S32.5: Intron variation and population genetics of birds
Vicki L. Friesen1 & Brad C. Congdon1,2
1Department of Biology, Queen's University,Kingston, Ontario K7L, 3N6, Canada, fax 613 545 6617, e-mail
friesen@biology.queensu.ca ; 2School of Biological Sciences,University of Auckland, Private Bag 92019, Auckland, New Zealand. Friesen, V.L., & Congdon, B.C. 1999. Intron variation and population genetics of birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1922-1929. Johannesburg: BirdLife South Africa.Both the study of evolutionary genetics and conservation biology require an inexpensive, rapid, versatile and sensitive method for assaying variation in the nuclear genome. We are using the polymerase chain reaction and the analysis of single-stranded conformational polymorphisms to analyse sequence variation in nuclear introns in birds. 'Universal' primers have been designed for 26 loci, and have been tested in surveys of murres (Uria spp.), murrelets (Brachyramphus spp.), guillemots (Cepphus spp.) and rheas (Rhea spp.). Results suggest that this approach presents a potentially powerful method for detecting genetic variation within and among local populations and species of birds: (1) a variety of genes can be surveyed, including genes of special interest such as those involved in disease resistance; (2) assays are rapid and relatively inexpensive; (3) large numbers of genes can be assayed, enabling accurate estimation of variation in the total genome; (4) almost any mutation can be detected in the genes amplified; (5) the exact nature of variation can be investigated by sequence analysis if desired; (6) statistical methods previously developed for proteins and/or sequence data can be used; (7) protocols can be transferred among species; and (8) assays can be performed on old or degraded samples, blood or museum skins, so that birds do not need to be killed. Results of analyses for murrelets support earlier evidence that North American and Asiatic subspecies represent reproductively isolated species, and that genetic differences exist among murrelets from different sites within North America.
INTRODUCTION
A wide variety of molecular tools have become available for the study of ecology and evolution in recent years (e.g. Avise 1994; Hillis et al. 1996). All have their strengths, but most also have serious limitations. For example, levels of variability detected through protein electrophoresis are often too low to be informative, especially for species breeding in previously glaciated areas or on islands (e.g. Evans 1987). Mitochondrial DNA (mtDNA) is more variable than allozymes, but represents essentially a single supergene whose mode of inheritance is not typical of the majority of the genome. Randomly amplified polymorphic DNA (RAPDs) and microsatellite loci are generally highly variable and can be screened efficiently, but either involve unknown genes (RAPDs) or require laborious and expensive groundwork (microsatellite loci). In the present paper, we describe an alternate type of genetic marker, nuclear introns, which provide an inexpensive, rapid, versatile and sensitive new method for population genetic studies of birds. We will discuss their molecular genetics, methods of analysis, molecular evolution, and utility for avian population genetics.
Molecular genetics of introns
Introns are non-coding segments of DNA that interrupt the coding sequences (exons) of structural genes of all eukaryotes (Fig. 1; Alberts et al. 1994). These segments are transcribed into messenger RNA along with the exons, but are spliced out prior to translation. They range in size from a few base pairs to tens of thousands of base pairs, and may contain microsatellites or exons for other loci. Their function is uncertain, but they probably provide sites for recombination between the functional domains of proteins.
