georgia, the nature of overall foraging range was broadly similar, being north to 29 S and with few trips venturing south of 56 °S or across the Antarctic Circumpolar Current (Fig. 7; Prince et al. 1998). However, the distinction between long and short trips was less clear cut at South Georgia. Nevertheless trips confined to the shelf area (and environs) and/or the APFZ were interspersed with very long trips mainly to the vicinity of the Falkland Islands and South America. The latter trips remained strictly oceanic, birds rarely coming closer inshore than waters of 1000m depth (Prince et al. 1998), except for some birds in the vicinity of the Falkland Islands.
Both at Crozet and South Georgia females tended to forage over more northerly waters than males. In detailed studies (Salamolard & Weimerskirch 1993, Weimerskirch 1995, Weimerskirch et al. 1997b) it was shown that during incubation females travel greater distances and have higher foraging effort than males, as they have during the short foraging trips of the brooding period when they also make longer trips than males.
In both studies the Wandering Albatrosses were feeding on a typical mixture of squid (particularly Moroteuthis ingens from the shelf-slope area near Crozet but more typically Kondakovia and Galiteuthis around South Georgia), fish and carrion/offal.
DISCUSSION
The data acquired from satellite tracking (and concurrent biological) studies of albatrosses exemplify the revolution that has taken place in our understanding of interactions between albatrosses and their marine environment. The three species reported on here give some indication of the diversity in the family in this regard.
Thus the Black-browed Albatross appears to be a relatively straightforward example of a species principally associated with shelves and shelf-slope areas, largely neglecting oceanic waters (which offer feeding opportunities for other albatross species) in between these shelf habitats. They are typical short-distance commuters (see Weimerskirch 1998). The affinity for shelf habitat is true also of the large population at the Falkland Islands, which is apparently confined year-round to the waters of the Patagonian Shelf, and largely so for birds breeding at Campbell Island, New Zealand, which spend most time over the Campbell Plateau , though also foraging at the APFZ (Waugh et al. in press).
It is noteworthy that even in winter, when the South Georgia Black-browed Albatross population migrates to South African waters, they are largely, if not exclusively, found in the inshore waters of the Benguela and Agulhas Currents (Prince et al. 1998). Similarly Kerguelen Black-browed Albatrosses frequent the neritic shelf waters around Australia (Weimerskirch et al. 1985).
The shelf habitats involved are characterised by high biomass of shoaling fish species (and also crustaceans around the Falklands and South Georgia). Not surprisingly epipelagic fish and crustaceans, where available, become the main prey of Black-browed Albatrosses in these habitats. Although some squid occur (and are also taken) over shelves, such species are more common in the diet of Black-browed Albatrosses when they venture to the APFZ. At least during the chick-rearing period, such options, including travel to shelves >500km distant from the breeding site, appear to be chiefly available to birds breeding at South Georgia.
The area of shelf surrounding breeding sites of Black-browed Albatrosses may also substantially effect the size of breeding population that can be sustained (Weimerskirch et al. 1989). Thus 85% of the world population breeds at the Falkland Islands and is associated with the vast area of the Patagonian Shelf. Another 10% of the world population depends on the relatively small South Georgia-Shag Rock shelf area with only a few thousand pairs associated with the much smaller shelves in the vicinity of Kerguelen and very small populations at Crozet where the shelf is very small. All these shelf systems are also exploited by commercial fin-fisheries (and also krill fisheries at South Georgia) and these may provide Black-browed Albatross with substantial opportunities for feeding on offal, waste and discards (Thompson 1992, Thompson & Riddy 1995). However the link between Black-browed Albatross and shelf systems is clearly independent of the presence of fisheries. At Kerguelen Black-browed Albatrosses forage from one year to the next in the same area where a fishery takes place some year (Weimerskirch 1997). In several recent years of satellite tracking at South Georgia no commercial fishing was taking place in the vicinity. Similarly no fisheries operate at present on the South Orkney Island shelves. We can be confident, therefore, that although Black-browed Albatrosses doubtless exploit fishing vessels wherever they can locate them, their basic feeding habitat is that of the oceanic (and continental off South America and southern Africa) shelves and shelf breaks.
