S31.2: Maximum hovering performance of hummingbirds: Capacities, constraints, and trade-offs
Peng Chai
Department of Zoology, University of Texas at Austin, Austin, Texas 78712-1064, USA, fax 512 471 9651, e-mail pengchai@utxvms.cc.utexas.edu
Chai, P. 1999. Maximum hovering performance of hummingbirds: Capacities, constraints, and trade-offs. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1810-1822. Johannesburg: BirdLife South Africa.Aerodynamic and energetic concerns of flight strongly mold the body design of flying animals. However, avian fliers also face other survival challenges, such as courtship, migration, and plumage renewal. Morpho-physiological trade-offs to these activities can compromise their flight performance in the face of ecological demands such as competition and predator avoidance. Intraspecific differences of Ruby-throated Hummingbirds in maximum hovering performance and associated capacities in kinematics, aerodynamics, muscle mechanics, and metabolism were investigated. Hummingbirds were tested with the strenuous activity of hovering in low-density gas mixtures - a lift assay for the capacity to generate vertical force. Adult males having shorter wings were less capable of generating lift force while hovering in hypodense air, despite displaying muscle mechanical and metabolic capacities equivalent to those of females. Body mass is the primary constraint on maximum hovering performance, and heavier birds showed aerodynamic failure at higher air densities. Muscle mechanical power output was constrained by wing kinematics because wingbeat frequency operated only within a narrow range, and wingstroke amplitude at maximum hovering reached its limit of near 180o, regardless of birds' mass and air density. Moulting birds with reduced wing area suffered from weight loss and reduction in hovering capacity and muscle power, reflecting transiently high cost of moults. Under multiple functional constraints, the study hummingbirds demonstrated versatile reserve capacities in their hovering performance. However, morpho-physiological adaptations to multiple survival requirements could reduce the biomechanical margin of safety during hovering and potentially impose fitness costs.
INTRODUCTION
Locomotor specialisations of an animal essentially determine its ecological and physiological characters. Hummingbirds are the only avian taxon capable of prolonged hovering and function as effective nectarivores and pollinators. Hovering flight is frequently viewed as a measure of extreme locomotor performance with very high demand of rates of lift and mechanical/metabolic power production. Hummingbirds operate close to the lower limit of body mass in vertebrate homeotherms, while during hovering, they exhibit mass-specific metabolic rate close to the upper limit (Suarez 1992; Chai & Dudley, 1995). They thus serve as ideal models for testing hypotheses concerning performance capacities, constraints, and trade-offs.
It has been suggested that the structure and function of these animals are tightly integrated to maximise flight performance but with less reserve capacity (Diamond 1990; Hochachka 1994; Suarez et al. 1997). Under limiting resources, excess capacity inflicts extra costs. Natural selection should favor reduced reserve when the survival cost of such excess relative to its benefit is high (Diamond & Hammond 1992). Thus, there should exist strong selection against excess capacity in this energetic-costly functional system. Here, reserve capacity is defined as excess hovering performance relative to that exhibits at normal hovering, and indeed power requirements in vertical ascent or load carrying can exceed values that characterise hovering.
Reserve hovering capacity may also represent safety margin and provide excess power to aid hummingbirds in predator avoidance, interference competition, carrying load, and overcoming pathological situations. However, diverse adaptive challenges such as escape flight, courtship, migration, and plumage renewal faced by individual hummingbirds and morpho-physiological specialisations associated with such activities can compromise flight machinery and impose limits as well as trade-offs to aerodynamic and energetic performance. For example, flight is energetically expensive, and fliers need to carry fuel (e.g. fat) loads to ensure energy security. Yet such increased loads can be deleterious to flight performance and will also increase the energetic costs of flight (Ellington 1984a; Rayner 1990; Hedenström 1992; Norberg 1995). Furthermore, small endothermic fliers such as hummingbirds run the risk of energy deficits, but must be light to maintain agility in the face of mass-dependent predation risks (Gosler et al. 1995; Kullberg et al. 1996). Hummingbird wing morphology also reflects an aerodynamic trade-off because the two extreme forms of flight along the airspeed spectrum (hovering and fast forward flight) differ substantially in their mechanical power requirements. During hovering, flapping wings maximise vertical forces (lift) by moving air downwards for weight support. Longer wings can enhance lift production, whereas weight loss and concomitantly lower wing loading (body weight relative to wing area) can reduce the cost of lift generation. During fast forward flight, horizontal force (thrust) is increased to offset increased drag forces primarily over the body and secondarily over the wings. A streamlined body, shorter wings, and a higher wing loading should decrease frictional resistance and thereby promote faster flight (Pennycuick 1975; Rayner 1988; Norberg 1990).