The potential of introns as molecular markers for studies of ecology and evolution results primarily from the fact that they are non-coding and therefore essentially neutral to selection; although introns are probably affected by selection on the exons to which they are linked, substitution rates for introns are substantially greater than for other single-copy nuclear DNA. The fact that they are nuclear means that their effective population size is 4X greater than mtDNA, which tends to increase their variability relative to mitochondrial genes with similar substitution rates. Because they are flanked by exons, which are generally under stabilizing selection, conserved sites are usually available for the design of PCR primers, and sequences of flanking regions are often available for a diversity of species for a variety of loci; this greatly reduces the amount of groundwork required for laboratory analyses compared to microsatellites. Furthermore, because PCR can be performed on old or degraded DNA samples, introns provide a more versatile tool than allozymes, which require high quality tissue samples for analyses. Introns occur in virtually all structural genes and are scattered throughout the genome, so they can provide an almost unlimited number of independent markers. Also, because analysis of introns involves direct analysis of the nuclear material, all or almost all mutations are detected, whereas indirect methods such as protein electrophoresis or analysis of RFLPs may miss up to 90% of genetic variation (Richardson et al. 1986; Birt et al. 1995). Finally, data from introns can be analyzed using sequence-based methods (Swofford et al. 1996), frequency-based methods (Swofford & Selander 1981), or methods that combine allele frequencies and sequences (e.g. analysis of molecular variance, Excoffier et al. 1992; Michalakis & Excoffier 1996; mismatch analysis [frequencies of different levels of sequence divergence among pairs of individuals], Slatkin & Hudson 1991; Rogers & Harpending 1992; Rogers et al. 1996); the later provide the greatest analytical power, and cannot be applied to frequency-based data such as variation in allozymes or microsatellites.
METHODS
The most efficient method of analysing introns involves DNA amplification and either direct or indirect sequence analysis. PCR primers can be placed in conserved sites within exons flanking an intron of interest, and are generally useful for a variety of taxa. A number of general PCR primers are already available through the efforts of a number of research groups (Lessa 1992; Lessa & Applebaum 1993; Palumbi & Baker 1994; Hillis et al. 1996; Friesen et al. 1997; Prychitko et al. 1997). Sequence variation can be assayed by direct sequencing and/or by various indirect mutation detection methods, such as the analysis of restriction fragment length polymorphisms (RFLPs), temperature gradient gel electrophoresis (TGGE), denaturing gradient gel electrophoresis (DGGE), or analyses of single-stranded conformational polymorphisms (SSCPs) or heteroduplexes (HD; Lessa & Applebaum 1993; Hillis et al. 1996). These indirect methods greatly reduce the amount of time and money required for large-scale population surveys without greatly reducing the sensitivity of the approach. We have found that combination of analyses of SSCPs with direct sequencing of variant genotypes provides a powerful approach for population-level studies (Friesen et al. 1997; see also Lessa 1992; Palumbi & Baker 1994).
RESULTS & DISCUSSION
Molecular evolution
Except in special cases, the use of molecular markers to study ecology and evolution assumes that substitutions are neutral to selection, usually that the identity of the target is known, and often that substitutions fit a molecular clock with a particular rate. To date, little has been published regarding the molecular evolution of introns in birds. We used PCR, analysis of SSCPs and direct sequencing to study patterns of substitutions in nine introns among Marbled Murrelets (Brachyramphus marmoratus; Charadriiformes: Alcidae), and between Marbled Murrelets and their congeners (Kittlitz's Murrelet B. brevirostris and the Long-billed Murrelet B. perdix; Friesen et al. 1997). Several lines of evidence suggest that amplification products represented the intended targets for most loci (e.g. Fig. 2): sequences of putative introns began with 'GT', ended with 'AG', and contained a potential lariat site, as expected for introns (Alberts et al. 1994), and sequences of putative exons matched those of the reference bird sequence almost perfectly. Prychitko & Moore (1997) analysed variation in an intron in the b -fibrinogen gene in woodpeckers, and concluded that substitutions were probably adaptively neutral.
