S33.4: Morphological and functional flexibility of the gastrointestinal tract in Garden Warblers (Sylvia borin) crossing the Sahara desert
Herbert Biebach
Research Unit for Ornithology of the Max-Planck-Society, Von-der Tann-Str. 7, 82346 Andechs, Germany, fax 8152 37333, e-mail Biebach@erl.mpi-seewiesen.mpg.de
Biebach, H. 1999. Morphological and functional flexibility of the gastrointestinal tract in Garden Warblers Sylvia borin crossing the Sahara desert. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1977-1982. Johannesburg: BirdLife South Africa.Small passerine birds cross the eastern Mediterranean Sea and the Sahara Desert either in one long non-stop flight during two nights and days or by an intermittent flight strategy with one or two stopover days. During such long flights with no feeding opportunities they use up their fat stores but also draw upon various organs, probably to satisfy a concomitant need of protein. One-third of the metabolised protein comes from the gastrointestinal tract. The gut and the gizzard shrink by 39% in mass in Garden Warblers Sylvia borin during the Sahara Desert crossing. When a long, continuous flight is simulated in captive Garden Warblers by depriving them of food for two days and nights, their gastrointestinal tract is also reduced by about 44%. After long flights the fat stores have to be replenished during stopover. However, the reduced gastrointestinal tract allows only limited food to be processed on the first stopover day. During subsequent days gross energy intake rises, resulting in a higher fat accumulation rate than during the first day of stopover. These effects are expected to have consequences for the decision about the duration of flight and stopover periods.
INTRODUCTION
Passerine birds that encounter an ecological barrier on their migratory route accumulate large amounts of fat (Biebach 1996). An increase in fat amounting to 50% of lean body mass is typical of birds preparing to cross regions such as the Gulf of Mexico, the West Atlantic Ocean or the Mediterranean Sea and the Sahara Desert (Biebach 1996). Maximal fat loads of up to 100% of lean body mass have been reported. The Garden Warbler (Sylvia borin), with a lean body mass of about 15 g (Bairlein 1987) increases to about 25 g before the desert crossing in autumn and spring (Bairlein 1991). Most of the mass increase, about 70%, is due to fat (Klaassen and Biebach 1994).
The fat stores serve as an energy supply to power migratory flights along migration legs where feeding is limited or impossible. Across the Mediterranean Sea and the Sahara Desert, a stretch of about 2000 km, passerines adopt either a non-stop flight strategy or an intermittent flight strategy with flight at night and rest during the day (Bairlein 1988; Biebach et al. 1991). Behavioural observations of resting birds at the coast of Egypt and in the Libyan desert make it unlikely that feeding plays any role in most species in this area. Among them are those with the highest fat loads, like the Garden Warbler (Biebach pers. obs.)
Those individuals that adopt a non-stop strategy fly continuously for about 28 h, as calculated from a ground speed of about 20 m/s. Ground speed is composed of air speed of about 12 m/s and a tailwind component of 8 m/s . Birds with an intermittent flight strategy would take about 40 h before they reach suitable foraging areas (Biebach et al. in prep.).
During recent years evidence has been accumulating that during prolonged flights not only fat is metabolised but also a considerable amount of protein (Jenni-Eiermann and Jenni 1991; Piersma and Jukema 1990; Lindström and Piersma 1993; Carpenter et al. 1993; Schwilch et al. 1996). In Garden Warblers the proportion of protein breakdown in relation to fat is about 1:3 (Klaassen and Biebach 1994). This ratio seems to apply even if the energy expenditure for temperature regulation is artificially increased by low ambient temperatures. The proportion of fat to protein metabolism in Thrush Nightingale Luscinia luscinia is 1:4 at thermoneutrality as well as in ambient temperatures so cold that metabolism was doubled.