In contrast, the Grey-headed Albatross, although of similar size to Black-browed Albatross and sympatric with it at many breeding sites, requires a very different balance of foraging habitat. This species, at least while breeding, is particularly closely associated with the APFZ, where it preys on species also characteristically abundant in this habitat, like lanternfish and ommastrephid squid, especially Martialia hyadesi (Cherel et al. 1996, Rodhouse et al. 1996, Guinet et al. 1997). Shelf habitats are also exploited, especially for icefish and crustaceans (at South Georgia, at least) but to a much lesser extent than the Frontal Zone. Even within this zone, however, preferred foraging areas may still be very localised. Rodhouse et al. (1996) suggest that the aggregations of Martialia hyadesi (on which Grey-headed Albatrosses were feeding) were within warm core rings in the APFZ and that these may reflect locally favourable conditions for the existence of high densities of myctophid fish and ommastrephid squid in this zone. This is also consistent with other observations of similar mesoscale features, in particular eddy structures at fronts, which favour aggregations of certain types of squid and lanternfish (see Rodhouse et al., 1996).
Thus of these two sympatric congeneric mollymawks, one chiefly exploits the shelf system characterised by epipelagic prey, the other an adjacent system characterised by the absence of epipelagic fish and the presence of squid as the dominant top predators. We have insufficient information about the distribution of Grey-headed Albatrosses at other times of year to indicate whether or not it remains associated with the Antarctic Polar Frontal Zone and/or similar associations of pelagic prey.
The Wandering Albatross stands considerably apart from the two mollymawks in the scale, diversity and apparent complexity of the foraging patterns it reveals. Thus at different times of its breeding cycle it appears to show:
(1) Largely random movement (see Viswanathan et al. 1996) on looping courses, except as dictated by the prevailing weather systems (see Weimerskirch et al. 1993, Nicholls et al. 1997), without particular focus on any obvious large or mesoscale oceanographic features. This mode appears characteristic of long trips during chick-rearing and of some birds (and possibly all birds at some periods) during incubation.
(2) Random movement interspersed with prolonged (3-6 day) bouts of foraging in relatively restricted areas. This mode seems characteristic of some (if not most) birds in some parts of foraging trips during incubation, as well as birds on post-breeding migration (see Prince et al. 1998, Croxall 1998). The nature of foraging activities at these localised areas may well relate to association with fishing activity (Weimerskirch et al. 1993, Prince et al. 1998, Croxall 1998).
(3) Directed short movements associated with shelves and/or the APFZ (at South Georgia only). This appears to be the typical mode of foraging for birds of both sexes during brooding and during short trips in the main chick-rearing period.
(4) Directed long movements (long commuting, Weimerskirch 1997) associated with shelves, for example in the case of Crozet Wandering Albatrosses commuting to the Kerguelen shelf.
Given this diversity of foraging areas and habits it is hardly surprising that the diet of the Wandering Albatross, especially in respect of cephalopods, is very much more diverse than the other two species reported on here (see e.g. Table 2). Weimerskirch et al. (1997c), using activity and prey-ingestion recorders in conjunction with satellite tracking, suggested that there may be 2 distinct feeding techniques used by Wandering Albatrosses: a sit and wait technique mainly associated with night time feeding and in-flight location followed by surface seizing, mainly used in daytime. They also recognised the distinction between the use of curvilinear search routes (random searching) and the use of concentrated (directed) searching. These different modes of behaviour are doubtless associated with the availability of different types of prey and the relative cost-effectiveness of encountering and exploiting them. There are likely to be major differences in regards of scavenged and live-caught prey. Given that the Wandering Albatross is the most omnivorous of albatrosses it is not surprising that such versatility in foraging behaviour should have co-evolved.
Although we have this wealth of new data on interactions between albatrosses and their marine environments, we are still some way from understanding how they structure their foraging trips to ensure cost-effective exploitation of prey in terms of maximising (or optimising) rates of chick provisioning while maintaining their own body reserves and condition (e.g. Weimerskirch et al. 1997a, b). We are, however, moving rapidly towards a situation where the overall foraging range of many albatrosses at different stages of their breeding cycle will be well documented (Croxall 1998). In addition the mesoscale features of relevance to their foraging ecology are becoming well understood. Combining these data will make it possible to assess much more precisely the nature of the problems albatrosses may encounter while foraging, especially those deriving from the effects of interactions with commercial fisheries, many of which are likely to be co-extensive with the features of particular interest to albatrosses. The characterisation of albatross foraging habitats is, therefore, of prime importance to their conservation as well as to the understanding of the dynamics of their predator-prey interactions.