For most loci that we studied, substitution patterns described a 'starburst' pattern with one or two hubs (e.g. Fig. 3; Friesen et al. 1997). Substitution patterns appeared to conform to predictions of the neutral theory (Fig. 2): most substitutions within exons were silent; substitutions were distributed randomly within introns; transitions outnumbered transversions; and Tajima's D statistic (Tajima 1989; Simonsen et al. 1995) did not differ significantly from 0 (D = -0.012, P > 0.1), suggesting an absence of strong selective forces. Divergence rates averaged 0.5 + 0.06 % MY-1, which is approximately one-quarter the mean for mtDNA and for cytochrome b; Prychitko & Moore (1997) reached a similar conclusion for a b -fibrinogen intron in woodpeckers. Rates differed significantly among loci for murrelets, (Fst, df = 1,51, P < 0.001), being highest for P40 (0.72 + 0.04 % MY-1) and lowest for tropomyosin (0.16 +0.01% / MY). This mutation rate is lower than the mean for mtDNA; however, because the effective population size of nuclear genes is 4X that of mtDNA, variabilities for introns were equivalent to those for mtDNA (see below).
Examples
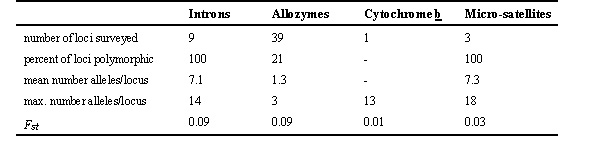
Because analyses of introns are relatively new to molecular genetics, few studies have been published in which intron variation was used to measure genetic differentiation and gene flow among populations of plants or animals. Our investigation of Marbled Murrelets (Friesen et al. 1997) represents the first avian study of which we are aware, and is especially useful in that our data can be compared directly to results of earlier studies of mtDNA, allozymes and microsatellites. Marbled Murrelets are pursuit-diving seabirds that feed inshore and nest predominantly in large trees in old-growth forest along the Pacific coast of North America (Ralph et al. 1995). They are a conservation concern because their breeding habits place them in direct conflict with logging interests, and their feeding ecology makes them vulnerable to oil pollution (Ralph et al. 1995). They are also of interest in the study of evolutionary genetics since birds in western Alaska nest on the ground, but the genetic distinctiveness and evolutionary origin of ground-nesting murrelets are unknown. Pitocchelli et al. (1995) compared variation in morphometrics and mitochondrial RFLPs among murrelets nesting in Alaska; they found that tree-nesting and ground-nesting murrelets differed slightly in morphometrics, but they did not find any divergence of mtDNA. We compared variation in allozymes and mitochondrial cytochrome b sequences among murrelets from Oregon to western Alaska (Friesen et al. 1996) and found extensive variation in cytochrome b genotypes, but no evidence of population differentiation; in contrast, we found that only eight of 39 protein loci were variable (Table 1), but that significant population genetic structure existed (Table 1). Data were insufficient to investigate the exact nature of the genetic structure. In a later analysis of a larger number of murrelets from British Columbia to the western Aleutian Islands, we found between two and 18 alleles at three microsatellite loci, and reported weak but significant differentiation between tree-nesting and ground-nesting murrelets (Table 1).
We also surveyed variation in nine introns, and found that levels of variability were high compared with other loci (Table 1): percent loci polymorphic, and mean and maximum number of alleles per locus were 5X higher for introns than for allozymes, and the number of alleles for the most variable locus (GAPD) was similar to that both for cytochrome b and for the most variable microsatellite locus. Both analyses of molecular variance and phylogenetic analyses based on genotype frequencies and sequence divergence among alleles indicated that murrelets from the Aleutian Islands were significant different than those from mainland North America. Results of analyses of mismatch distributions, differences in mutation rates among loci, and tests for selection suggested that murrelets survived the Pleistocene glaciations in two or more refugia. Estimates of gene flow suggested that net gene flow is from east to west, is lower in the Aleutian Islands than in the mainland, and decreases with distance between populations. We concluded that murrelets recolonised Alaska and British Columbia from at least two Pleistocene refugia, and that gene flow is currently restricted both between murrelets breeding in the Aleutian Islands and those from mainland North America, and among murrelets breeding throughout the Aleutian Islands. Analysis of allozymes and cytochrome b sequences failed to detect any of these effects (Friesen et al. 1996), possibly because of the lower mutation rates of allozymes and the greater susceptibility of mtDNA to population bottlenecks.