RESULTS
Morphological flexibility
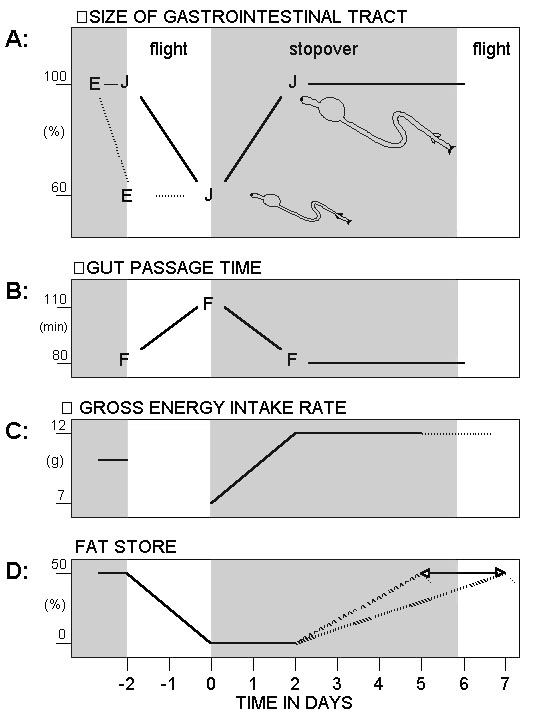
The source of protein within the bird’s body during a long-distance flight primarily comprises the gastrointestinal tract, the flight muscles and the leg muscles (Biebach 1998; Bauchinger and Biebach 1998). The loss of protein from the gastrointestinal tract itself contributes 37%. This conclusion was drawn by comparing the different body components of Garden Warblers just before they started to cross the Mediterranean Sea and the Sahara Desert in autumn with those in birds of the same species after the desert crossing in spring. The latter had a 39% lower mass of the gastrointestinal tract. Further support of these findings comes from lab experiments during which a flight period of Garden Warblers was partially simulated by 2 days without food, which corresponds to about the length of a non-stop flight across the Sahara Desert. In this case the mass of the small intestine + gizzard was reduced by 44%.
The extent of reduction in gastrointestinal tract mass is the same in birds exposed to a two-day food deprivation period and those who actually performed a long-distance flight of 28 to 40 h without food (Biebach 1998). It seems that this duration of food deprivation is sufficient to simulate the conditions that reduce the gastrointestinal tract of birds in the field.
Direct information about the rate at which the morphology of the gastrointestinal tract is restored after an extended flight is limited; only a simulation experiment with relatively few Garden Warblers is available (Biebach 1998). The size of the gastrointestinal tract was inspected directly before (day minus 2) and after (day 0) a 2-day food deprivation period and on day 1, day 3 and day 5 after the onset of feeding. On day 0 small intestine + gizzard fresh mass was reduced by about 30% compared to the pre-fast value. Not until the birds had fed for 3 days was the pre-fast value regained. That is, it took about two to three days to restore the gastrointestinal tract morphologically under ad libitum food conditions in captivity.
What is currently known about the morphological changes in the gastrointestinal tract before, during and after a long distance flight in Garden Warblers can be summarised as follows. Garden Warblers approaching their ecological barrier in autumn reach the Mediterranean Sea with a full-size gastrointestinal tract. After the desert and sea crossing on the return journey in spring, the gastrointestinal tract is reduced by 40%. The time progression of the reduction is not known. Two extremes are possible: a reduction before setting out for the crossing (for waders see Piersma 1998) or a reduction in the course of the crossing. Restoration of the gastrointestinal tract in a subsequent stopover site is probably fast, requiring only two to three days.
Functional consequences
Functional aspects of the gastrointestinal tract reduction significantly affect the bird’s behaviour during the flight phase and during the following stopover. During flight a reduced gastrointestinal tract has a threefold effect:
1. Metabolic maintenance costs of a fully functional gut are the highest among all organs (Stevens and Hume 1995). Lowering the mass and the length of the gut probably involves all the layers: the mucosa, the muscles, and the serosa with its microvilly structure and its very high turnover rate. Even without detailed knowledge about the involvement of these components during reduction, we can be sure that the total maintenance costs will be much lower if the tissue mass is reduced.
2. Decreasing the total mass a bird must transport during flight, by lowering the mass of the gastrointestinal tract (and other compartments), should have an effect on various aspects of flight performance. Cost of transport as well as optimal flight speed should decrease (Pennycuick 1978) whereas e.g. acceleration, maximum speed and rate of climb should increase (Hedenström and Alerstam 1995).
3. The substrate made available by gastrointestinal tract reduction is primarily protein. In the context of the bird’s crossing of an ecological barrier four hypotheses about its use have been put forward (summary in Bauchinger and Biebach 1998): the protein pool hypothesis, the gluconeogenesis hypothesis, the promotion of fat catabolism hypothesis and the water hypothesis, non of which are mutually exclusive.