In the present study, intraspecific differences of Ruby-throated Hummingbirds in hovering capacity were compared to investigate how different functional levels including wing morphology, wingbeat kinematics, flight muscle contractile mechanics, and whole-body metabolism integrate during maximum hovering performance. Maximum biomechanical and energetic capacities of hummingbirds with shorter wings (adult males), with increased body weight, and with loss of wing area (during moult) are systematically compared with aerodynamic predictions. Birds with reduced wing length and area or with increased body weight are predicted to exhibit inferior hovering capacity.
Hummingbirds were tested with the strenuous activity of hovering in low-density gas mixtures - a lift assay for the capacity to generate vertical force. We have non-invasively induced maximum hovering performance in individual Ruby-throated Hummingbirds Archilochus colubris L. using normoxic but hypodense mixtures of air and heliox (21% O2 and 79% He). Oxygen availability is not limiting in such studies, but the density of heliox is only one-third that of normal air (Chai & Dudley 1995; Dudley & Chai, 1996). Hovering in low-density air is analogous to increasing treadmill speeds for runners in that hummingbirds must increase their mechanical power output to generate lift force sufficient to stay airborne. Limits to the locomotor capacity of hovering hummingbirds are unequivocally indicated by aerodynamic failure at low air densities (Chai & Dudley, 1995).
During maximum hovering, reserve capacities of multiple functional systems of kinematics, mechanics, and metabolism reflect the overall design of the flight machinery of hummingbirds as shaped by both physical laws and natural selection (see Arnold 1983; Bennett 1991; Diamond & Hammond 1992; Wainwright 1994). How much reserve capacity is built into each functional level, and what are the invariant vs. malleable elements of flight design in hummingbirds? Thus, the goal of this study is to explore the links between aerodynamic and energetics of hummingbird flight on the one hand, and on the other hand evaluate potential behavioural and ecological influences on hovering performance.
METHODS
Experimental procedures for manipulating air densities have been described previously (Dudley 1995; Chai & Dudley 1995). Flight experiments were implemented within an airtight plexiglas cube (90 x 90 x 90 cm). The bird was trained to hover-feed approximately every 20 min through a mask which allowed for collection of respiratory gases using an open-flow feeder-mask respirometry system (Chai & Dudley 1995). Oxygen consumption rates during hovering were obtained from the expired respiratory gases. At the same time, wingbeat kinematics were video-recorded (Sony CCD-TR600 at 60 fields s-1 with a high-speed shutter of 1/4000 s). Data were collected initially from birds hover-feeding in unmanipulated sea-level air (density 1.20 kg m-3). Air within the cube was then gradually replaced by filling with normoxic heliox (21% O2 and 79% He, density 0.40 kg m-3). As the air became thinner and hovering flight became more strenuous, the bird gradually reduced its hover-feeding duration (Chai & Dudley 1995). Heliox filling was terminated after the bird showed aerodynamic failure while hover-feeding in hypodense air. The bird drastically descended to the chamber floor and momentarily lost the ability to fly. Maximum transient hovering performance was taken as the short hover-feeding sequence (2 - 4 s) recorded immediately prior to aerodynamic failure. Wingbeat kinematics (i.e. wingbeat frequency n and stroke amplitude
F ) and morphological parameters of individual birds then were used to estimate the mechanical power requirements at maximum hovering performance (see Ellington 1984a).Morphological parameters used in aerodynamic
calculations included body mass m, wing length R, total wing area S
(the area of both wings), aspect ratio AR (=4R2/S), and
wing loading pw (= mg/S, where g is
gravitational acceleration). Aerodynamic calculations assumed simple harmonic motion and
horizontal stroke plane of the wings, and the mean lift coefficient ![]() (derived by assuming that vertical
force production averaged over the wingbeat period equal the bird's body weight; see
Ellington 1984a) is presented. Mechanical power requirements were estimated by
evaluating individual components of body mass-specific profile (P*pro)
and induced (P*ind) power requirements. P*pro
represents energetic expenditure to overcome frictional drag forces on the flapping wings,
and P*ind is the power needed to generate downward momentum to the
surrounding air so as to offset the body weight (for Ruby-throated Hummingbirds, P*ind
is 3 - 4 times greater than P*pro; Chai et al. 1997). Total power
expenditure (P*per) was calculated assuming perfect elastic storage of
wing inertial energy, representing minimum estimates of required mechanical power. Thus, P*per
= P*pro + P*ind, and P*per is equal
to the aerodynamic power requirement (i.e. power required to move the air). Hummingbirds
can probably store kinetic energy elastically during the deceleration phase of the
wingstroke, so that inertial power requirements are probably negligible (see Greenewalt
1975; Ellington, 1984a; Wells 1993).