The only other study of which we are aware that used intron variation to investigate population differentiation in a bird involved a study of Rock Ptarmigan Lagopus mutus; Holder et al. (unpubl.) used SSCPs and direct sequencing to compare variation in a GAPD intron as well as the mitochondrial control region among ptarmigan from throughout the Nearctic. Their results suggest that ptarmigan were isolated and diverged from each other both genetically and morphologically in multiple refugia during the Pleistocene glaciations. In this study, results for introns were less conclusive than those for the control region, but served to confirm the results for mtDNA. Introns have also been used extensively in population genetic studies of other vertebrates, invertebrates and plants. For example, Palumbi & Baker (1994) used cloning and direct sequencing to compare variation in actin introns among Humpback Whales Megaptera novaengliae from various sites, and found significant differences in allele frequencies of whales from the Atlantic and Pacific Oceans, in agreement with results from mtDNA; however they found little population differentiation in intron variation within the Pacific, despite significant differentiation in mtDNA. In another study, Moran et al. (1997) used RFLPs in eight introns to distinguish populations of Chinook Salmon Oncorhynchus tshawytscha and Steelhead (O. mykiss) from northwestern North America. Introns also are being used extensively in phylogenetic studies, including studies of birds. For example, Prychitko & Moore (1997) found that sequences of an intron from the b -fibrinogen gene were as useful as those from cytochrome b in reconstructing the phylogenetic relationships among the woodpeckers. Currently, we are using introns to study population differentiation in Kittlitz's Murrelets, Common Murres Uria aalge, Thick-billed Murres U. lomvia and Pigeon Guillemots Cepphus columba. Results of studies such as these suggest that introns will provide a sensitive, efficient and versatile tool for studying population differentiation, as well as phylogenetic relationships, hybridization, and possibly kinship (including parentage) in birds; combination of analyses of introns and mtDNA promise to be especially powerful.
REFERENCES
Alberts, B., Bray, D., Lewis, J., Raff, M., Roberts, K., & Watson, J.D. 1994. Molecular biology of the cell. London; Garland Publishing.
Avise, J.C. 1994. Molecular markers, natural history and evolution. New York; Chapman and Hall.
Birt, T.P., Friesen, V.L., Green, J.M., & Davidson, W.S. 1995. Mitochondrial DNA variation in Newfoundland and Norwegian populations of Atlantic capelin, Mallotus villosus, detected using two techniques. Molecular Ecology 4: 771-776.
Evans, P.G.H. 1987. Electrophoretic variability of gene products. In: Cooke, F., & Buckley, P. A. (eds) Avian genetics. London; Academic Press: 105-162.
Excoffier, L., Smouse, P.E., & Quattro, J.M. 1992. Analysis of molecular variance inferred from metric distance among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 131: 479-491.
Friesen, V.L., Baker, A.J., & Piatt, J.F. 1996. Molecular evidence for a 'new' species of alcid: the Long-billed Murrelet (Brachyramphus perdix). Condor 98: 681-690.
Friesen, V.L., Congdon, B.C., Walsh, H.E., & Birt, T.P. 1997. Intron variation in Marbled Murrelets detected using analysis of single-stranded conformational polymorphisms. Molecular Ecology 6: 1047-1058.
Hillis, D.M., Moritz, B.K., & Mables, C. (eds) 1996. Molecular systematics. Sunderland, Massachusetts; Sinauer Associates.
Lessa, E.P. 1992. Rapid surveying of DNA sequence variation in natural populations. Molecular Biology and Evolution 9: 323-330.
Lessa, E.P., & Applebaum, G. 1993. Screening techniques for detecting allelic variation in DNA sequences. Molecular Ecology 2: 121-129.
Michalakis, Y., & Excoffier, L. 1996. A generic estimation of population subdivision using distance between alleles with special interest to microsatellite loci. Genetics 142: 1061-1064.