ACKNOWLEDGEMENTS
We thank all those who have helped us with albatross research at South Georgia, Iles Crozet and Kerguelen. We dedicate this paper to the memory of our long-term friend and collaborator, Peter Prince, whose untimely death is as much a loss to albatrosses as to ourselves.
REFERENCES
Arnould, J.P.Y., Briggs, D.R., Croxall, J.P., Prince, P.A. & Wood, A.G. 1996. The foraging behaviour and energetics of wandering albatrosses brooding chicks. Antarctic Science 8: 229-236.
Cherel, Y., Ridoux, V. & Rodhouse, P.G. 1996. Fish and squid in the diet of king penguin chicks, Aptenodytes patagonicus, during winter at sub-antarctic Crozet Islands. Marine Biology 126: 559-570.
Cherel, Y. & Klages, N. 1998. A review of the food of albatrosses (Diomedeidae). In: Robertson, G. & Gales, R. (eds) Albatross Biology and Conservation: 113-136. Chipping Norton, Australia, Surrey Beatty & Sons.
Cherel, Y. & Weimerskirch, H. 1996. Seabirds as indicators of marine resources: black-browed albatrosses feeding on ommastrephid squids in Kerguelen waters. Marine Ecology Progress Series 129: 295-300.
Cooper, J., Henley, S.R. & Klages, N.T.W. 1992. The diet of the Wandering Albatross Diomedea exulans at Subantarctic Marion Island. Polar Biology 12: 477-484.
Croxall, J.P. & Prince, P.A. 1980. Food, feeding ecology and ecological segregation of seabirds at South Georgia. Biological Journal of the Linnean Society 14: 103-131.
Croxall, J.P. & Prince, P.A. 1994. Dead or alive, night or day; how do albatrosses catch squid? Antarctic Science 6: 155-162.
Croxall, J.P. & Prince, P.A. 1996. Cephalopods as prey: seabirds. Philosophical Transactions of the Royal Society Series B 351: 1023-1043.
Croxall, J.P., McCann, T.S., Prince, P.A. & Rothery, P. 1988b. Reproductive performance of seabirds and seals at South Georgia and Signy Island, South Orkney Islands 1976-1986: Implications for Southern Ocean monitoring studies. In: Sahrhage, D. (ed.) Antarctic Ocean and Resources Variability. Springer-Verlag, Berlin: 261-285.
Croxall, J.P., North, A.W. & Prince, P.A. 1988a. Fish prey of the wandering albatross Diomedea exulans at South Georgia. Polar Biology 9: 9-16.
Croxall, J.P., Prince, P.A. & Reid, K. 1997. Dietary segregation in South Georgia seabirds. Journal of Zoology, London, 24: 531-556.
Croxall, J.P. 1998. Research and conservation: a future for albatrosses? In: Robertson, G. & Gales, R. (eds) Albatross Biology and Conservation: 267-288. Chipping Norton, Australia, Surrey Beatty & Sons.
Croxall, J.P., Prince, P.A., Rothery, P. & Wood, A.G. 1998. Population changes in albatrosses at South Georgia.In: Robertson, G. & Gales, R. (eds) Albatross Biology and Conservation: 68-83. Chipping Norton, Australia, Surrey Beatty & Sons.
Croxall, J.P., Reid, K. & Prince, P.A. 1999. Diet and productivity responses of predators to differences in availability of Antarctic krill. Marine Ecology Progress Series (in press).
Gales, R. 1998. Albatross populations: status and threats. In: Robertson, G. & Gales, R. (eds.). Albatross Biology and Conservation: 20-45. Chipping Norton, Australia, Surrey Beatty & Sons.
Guinet, C., Koudil, M., Bost, C.A., Durbec, J.P., Georges, J.Y., Mouchot, M.C. & Jouventin, P. 1997. Foraging behaviour of satellite-tracked king penguins in relation to sea-surface temperatures obtained by satellite telemetry at Crozet Archipelago, a study during three austral summers. Marine Ecology Progress Series 150: 1-20.
Harrison, N.M., Whitehouse, M.J., Heinemann, M.J., Prince, P.A., Hunt, G.L. Jr, & Veit, R.R. 1991. Observations of multispecies seabird flocks around South Georgia. Auk 108: 801-810.
Huin, N. & Prince, P.A. 1997. Diving behaviour of the grey-headed albatross, Diomedea chrysostoma at Bird Island, South Georgia. Antarctic Science, 9: 243-249.