(derived by assuming that vertical
force production averaged over the wingbeat period equal the bird's body weight; see
Ellington 1984a) is presented. Mechanical power requirements were estimated by
evaluating individual components of body mass-specific profile (P*pro)
and induced (P*ind) power requirements. P*pro
represents energetic expenditure to overcome frictional drag forces on the flapping wings,
and P*ind is the power needed to generate downward momentum to the
surrounding air so as to offset the body weight (for Ruby-throated Hummingbirds, P*ind
is 3 - 4 times greater than P*pro; Chai et al. 1997). Total power
expenditure (P*per) was calculated assuming perfect elastic storage of
wing inertial energy, representing minimum estimates of required mechanical power. Thus, P*per
= P*pro + P*ind, and P*per is equal
to the aerodynamic power requirement (i.e. power required to move the air). Hummingbirds
can probably store kinetic energy elastically during the deceleration phase of the
wingstroke, so that inertial power requirements are probably negligible (see Greenewalt
1975; Ellington, 1984a; Wells 1993).
Metabolic cost during hovering was derived
from rates of oxygen consumption (![]() ). As hovering became
more strenuous due to air density reduction, birds gradually reduced their feeding
duration. Oxygen consumption rates at aerodynamic failure could not be reliably obtained
given the short duration of hover-feeding. Instead, measurements were taken from
hover-feeding bouts greater than 5 s in duration but at densities similar to the value at
which birds failed aerodynamically. Flight efficiency was then taken as the ratio of
mechanical power output to metabolic power input for the same hover-feeding sequence.
Furthermore, reserve capacities of wingbeat frequency, stroke amplitude, lift capacity of
the wings, mechanical power output, and rates of oxygen consumption were expressed as the
% increase relative to values obtained when hovering in unmanipulated sea-level air prior
to replacement with heliox.
). As hovering became
more strenuous due to air density reduction, birds gradually reduced their feeding
duration. Oxygen consumption rates at aerodynamic failure could not be reliably obtained
given the short duration of hover-feeding. Instead, measurements were taken from
hover-feeding bouts greater than 5 s in duration but at densities similar to the value at
which birds failed aerodynamically. Flight efficiency was then taken as the ratio of
mechanical power output to metabolic power input for the same hover-feeding sequence.
Furthermore, reserve capacities of wingbeat frequency, stroke amplitude, lift capacity of
the wings, mechanical power output, and rates of oxygen consumption were expressed as the
% increase relative to values obtained when hovering in unmanipulated sea-level air prior
to replacement with heliox.
Analysis of covariance (ANCOVA) was conducted on kinematic, aerodynamic, mechanical, and metabolic variables relating to maximum hovering performance in hypodense gas mixtures. ANCOVA was used to assess effects of body mass and bird group. Four bird groups were identified given different sex and plumage conditions: moulting birds, non-moult females, juvenile males, and adult males. Least-squares means adjusted for the covariate effect of body mass as well as for unbalanced sample sizes were calculated to indicate the expected mean and estimated standard error for each bird group (SAS PROC GLM, SAS Institute 1989). Least-squares means (i.e. population marginal means) are the expected value of bird group means in a balanced design involving bird groups with the body mass covariate set at its mean value (Milliken & Johnson 1992).
RESULTS
We conducted a total of 34 density reduction experiments on 18 individual hummingbirds with varying body mass and wing condition. Each individual bird received 1 - 3 density reduction trials either with unimpaired wings or during the annual moult (measured during peak moult of flight feathers in the spring). Dissimilar plumage during moult justifies treating performance capacity measured from each density reduction trial as an independent observation (i.e. n = 34). Adult males had the shortest and smallest wings; wing loading was correspondingly higher than those of juvenile males and females (Table 1 and Fig. 1). Juvenile males had wing lengths between those of females and adult males (wing morphology of juvenile and adult females is indistinguishable). Non-moulting birds were thus divided into three groups (Table 1). Moulting birds still possessed 2 - 3 outer primaries (and thus exhibited constant wing length), but on average retained only 78± 8% (mean ± 1 s.d.) of non-moult wing area, yielding high aspect ratio (Fig. 1 and Chai 1997).
The capacity to hover in hypodense air inversely varied with body mass, with heavier birds displaying aerodynamic failure at higher air densities (Table 1 and Fig. 1). Weight effect was confounded with effects due to differences in wing morphology. For non-moult birds, adult males were least capable of hovering, whereas females were most capable. Moulting birds with reduced wing area also lost weight, yielding values of wing loading similar to that of non-moult birds (Table 1 and Fig. 1). Despite such presumably adaptive weight loss, moulting birds still failed at higher air densities relative to non-moult birds (Table 1 and Fig. 1).
Hovering birds met the challenge of
low-density air by modulating wingbeat kinematics. However, wingbeat frequency varied by
only 4 - 6%, and modulation of wingstroke amplitude was a more important means of
generating mechanical power. Maximum power was attained at a stroke amplitude near 180o
(Table 1 and Fig. 2). Wing length and
wingbeat frequency were negatively correlated. Males showed inferior lift performance of
the wings (lower ![]() >) and failed at higher air
densities. Moulting birds showed the least capacity for lift generation (Table 1 and Fig. 2).
>) and failed at higher air
densities. Moulting birds showed the least capacity for lift generation (Table 1 and Fig. 2).