Near the beginning of stopover a reduced gastrointestinal tract results in a reduced digestive capacity. Mean retention time on day 1 after a two-day food deprivation is lengthened from 78 min to 110 min in Garden Warblers kept in captivity (Hume and Biebach 1996). Additional consequences are expected such as diminished enzyme activity. Taken together these effects restricted the bird’s total intake rate, so that even if the digestive efficiency is unchanged, there is a lowered total resorption rate of nutrients and energy. Similar results were obtained in an experiment with Blackcaps Sylvia atricapilla that were caught immediately after having crossed the Sahara Desert in spring, so that several gut functions could be measured in captivity (Biebach et al. 1998 in prep.). As in the Garden Warblers, gut passage time, gross energy intake rate and mass gain on day 1 (day of capture) were only about half of the values measured after five days of ad lib. food.
DISCUSSION
Costs and benefits
Several different currencies of costs and benefits can be distinguished. During the flight phase there is an energetic saving due to the lower maintenance costs of a reduced gastrointestinal tract, and because of the total mass reduction the cost of transport will diminish. At the same time optimal flight speed is lowered, suggesting time costs of marginal value. Flight mechanical theory also suggests that lower mass will be associated with faster acceleration and a higher maximum speed and rate of climb, all of which are important characteristics with regard to escaping from predators (Hedenström 1992). A reduced risk of predation is therefore expected. No matter how the protein freed by gastrointestinal tract reduction is used metabolically, it will allow the bird to sustain flight or go without food or water for longer periods.
During stopover the main task is to restore the fat depots and rebuilding various organs such as the liver, kidney and muscles. This reconstruction is reflected in high rates of mass gain, amounting to 4 to 5% of lean body mass in passerine migrants (Lindström 1991). Under these circumstances a reduced gastrointestinal tract with a reduced assimilation capacity must result in a lower restoration rate and a lower rate of mass gain. That is, it takes longer to reach the physiological conditions that will allow the next flight to be initiated. During a total stopover period of about 10 days, given that it takes two to three days before the gastrointestinal tract has reached its full capacity after a long flight, considerable time costs can be expected. There are several other hypotheses to explain the mass loss of newly arrived birds during the first days before mass gain is achieved. The time delay before fat is put on has been suggested to result from establishing a territory, becoming familiar with the habitat to effectively exploit resources (Loria and Moore 1990) or handling stress (Clark 1997; Winker et al. 1990). But these can be ruled out especially in light of the simulation experiments in which mass gain was delayed even in captive birds, where none of these possibilities applied. Therefore it seems likely that the main cause of the time delay in restoration of fat and organs during stopover is the reduced digestive capacity of the gastrointestinal tract after an extended fast period. In addition to the time costs, energetic costs and/or specific nutritional needs are also involved in rebuilding the gastrointestinal tract. It remains an unsolved question from which resources the substrate for gastrointestinal restoration is derived: directly from the food ingested by the only partially functioning gastrointestinal tract or from rearranging internal components, as has been shown for the python (Secor et al. 1994) in a similar context.
A quantitative cost-benefit analysis could shed some light on the selective forces that have promoted this flexibility in morphology and function of the gastrointestinal tract during migration. Unfortunately, at present the underlying mechanisms and time courses are not well enough known to allow such a quantitative analysis.
Nevertheless, gastrointestinal tract flexibility can be expected to play an important role in determining flight strategy, in particular the relative duration of flight and stopover phases. The gastrointestinal mass loss may occur before or during flight and in the latter case may have a linear or an exponential time course. Furthermore, depending on the state of the gastrointestinal tract at arrival in a stopover site, the cost and time course of recovery may change. And finally all this may depend on factors such as the flight conditions and the habitat quality and food availability during stopover. Knowing these interactions, we may be able to explain the various flight strategies.
The Garden Warbler is the only passerine migrant so far known to have evolved a high flexibility in the gastrointestinal tract during migration. A similar degree of flexibility has also been found in studies of the gastrointestinal tract response to food quality. In the extreme case birds deprived of food respond with a regression of the digestive system (Karasov 1996). 'No food' might also be the signal to which the gastrointestinal tract responds during migration. The quantitative agreement between the effects of two days of food deprivation in captive birds and 2 to 3 days of in-flight starvation in freely ranging birds supports this possibility. If this is true we can expect gastrointestinal tract flexibility in those migrant species that experience extensive periods of starvation during flight. More specifically, all migratory species that accumulate extensive fat stores to support long flight periods without food would be expected to show a gastrointestinal tract reduction during these periods and its consequences during stopover (Fig. 1).