Imber, M.J. 1992. Cephalopods eaten by wandering albatrosses (Diomedea exulans L.) breeding at six circumpolar localities. Journal of the Royal Society of New Zealand 22: 243-263.
Jouventin, P & Weimerskirch, H. 1990 - Satellite tracking of wandering albatrosses. Nature 343: 746-748.
Marchant, S. & Higgins, P.J. 1990. Handbook of Australian, New Zealand and Antarctic birds. Volume 1: Ratites to Ducks. Part A: Ratites to Petrels. Oxford University Press, Melbourne.
Martin, G.R. 1998. Eye structure and amphibious foraging in albatrosses. Proceedings of the Royal Society of London, Series B 265: 665-671.
Nicholls, D.G., Murray, M.D., Butcher, E. & Moors, P. 1997. Weather systems determine the non-breeding distribution of wandering albatrosses over Southern Oceans. Emu 97: 240-244.
Prince, P.A. 1980. The food and feeding ecology of grey-headed albatross Diomedea chrysostoma and black-browed albatross, D. melanophris. Ibis 122: 476-488.
Prince, P.A., Huin, N. & Weimerskirch, H. 1994. Diving depths of albatrosses. Antarctic Science 6: 353-354.
Prince, P.A., Croxall, J.P., Trathan, P.N. & Wood, A.G. 1998. The pelagic distribution of South Georgia albatrosses and their relationships with fisheries. In: Robertson, G. & Gales, R. (eds) Albatross Biology and Conservation: 137-167. Chipping Norton, Australia, Surrey Beatty & Sons.
Reid, K., Croxall, J.P. & Prince, P.A. 1996. The fish diet of black-browed and grey-headed albatrosses Diomedea melanophris and D. chrysostoma at South Georgia. Polar Biology 16: 469-477.
Ridoux, V. 1994. The diets and dietary segregation of seabirds at the subantarctic Crozet Islands. Marine Ornithology 22: 1-192.
Rodhouse, P.G., Clarke, M.R. & Murray, A.W.A. 1987. Cephalopod prey of the wandering albatross Diomedea exulans. Marine Biology 96: 1-10.
Rodhouse, P., Prince, P.A., Clarke, M.R. & Murray, A.W.A. 1990. Cephalopod prey of the grey-headed albatross Diomedea chrysostoma. Marine Biology 104: 353-362.
Rodhouse, P.G. & Prince, P.A. 1993. Cephalopod prey of the black-browed albatross Diomedea melanophris at South Georgia. Polar Biology 13: 373-376.
Rodhouse, P.G., Prince, P.A., Trathan, P.N., Hatfield, E.M.C., Watkins, J.L., Bone, D.G., Murphy, E.J. & White, M.G. 1996. Cephalopod community associated with mesoscale oceanographic features at the Antarctic Polar Front: use of satellite-tracked avian predators to locate pelagic food web activity. Marine Ecology Progress Series 136: 37-50.
Salamolard, M. & Weimerskirch, H. 1993. Relationship between foraging effort and energy requirement throughout the breeding season in the wandering albatross. Functional Ecology 7: 643-652.
Thompson, K.R. 1992. Quantitative analysis of the use of discards from squid trawlers by Black-browed Albatrosses Diomedea melanophris in the vicinity of the Falkland Islands. Ibis 134: 11-12.
Thompson, K.R. & Riddy, M.D. 1995. Utilization of offal and discards from 'finfish' trawlers around the Falkland Islands by the Black-browed Albatross Diomedea melanophris. Ibis 137: 198-206.
Viswanathan, G.M., Afanasyev, V., Buldyrev, S.V., Murphy, E.J., Prince, P.A. & Stanley, H.E. 1996. Levy flight search patterns of wandering albatrosses. Nature 381: 413-415.
Waugh, S.M., Weimerskirch, H., Cherel, Y., Shankar, U., Prince, P.A. & Sagar, P.M. Submitted. The exploitation of the marine environment by two sympatric albatrosses in the Pacific Southern Ocean. Marine Ecology Progress Series,
Weimerskirch, H. 1995. Regulation of foraging trips and incubation routine in male and female wandering albatrosses. Oecologia 102: 37-43.
Weimerskirch H. 1997. Foraging strategies of southern albatrosses and their relationship with fisheries. In: Robertson, G. & Gales, R. (eds) Albatross Biology and Conservation: 168-179. Chipping Norton, Australia, Surrey Beatty & Sons.
Weimerskirch H. & Wilson, R.P. 1992. When do wandering albatrosses Diomedea exulans forage? Marine Ecology Progress Series 86: 297-300.