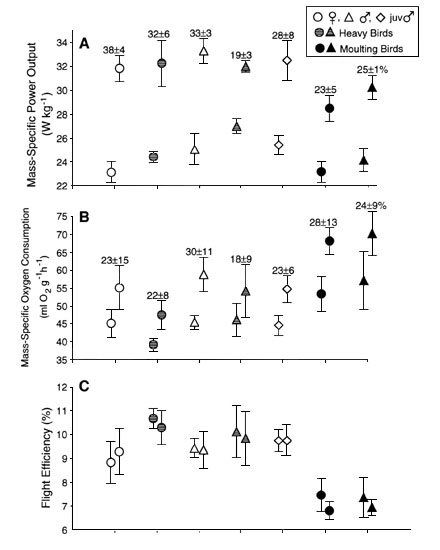
Despite variable hovering capacity in hypodense gas mixtures among bird groups (Fig. 1), maximum mass-specific mechanical power outputs (P*per) of non-moult bird groups were actually similar (Table 1). No statistical differences in power output were found among non-moult bird groups, and the moulting bird group was again the least capable of producing useful mechanical power (Table 1 and Fig. 3). Relative to hovering in normal air, heavier birds at maximum performance exhibited proportionally less power reserve (Table 1 and Fig. 3). Male birds also suffered from a reduced power reserve, while females showed the highest margin for excess power. Moulting birds during hovering also showed the least amount of power reserve (Table 1 and Fig. 3).
Maximum rates of oxygen consumption indicated
a significantly positive weight effect. No statistical differences existed among non-moult
bird groups in metabolic expenditure, whereas moulting birds showed a significant increase
in metabolism (![]() ; Table 1 and Fig. 3). Reserve capacity in mechanical power output (P*per
reserve) varied significantly among bird groups, yet metabolic reserve capacities (
; Table 1 and Fig. 3). Reserve capacity in mechanical power output (P*per
reserve) varied significantly among bird groups, yet metabolic reserve capacities (![]() reserve) were similar (Table 1 and Fig. 3). Increased mechanical power requirements due to weight gain
were accompanied by an increased rate of oxygen consumption, but this increase was not
commensurate with the rise in mechanical power. Overall flight efficiency (ratio of
mechanical power output to metabolic power input) was also improved with weight gain (Table 1 and Fig. 3). During strenuous hovering
performance, moulting birds were least capable of generating useful mechanical power and
also experienced the highest metabolic cost, thereby displaying the lowest flight
efficiency. Flight efficiency did not significantly vary among non-moult birds.
reserve) were similar (Table 1 and Fig. 3). Increased mechanical power requirements due to weight gain
were accompanied by an increased rate of oxygen consumption, but this increase was not
commensurate with the rise in mechanical power. Overall flight efficiency (ratio of
mechanical power output to metabolic power input) was also improved with weight gain (Table 1 and Fig. 3). During strenuous hovering
performance, moulting birds were least capable of generating useful mechanical power and
also experienced the highest metabolic cost, thereby displaying the lowest flight
efficiency. Flight efficiency did not significantly vary among non-moult birds.
Air densities at aerodynamic failure were significantly different among the four bird groups. Of the non-moult bird groups, females with the longest wings and lowest wing loading were the most capable of hovering, whereas adult males with the shortest wings and highest wing loading were the least (Table 1). Weight gain is associated with reduced hovering performance, and heavier birds failed at higher air densities. Moulting birds renewing their primary flight feathers reduced both wing area and body mass. Despite wing loading similar to that of non-moult birds, moulting birds exhibited the lowest capacity to hover. Thus, congruent with the aerodynamic predictions, hummingbirds with shorter wings (adult males), with increased body weight, and with loss of wing area (during moult) exhibited inferior hovering capacity (Table 1 and Fig. 1). However, aerodynamic predictions are based on energetic costs of flight and could be unsatisfactory because reserve capacities of energy supply are largely unknown. For example, our empirical measurements showed that moulting inflicted high energetic costs during hovering (Fig. 3). Such birds possess suboptimal wing shapes, which may interfere with neuromuscular co-ordination (Ellington 1984b, Swaddle et al. 1996; Chai 1997). Furthermore, moulting can exert multiple energetic costs, including loss of plumage insulation and an increase of basal metabolism (Walsberg 1983; Lindström et al. 1993; Jenni & Winkler 1994; Murphy 1996).