REFERENCES
Bairlein, F. 1988. How Do Migratory Songbirds Cross the Sahara? TREE 3(8): 191-194.
Bairlein, F. 1991. Body mass of Garden Warblers (Sylvia borin) on migration: a review of field data. Die Vogelwarte 36: 48-61.
Bauchinger, U. & Biebach, H. 1998. The role of protein during migration in passerine birds. Biol. Cons. Fauna 102: ..-..
Biebach, H. 1996. Energetics of winter and migratory fattening. In: Carey, C. (ed.) Avian Energetics and Nutritional Ecology. New York; Chapman & Hall pp: 280-323.
Biebach, H. 1998. Phenotypic organ flexibility in Garden Warblers Sylvia borin during long-distance migration. Journal of Avian Biology, in press.
Carpenter, F.L., Hixon, M.A., Beuchat, C.A., Russell, R.W. & Paton, D.C. 1993. Biphasic mass gain in migrant hummingbirds: body-composition changes, torpor, and ecological significance. Ecology 74(N4): 1173-1182.
Clark, G. A. 1979. Body weights of birds: A review. 193-202.
Hedenström, A. & Alerstam, T. 1995. Optimal flight speed of birds. Philosophical Transactions of the Royal Society of London Series B 348: 471-487.
Hume, I.D. & Biebach, H. 1996. Digestive tract function in the long-distance migratory garden warbler, Sylvia borin . J. Comp. Physiol. B 166: 388-395.
Karasov, W.H. 1996. Digestive plasticity in acian engergetics and feeding ecology. In: Carey, C. (ed.) Avian Energetics and Nutritional Ecology. New York; Chapman & Hall pp: 61-84.
Klaassen, M. & Biebach, H. 1994. Energetics of fattening and starvation in the long-distance migratory garden warbler, Sylvia borin, during the migratory phase. J. Comp. Physiol. B 164: 362-371.
Lindström, Å. 1991 Maximum fat deposition rates in migrating birds. Ornis Scandinavica 22: 12-19.
Lindström, Å. & Piersma, T. 1993. Mass changes in migrating birds: the evidence for fat and protein storage re-examined. Ibis 135(N1): 70-78.
Loria, D.L. & Moore, F.R. 1990. Energy demands of migration on Red-eyed Vireos, Vireo olivaceus. Behavioral Ecol. 1: 24-35.
Pennycuick, C.J. 1978. Fifteen testable predictions about bird flight. Oikos 30: 165-176.
Piersma, T. & Jukema, J. 1990. Budgetting the flight of a long-distance migrant: changes in nutrient reserve levels of Bar-tailed Godwits at successive spring staging sites. Ardea 78 (1-2): 315-338.
Piersma T. 1998. Phenotypic flexibility during migration: physiological optimization contingent on the risks and rewards of fuelling and flight. Journal of Avian Biology, in press.
Schwilch, R., Jenni, L. & Jenni-Eiermann, S. 1996. Metabolic responses of homing pigeons to flight and subsequent recovery. J. Comp. Physiol. B 166(N2): 77-87.
Secor, S.M., Stein, E.D. & Diamond, J. 1994. Rapid upregulation of snake intestine in response to feeding: a new model of intestinal adaptation. Am. J. Physiol. 266: G695-G705.
Stevens, C.E. & Hume, I.D. 1995. Comparative physiology of the vertebrate digestive system. Cambridge; Cambrige University Press: 2nd edn.
Winker, K., Warner D.W. & Weisbrod A.R. 1992. Daily mass gain among woodland migrants at an inland stopover site. Auk 109: 853-862.
Fig. 1. Schematic relationship of gastrointestinal tract size, gut passage time, gross energy intake rate and extent of fat store depending on flight and stopover phase of a migratory Garden Warbler. Abscissa is in days, 0 is the first stopover day after an extended non-stop flight of two days. Until day two is the delay phase after which fat accumulation is possible. Values (closed symbols and solid lines) originate from Garden Warblers either from the field or from a partial simulation experiment with captive birds.(A) Size is shown as mass of lean matter in %. Open symbols and dashed lines indicate a hypothetical time course. (C) Fat store is shown as mass in % above lean dry matter. Dashed lines indicate different fat accumulation rates, resulting in different takeoff times indicated by the endpoints of the double arrow.