Weimerskirch H., Jouventin P., Mougin J.L., Stahl J.C. & Van Beveren M. 1985 - Banding recoveries and the dispersion of seabirds breeding in French austral and antarctic territories. Emu 85: 22-23.
Weimerskirch, H., Jouventin, P. & Stahl, J.C. 1986. Comparative ecology of the six albatross species breeding on the Crozet Islands. Ibis, 128: 195-213.
Weimerskirch H., Zotier R. & Jouventin P. 1989 The avifauna of the Kerguelen Islands. Emu 89: 15-29.
Weimerskirch, H., Salamolard, M., Sarrazin, F. & Jouventin, P. 1993. Foraging strategy of wandering albatrosses through the breeding season: a study using satellite telemetry. Auk 110: 325-342.
Weimerskirch, H., Mougey, T. & Hindermeyer, X. 1997a. Foraging and provisioning strategies of black-browed albatrosses in relation to the requirements of the chick: natural variation and experimental study. Behavioural Ecology 8: 635-643.
Weimerskirch, H., Cherel, Y., Cuenot-Chaillet, F. & Ridoux, V. 1997b. Alternative foraging strategies and resource allocation by male and female wandering albatrosses. Ecology 78: 2051-2063.
Weimerskirch, H., Wilson, R.P., Lys, P. 1997c. Activity pattern of foraging in the wandering albatross: a marine predator with two modes of prey searching. Marine Ecology Progress Series 151: 245-254.
Wood, A.G., Naef-Daenzer, B., Prince, P.A. & Croxall, J.P. in press. Quantifying habitat use in satellite-tracked pelagic seabirds: use of kernel estimation with albatrosses. Journal of Avian Biology.
Table 1. Selected biological data on albatrosses at study sites 1.
Table 2. Diet composition (% by mass) and main prey (in approximate order of importance) of albatrosses at study sites. (Range given where multiyear data available)1.

Fig. 1. Distribution of locations of chick-rearing Black-browed Albatrosses around Kerguelen in 1994 (1290 locations) and 1995 (857 locations). A cluster of locations just off the colony is the result of birds aggregating in rafts. Depth of contours in meters.
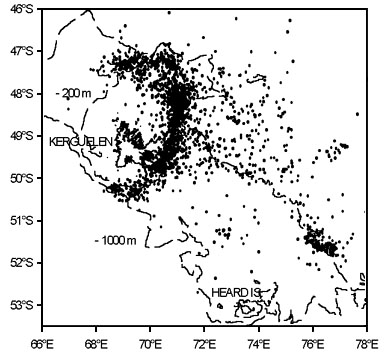
Fig. 2. Distribution of locations of chick-rearing Black-browed Albatrosses breeding at Bird Island, South Georgia in January - March 1993 (1819 uplinks for 19 birds tracked for 77 days). The position of the 1000m isobath is shown.
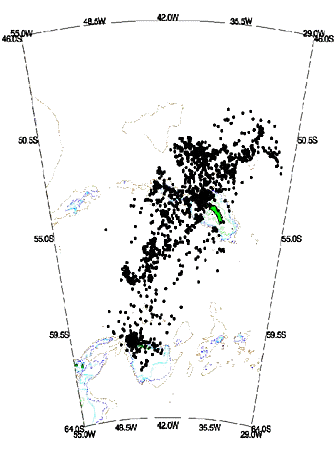
Fig. 3. Density contour results from kernel estimation of the location patterns given in Fig. 3 for Black-browed Albatrosses - contour levels at absolute density values of 5 - 30, 31 - 60, 61 - 92.60 locations. The position of the 200m, 500m, 1000m and 2000m isobaths are shown
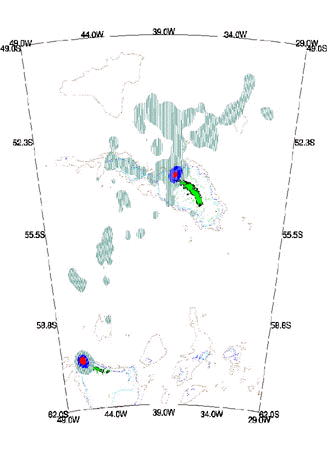
Fig. 4. Distribution of locations from chick-rearing Grey-headed Albatrosses breeding at Bird Island, South Georgia in January - March 1993 (1919 uplinks for 10 birds tracked for 90 days). The position of the 1000m isobath is shown.