The present study identified constraints relating to wingbeat kinematics, and muscle mechanical and metabolic capacities. Hovering in variable-density gas mixtures requires modification of wingbeat kinematics to attain the requisite aerodynamic force balance and associated mechanical power output. Constraints on the rate of muscle contraction are implicated by the limited range of values for wingbeat frequency, with the frequency reserve averaging only 5%. A strong negative correlation also existed between wing length and wingbeat frequency (Table 1). Such patterns of relatively fixed wingbeat frequency and but an inverse relationship between this parameter with wing length are typical of all flying birds (Pennycuick 1996). This result suggests that the flight muscles of hummingbirds are probably adapted to work at a particular resonant frequency to optimise mechanical power output of the oscillatory rhythm (Greenewalt 1975). The existence of such a resonant system should be strongly favored because the mechanical power requirement with perfect elastic energy storage (i.e. P*
per) is less than half of that with no such storage (Wells 1993; Chai & Dudley 1995).Modulation of wingstroke amplitude is the primary means of varying mechanical power during hovering, with maximum power output attained near a geometrical constraint of 180o (see also Chai & Dudley 1995). However, the duration of maximum hovering performance was brief. A negative relationship existed between magnitude and duration of mechanical power production, indicating limits to the aerobic capacity for sustainable performance (Josephson 1993; Chai & Dudley 1995).
Our data were derived from captive hummingbirds with food provided ad libitum during captivity. Indeed, body masses of the study birds were higher than those of wild Ruby-throated Hummingbirds (e.g. wild-caught males: 3.3± 0.2 g; wild-caught females: 3.5± 0.4 g in September; Robinson et al. 1996). Weight gain is highly detrimental to hovering because whole-bird power requirements increase with the 1.5 power of body mass given a fixed wing length (Weis-Fogh 1977). If our birds had not substantially gained weight in captivity, their hovering capacity would likely have been higher.
Among non-moult bird groups, females having
the longest wings were most efficient in generating lift force in low density gas mixtures
(highest ![]() and
and ![]() reserve) and displayed the highest reserve capacity, but incurred
metabolic costs similar to other bird groups (
reserve) and displayed the highest reserve capacity, but incurred
metabolic costs similar to other bird groups (![]() , Table 1 and Fig. 3). In spite of dissimilar
hovering capacities, maximum values of mechanical power output (P*per) among non-moult bird groups were surprisingly
similar. The major component of P*per is P*ind, which
varies in approximate proportion to the square root of wing disk loading (= mg/(FR2), see Ellington 1984a). Energetic costs of hovering thus vary inversely
with wing length and are highest in adult males with the shortest wings, and this group
displayed the lowest mechanical power reserve (P*per reserve) among non-moult birds. In contrast to variable mechanical power
reserves, the reserve capacity of the metabolic machinery (
, Table 1 and Fig. 3). In spite of dissimilar
hovering capacities, maximum values of mechanical power output (P*per) among non-moult bird groups were surprisingly
similar. The major component of P*per is P*ind, which
varies in approximate proportion to the square root of wing disk loading (= mg/(FR2), see Ellington 1984a). Energetic costs of hovering thus vary inversely
with wing length and are highest in adult males with the shortest wings, and this group
displayed the lowest mechanical power reserve (P*per reserve) among non-moult birds. In contrast to variable mechanical power
reserves, the reserve capacity of the metabolic machinery ( ![]() reserve) was invariant among bird groups, implying a similar
underlying constraint in metabolic flux capacity. Given equivalent muscle mechanical and
metabolic potential, reduced hovering performance in males probably reflects a trade-off
in wing morphology for increased speed and agility.
reserve) was invariant among bird groups, implying a similar
underlying constraint in metabolic flux capacity. Given equivalent muscle mechanical and
metabolic potential, reduced hovering performance in males probably reflects a trade-off
in wing morphology for increased speed and agility.
Maximum mass-specific mechanical power output (P*per) remained similar among non-moult bird groups, whole-bird power output was thus mass-dependent and increased linearly with body mass. Muscle power output is generally proportional to the product of contraction frequency (i.e. wingbeat frequency), muscle strain (proportional to stroke amplitude), and myofibrillar stress (Pennycuick & Rezende 1984). Because wingbeat kinematics (wingbeat frequency and stroke amplitude) were mass-independent at the point of maximal hovering (Table 1), difference in force production (muscle stress) may have been primarily responsible for difference in whole-bird power output between heavy and light birds. Mass-dependent whole-bird power output could also be due to a proportional increase in flight muscle mass with body weight gain, and a morphological study investigating the link between flight muscle mass and weight gain is badly needed (see Carpenter et al. 1991).
Such knowledge of possible variation in flight muscle mass will serve to distinguish whether mass-dependent whole-bird power output derives from the presence of more muscle or from improved mechanical efficiency associated with weight gain. More importantly, maximum P*per in the present study were derived from hovering flight through manipulation of air density, and P*per only represents the minimum mechanical power necessary to hover and to overcome two aerodynamic forces: induced drag and profile drag. Mass-invariant maximum P*per derives from mass invariance in P*ind, a parameter that is proportional to the mean downwards velocity of air associated with weight support (see Ellington, 1984a). Heavier birds failed at higher air densities but imparted similar downwards velocities to this denser air, thus expending greater induced and thus total power. Failure at different air densities reflects constraints imposed by wingbeat kinematics which then set upper limits to P*per (Table 1 and Fig. 3). Thus, mass-invariant P*per at maximum performance in low-density flight media reflects complex interactions among kinematic constraints, available mechanical power, and aerodynamic force production (Ellington 1991). Furthermore, the brief duration of maximum P*per , as compared to relatively long periods of hovering in normodense air at reduced P*per, reflects interactions between anaerobic metabolic pathway for burst power and aerobic pathway for endurance. Hummingbirds are capable of anaerobic hovering (Chai & Dudley 1996). In load-lifting study, hummingbirds carrying maximum loads reached similar kinematic constraints as birds hovering in hypodense gas mixtures, but generated 50% more maximum muscle power (Chai et al. 1997). However, the duration was very brief, of the order of 1 s. This again demonstrated an inverse relationship between the magnitude and duration of maximum power output.