Moran, P., Dightman, D.A., Waples, R.S., & Park, L.K. 1997. PCR-RFLP analysis reveals substantial population-level variation in the introns of Pacific salmon (Oncorhynchus spp.). Molecular Marine Biology and Biotechnology 6: 315-327.
Palumbi, S.R., & Baker, C.S. 1994. Contrasting population structure from nuclear intron sequences and mtDNA of humpback wales. Molecular Biology and Evolution 11: 426-435.
Pitocchelli, J., Piatt, J., & Cronin, M. 1995. Morphological and genetic divergence among Alaskan populations of Brachyramphus murrelets. Wilson Bulletin 107: 235-250.
Prychitko, T.M., & Moore, W.S. 1997. The utility of DNA sequences of an intron from the b -fibrinogen gene in phylogenetic analysis of woodpeckers (Aves: Picidae). Molecular Phylogenetics and Evolution 8: 193-204.
Richardson, B.J., Baverstock, P.R., & Adams, M. 1986. Allozyme electrophoresis. London; Academic Press.
Rogers, A.R., & Harpending, H. 1992. Population growth makes waves in the distribution of pairwise genetic differences. Molecular Biology and Evolution 9: 552-569.
Rogers, A.R., Fraley, A.E., Bamshad, M.J., Watkins, W.S., & Jorde, L.B. 1996. Mitochondrial mismatch analysis is insensitive to the mutational process. Molecular Biology and Evolution 13: 895-902.
Simonsen, K.L., Churchill, G.A., & Aquadro, C.F. 1995. Properties of statistical tests of neutrality for DNA polymorphism data. Genetics 141: 413-429.
Slatkin, M., & Hudson, R.R. 1991. Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 129: 555-562.
Swofford, D.L., Olsen, G.J., Waddell, P.J., & Hillis, D.M. 1996. Phylogenetic inference. In: Hillis, D. M., Moritz, C., & Mable, B. K. (eds) Molecular systematics. Sunderland, Massachusetts; Sinauer Associates: 407-514.
Swofford, D.L., & Selander, R.B. 1981. BIOSYS-1: a Fortran program for the comprehensive analysis of electrophoretic data in population genetics and systematics. Journal of Heredity 72: 281-283.
Tajima, F. 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphisms. Genetics 123: 585-595.
Table 1. Indices of variabilities, and estimates of Wright's F
st obtained using different molecular markers for Marbled Murrelets.
Fig. 1. Structure of a typical eukaryotic gene, including introns and exons. 'Gppp' indicates the 5' cap; 'e' designates an exon; 'i' designates an intron; 'AAAAAAA' represents the poly-A tail.
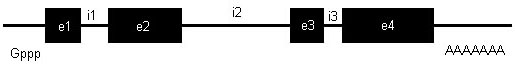
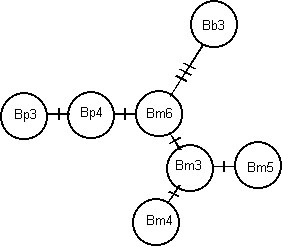
Fig. 2. (A). Nucleotide sequences and inferred amino acid sequences of a lamin intron and parts of the flanking exons for Marbled Murrelets and a reference taxon (the Chicken, Gallus gallus). Dots represent the intron of the reference taxon, which could not be easily aligned with that of murrelets. Sites that are underscored are sites of variation within murrelets. Site designations are positions relative to the 5' primer. (B). Sequence variation within a lamin intron for murrelets. 'N' is the number of individuals possessing a given allele. 'Bm' indicates an allele that occurs in Marbled Murrelets; 'Bb' indicates an allele that occurs in Kittlitz's Murrelets; 'Bp' indicates an allele that occurs in Long-billed Murrelets. From Friesen et al. (1997).

Fig. 3. Substitutional relationships among lamin alleles for brachyramphine murrelets. Cross-bars indicate substitutions. From Friesen et al. (1997).