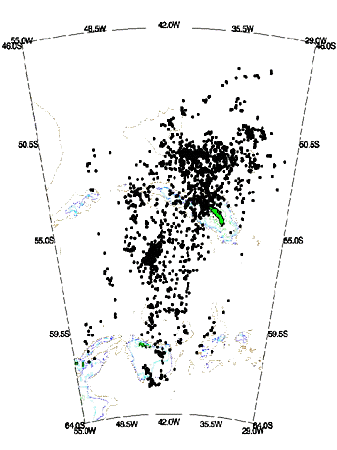
Fig. 5. Density contour results from kernel estimation of the location patterns given in Fig. 5 for Grey-headed Albatrosses - contour levels at absolute density values of 5 - 15, 16 - 30, 31 - 58.62 locations. The position of the 200m, 500m, 1000m and 2000m isobaths are shown
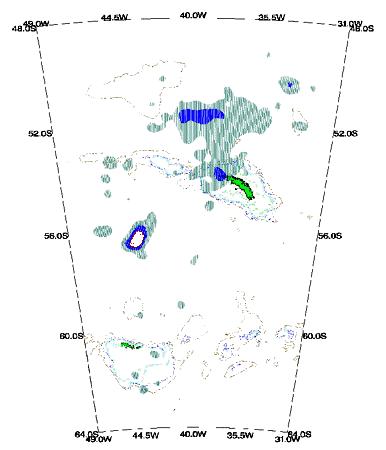
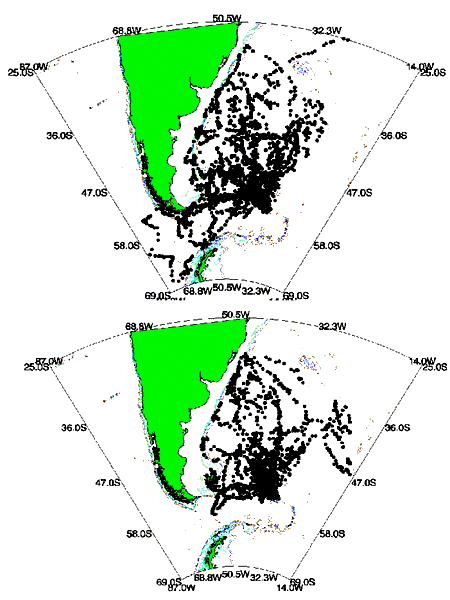
Fig. 6. Distribution of locations from satellite-tracked Wandering Albatrosses breeding in South Georgia during the brood - guard period. Arrow indicates the location of Bird Island. (a) Males; (b) Females.
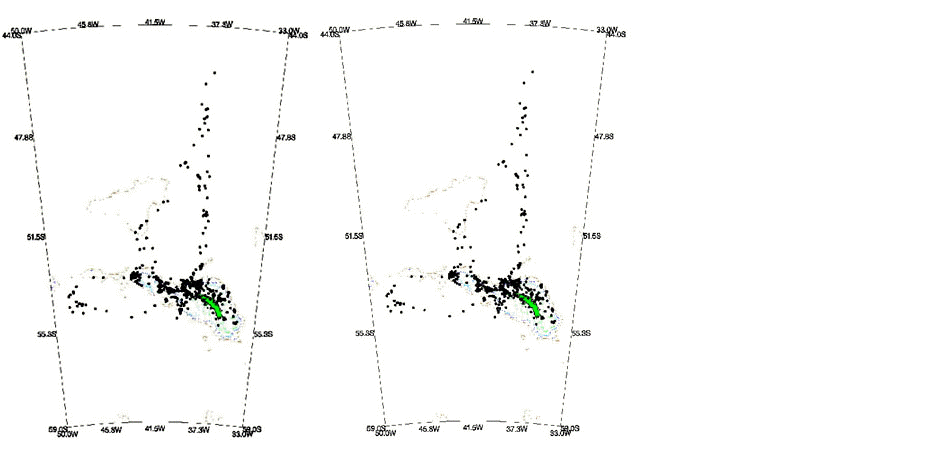
Fig. 7. Distribution of locations from satellite-tracked Wandering Albatrosses breeding at Bird Island, South Georgia. Based on 10.581 uplinks from 56 birds (34 males, 22 females) tracked for a total of 875 days between August 1990 and September 1994. The position of the 1000m isobath is shown. (a) Males; (b) Females.