Moulting birds generated less mechanical power at higher metabolic cost, in comparison to non-moult hummingbirds among which muscle mechanical and metabolic capacities were equivalent. Moulting birds thus displayed the lowest flight efficiency among the four bird groups (Table 1 and Fig. 3). Moult involves complex morpho-physiological changes, and high metabolic costs of hovering for moulting birds may be attributed to multiple causes. Moulting and associated reduction in flight performance is however transient. Since this study examined birds at their peak moult when wing area is at its minimum, the measured parameter values relating to hovering likely reflect the worst possible performance in this species.
Through intraspecific comparisons on hummingbirds with shorter wings (adult males), with increased body weight, and with wing area reduction (during moult), the present study demonstrated performance trade-offs that presumably reflect diverse flight challenges due to sexual dimorphism, migration, and plumage renewal. The integrated responses of multiple functional systems such as morphology, kinematics, muscle mechanics, and whole-bird metabolism define the range of flight activities that a hummingbird enjoys. The present study delineated reserve capacities of such systems at maximum hovering, reflecting functional design and safety margins shaped by both physical laws and natural selection. Future studies should explore if such varying hovering capacities are associated with behavioural and ecological compensation, particular in the context of foraging and resource utilisation as well as distribution and overall fitness of individuals.
ACKNOWLEDGMENTS
I thank R. Dudley for reviewing the manuscript. This work was supported by NSF grant IBN-9601089.
REFERENCES
Arnold, S.J. 1983. Morphology, performance and fitness. American Zoologist 23: 347-361.
Bennett, A.F. 1991. The evolution of activity capacity. Journal of Experimental Biology 160: 1-23.
Carpenter, F.L., Hixon, M.A., Beuchat, C.A., Russell, R.W. & Paton, D.C. 1993. Biphasic mass gain in migrant hummingbirds: body composition changes, torpor, and ecological significance. Ecology 74: 1173-1182.
Chai, P. 1997. Hummingbird hovering energetics during moult of primary flight feathers. Journal of Experimental Biology 200: 1527-1536.
Chai, P. & Dudley, R. 1995. Limits to vertebrate locomotor energetics suggested by hummingbirds hovering in heliox. Nature (London) 377: 722-725.
Chai, P. & Dudley, R. 1996. Limits to flight energetics of hummingbirds hovering in hypodense and hypoxic gas mixtures. Journal of Experimental Biology 199: 2285-2295.
Chai, P., Chen, J.S.C. & Dudley, R. 1997. Transient hovering performance of hummingbirds under conditions of maximal loading. Journal of Experimental Biology 200: 921-929.
Diamond, J.M. 1990. How to fuel a hummingbird. Nature (London) 348: 392.
Diamond, J.M. & Hammond, K.A. 1992. The matches, achieved by natural selection, between biological capacities and their natural loads. Experientia 48: 551-557.
Dudley, R. 1995. Extraordinary flight performance of orchid bees Apidae: Euglossini hovering in heliox 80 % He/20 % O
2. Journal of Experimental Biology 198: 1065-1070.Dudley, R. & Chai, P. 1996. Animal flight mechanics in physically variable gas mixtures. Journal of Experimental Biology 199: 1881-1885.
Ellington, C.P. 1984a. The aerodynamics of hovering insect flight. VI. Lift and power requirements. Philosophical Transactions of the Royal Society of London B, Biological Sciences 305: 145-181.
Ellington, C.P. 1984b. The aerodynamics of hovering insect flight. II. Morphological parameters. Philosophical Transactions of the Royal Society of London B, Biological Sciences 305: 17-40.
Ellington, C.P. 1991. Limitations on animal flight performance. Journal of Experimental Biology 160: 71-91.
Gosler, A.G., Greenwood, J.J.D. & Perrins, C. 1995. Predation risk and the cost of being fat. Nature (London): 377: 621-623.
Greenewalt, C.H. 1975. The flight of birds. Transactions of the American Philosophical Society 65: 1-67.
Hedenström, A. 1992. Flight performance in relation to fuel load in birds. Journal of Theoretical Biology 158: 535-537.
Hochachka, P.W. 1994. Muscles as Molecular and Metabolic Machines. Boca Raton, Florida; CRC Press: 158pp.
Jenni, L. & Winkler, R. 1994. Moult and aging of European passerines. London; Academic Press: 224pp.
Josephson, R.K. 1993. Contraction dynamics and power output of skeletal muscle. Annual Review of Physiology 55: 527-546.
Kullberg, C., Fransson, T. & Jakobsson, S. 1996. Impaired predator evasion in fat blackcaps (Sylvia atricapilla). Proceedings of the Royal Society of London B, Biological Sciences 263: 1671-1675.
Lindström, Å , Visser, G.H. & Daan, S. 1993. The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiological Zoology 66: 490-510.
Milliken, G.A. & Johnson, D.E. 1992. Analysis of messy data. New York; Von Nostrand Reinhold: 473pp.
Murphy, M.E. 1996. Energetics and nutrition of molt. In: Carey, C. (ed.) Avian energetics and nutritional ecology. New York; Plenum Press: 158-198.
Norberg, U.M. 1990. Vertebrate flight: mechanics, physiology, morphology, ecology and evolution. Berlin; Springer-Verlag: 291pp.
Norberg, U.M. 1995. How a long tail and changes in mass and wing shape affect the cost of flight in animals. Functional Ecology 9: 48-54.
Pennycuick, C.J. 1975. Mechanics of flight. In: Farner, D.S. & King, J.R. (eds) Avian biology. Vol. 5; New York; Academic Press: 1-75.
Pennycuick, C.J. 1996. Wingbeat frequency of birds in steady cruising flight: new data and improved predictions. Journal of Experimental Biology 199: 1613-1618.
Pennycuick, C.J. & Rezende, M.A. 1984. The specific power output of aerobic flight muscle, related to the power density of mitochondria. Journal of Experimental Biology 108: 377-392.
Pennycuick, C.J., Fuller, M.R., Oar, J.J. & Kirkpatrick, S.J. 1996. Wingbeat frequency and the body drag anomaly: wind tunnel observations on a thrush nightingale (Luscinia luscinia) and a teal (Anas crecca). Journal of Experimental Biology 199: 2757-2765.
Rayner, J.M.V. 1988. Form and function in avian flight. In: Johnston, R.F. (ed.) Current ornithology. Vol. 5; New York; Plenum Press: 1-66.
Rayner, J.M.V. 1990. The mechanics of flight and bird migration performance. In: Gwinner, E. (ed.) Bird migration: physiology and ecophysiology. Berlin; Springer-Verlag: 283-299.
Robinson, T.R., Sargent, R.R. & Sargent, M.B. 1996. Ruby-throated Hummingbird (Archilochus colubris). In: Poole, A. & Gill, F. (eds) The birds of North America, No. 204. Washington, D. C.; The Academy of Natural Sciences, Philadelphia, and the American Ornithologists' Union: 1-16.
SAS. 1989. SAS/STAT User's Guide, Version 6, 4th ed., Vol. 2; Cary, NC; SAS Institute Inc: 846pp.
Slagsvold, T. & Dale, S. 1996. Disappearance of female pied flycatchers in relation to breeding stage and experimentally induced molt. Ecology: 77: 461-471.
Suarez, R.K. 1992. Hummingbird flight: sustaining the highest mass-specific metabolic rates among vertebrates. Experientia 48: 565-570.
Suarez, R.K., Staples, J.F., Lighton, J.R.B. & West, T.G. 1997. Relationships between enzymatic flux capacities and metabolic flux rates: nonequilibrium reactions in muscle glycolysis. Proceedings of the National Academy of Sciences (USA) 94: 7065-7069.
Swaddle, J.P., Witter, M.S., Cuthill, I.C., Budden, A. & McCowen, P. 1996. Plumage condition affects flight performance in Common Starlings: implications for developmental homeostasis, abrasion and moult. Journal of Avian Biology 27: 103-111.
Wainwright, P.C. 1994. Functional morphology as a tool in ecological research. In: Wainwright, P.C. & Reilly, S.M. (eds) Ecological morphology, integrative organismal biology. Chicago; University of Chicago Press: 42-59.
Walsberg, G.E. 1983. Avian ecological energetics. In: Farner, D.S., King, J.R. & Parkes, K.C. (eds) Avian biology. Vol. 7; New York; Academic Press: 161-220.
Weis-Fogh, T. 1977. Dimensional analysis of hovering flight. In: Pedley, T.J. (ed.) Scale effects in animal locomotion. London; Academic Press: 405-420.
Wells, D.J. 1993. Muscle performance in hovering hummingbirds. Journal of Experimental Biology 178: 39-57.
Table 1. Statistical results assessing effects of body mass and bird group on morphological, kinematic, aerodynamic, and mechanical parameters relating to maximum hovering performance in hypodense air (see text for explanation of parameters). For morphological variables, ANOVA was used (body mass was not included as a covariate); for kinematic, aerodynamic, and mechanical variables, ANCONA was used. Least-squares means adjusted for the covariate effect of body mass as well as unbalanced sample size are used to show the mean and estimated standard error at maximum performance for each bird group. Moulting birds include 5 females, 3 juv. males, and 1 male.
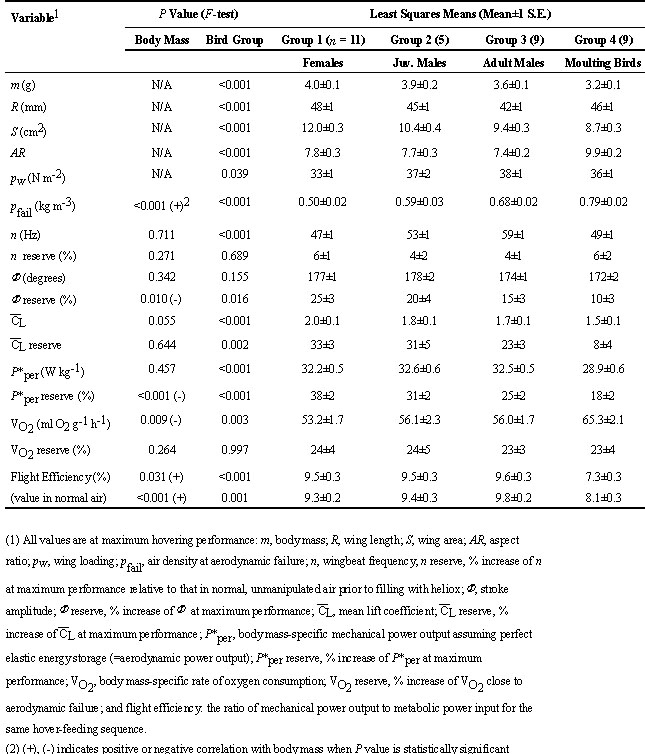
Fig. 1. Air density at maximum hovering performance prior to aerodynamic failure (A) of Ruby-throated Hummingbirds and their morphological indicators (B, body mass; C, wing length; D, wing area; E, aspect ratio; and F, wing loading) by bird groups. Values are means± 1 S.D. Four major bird groups with dissimilar wing morphology are identified: females, adult males, juvenile males (n = 5 birds), and molting birds. To demonstrate body mass effect, heavy females (> 4 g, n = 6) are separated from light ones (n = 5), and heavy males (> 3.5 g, n = 5) from light ones (n = 4). Molting birds are separated by sex: 5 females and 4 males (including 3 juvenile males). Moulting females on average possessed 77± 10% of non-moult wing area; moulting males 79± 5%. Their wing length did not change because 2 - 3 old, outer primaries still remained.
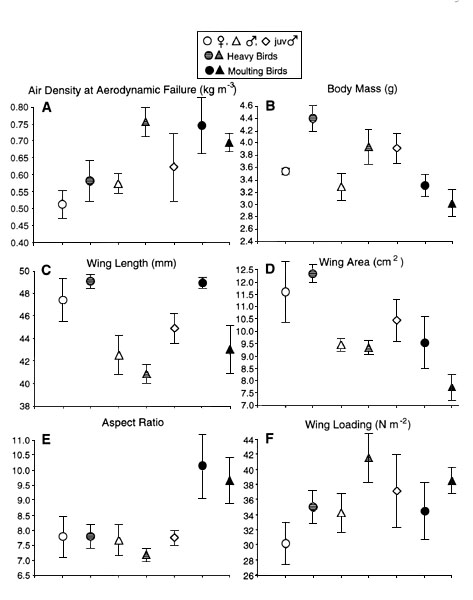
Fig. 2. Wingbeat frequency (A), stroke amplitude (B), and mean lift coefficient (C) by bird groups. Values are means± 1 S.D. Two values are presented for each bird group: left value comes from hovering in sea-level, unmanipulated air; right one at maximum hovering performance. Reserve capacity as % increase at maximum performance relative to normal hovering is indicated on the top.
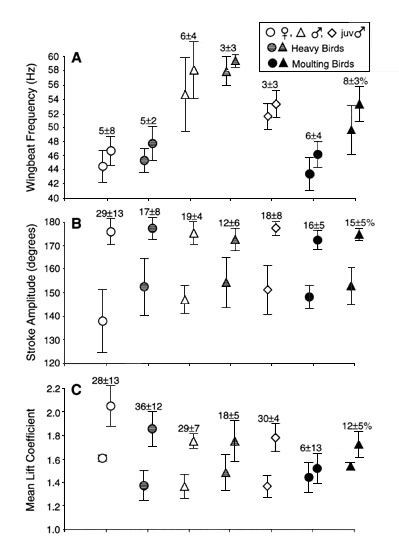
Fig. 3. Body
mass-specific power output assuming perfect elastic energy storage (A), rate of oxygen
consumption ![]() (B), and flight efficiency as ratio of
mechanical power output to metabolic power input (C). Values are means± 1 S.D.
(B), and flight efficiency as ratio of
mechanical power output to metabolic power input (C). Values are means± 1 S.D.