S30.5: Gonadal and hormonal patterns in the annual cycle of an Australian honeyeater
Lee B. Astheimer1 & William A. Buttemer2 1Australian Flora and Fauna Research Centre, Department of Biomedical Science, University of Wollongong, Wollongong 2522, Australia, fax 61 2 4221 4096, e-mail Lee_Astheimer@ uow.edu.au; 2Department of Biological Sciences, University of Wollongong, Wollongong 2522, Australia, e-mail buttemer@ uow.edu.au Astheimer, L.B., & Buttemer, W.A. 1999. Gonadal and hormonal patterns in the annual cycle of an Australian honeyeater. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1768-1783. Johannesburg: BirdLife South Africa.The White-plumed Honeyeater Lichenostomus penicillatus an insectivorous, old-endemic member of the Meliphagidae, is widely distributed in Australian riparian habitats. This resident honeyeater breeds most of the year and breeding/moult overlap is typical. Annual changes in reproductive condition in an arid-zone population were assessed by unilateral laparotomy and plasma homone levels. Females undergo cycles of gonadal recrudescence and regression, showing complete regression briefly in mid-winter. In contrast, adult males maintain enlarged testes all year, without gonadal regression. Testis size was not diminished during the post-nuptial moult, though it was reduced during a severe drought. Plasma levels of testosterone (T) in males were consistently low (less than 0.5 ng/ml in 80% of adult males) and did not correlate with testis size, moult condition, or female ovarian status. Free-living males challenged with intrajugular injections of LHRH showed consistently elevated LH levels compared with saline-injected controls, demonstrating efficacy of the hypothalamic-pituitary-gonadal axis. Captive birds given T-implants slowed their rate of primary moult, but continued the normal pattern. In contrast, similar implants in House Sparrows completely arrested moult. Apparently, in this honeyeater, tonic low levels of T maintain reproductive potential of males year round and its effects may be uncoupled from an inhibitory action on moult.
INTRODUCTION
Although less than one-third of Australia’s land mass lies in tropical latitudes, the life history patterns of most Australian passerines are remarkably similar to the strategies adopted by tropical birds generally. As in the latter group, many Australian passerines have characteristics such as flexible breeding schedules, limited migration, smaller than expected clutch sizes, longer than expected incubation, nestling and fledgling periods, moult/breeding overlap, and a high incidence of social co-operation (Yom Tov 1987; Ford 1989). We have been examining the morphological, physiological, and hormonal correlates of breeding in selected Australian passerines. The marked departure of our observations from those reported for north temperate avian species from similar latitudes have provoked us to reexamine the functional significance of interspecific variation in secretion and use of reproductive hormones to co-ordinate avian life history events.
Australia has a very high degree of endemism within its passerine avifauna. We have focused our research efforts on the so-called ‘ old endemic’ passerines which have a long evolutionary history in Australia. This group, within the Parvorder Corvida, is thought to have radiated in Australia about 65 MYA, giving rise to the Corvidae as well as many endemic Australo-Papuan families including Meliphagidae (honeyeaters), Maluridae (fairy wrens), Pomatostomidae (babblers) and Pachycephalidae (whistlers) from which our examples will be drawn. In contrast, the ‘ new endemic’ members of the Parvorder Passerida arose from Eurasian passeriform invasions around 25 MYA (Sibley & Alquist 1985; Christidis & Boles 1994). The extended endemism of Australia’s passerines provides the opportunity to examine how the phylogenetic and habitat differences of these birds might be reflected by differences in how they schedule and regulate reproductive events compared to their north-temperate counterparts.
Life history schedulesPopularisation of early observations of the opportunistic breeding behaviour of the Zebra Finch (a ‘new endemic’ Estrildid finch; Christidis 1987) has led to the widespread assumption that large scale nomadism is a characteristic common to most Australian passerines and that these birds are physiologically opportunistic, able to initiate breeding rapidly in response to locally erratic rainfall. Although such responses do occur in Zebra Finches (Zann 1996) and certain arid-zone species and/or populations (e.g. chats and some honeyeaters), only a small number of Australian passerines typically exhibit this extreme behaviour. In contrast, many Australian passerines are relatively sedentary. Yet many of these species maintain reproductive potential for extensive periods of the year. In fact, nearly 20% of Australia’s old endemic species are reported to breed year-round though they are neither nomadic nor inhabitants of the arid interior (Fig. 1).
Such apparent flexibility in breeding schedules raises a number of questions. How do these species schedule breeding and moulting events? Do they display gonadal regression and recrudescence as seen in seasonally breeding species? Does their pattern of reproductive steroid secretion differ from that of seasonal breeders? Before these questions can be addressed, however, one has to be certain that the species under study exhibits breeding flexibility at the individual rather than the population level. Given the widespread distribution of many of the Australian species reported to breed at any month of the year, it is always possible that individuals actually exhibit seasonal breeding, but that the seasons vary temporally between populations. Alternately, breeding flexibility within a population could result from staggered breeding schedules among a population of individuals having different inherent breeding rhythms, a pattern noted in noddies at Ascension Island (Ashmole 1961). Resolution of these concerns requires repeated capture of individuals over several annual cycles. Accordingly, we have focussed our attention on a sedentary population of White-plumed Honeyeaters Lichenostomus penicillatus, a species reported to breed year round (Simpson and Day 1993). We have studied a large resident population in arid western New South Wales where it occurs in association with stands of River Red Gum Eucalyptus camaldulensis, surrounding dams and along intermittent water courses. Over the past 3 years we have repeatedly captured free-living members of this population to assess plasma levels of reproductive hormones and gonadal condition by unilateral laparotomy. We report here a summary of this research along with pertinent information from studies of other old-endemic Australian passerines.
METHODSSpecies and Study Sites
The passerine species discussed in this paper were studied at several locations in Australia using common methodologies. White-plumed Honeyeaters and all other arid-zone species mentioned were studied at Fowler’s Gap Arid-Zone Research Station, New South Wales (latitude 31° S, longitude 142° E) operated by the University of New South Wales. Rainfall, minimum and maximum temperatures and other climatic parameters are measured daily by research station personnel. House Sparrows Passer domesticus is an introduced species in Australia and was captured in suburban gardens in Wollongong (34° 25'S, 150° 54'E). Rufous Whistlers Pachycephala rufiventris and White-browed Babblers Pomatostomus superciliosus were captured in Back Yamma State Forest, New South Wales (33° 20’S, 148° 10'E).
Capture, Blood Sampling and MorphometricsBirds were captured in Japanese mist nets and removed within 10 minutes of capture. A small blood sample (up to 200 ul depending upon species size) was taken by puncture of the alar vein with a 26 ga. needle immediately after capture. Blood was taken into microhaematocrit tubes and held on ice until centrifugation. Plasma was harvested and frozen until later hormone analyses by radioimmunoassay. Birds were fitted with individually numbered aluminium rings (Australian Birds and Bat Banding Scheme) and the following data were recorded: mass, wing and tarsal lengths, furcular and abdominal fat scores, plumage status (juvenile, gender) and moult description along with primary moult scores.
Gender and gonadal condition were assessed by unilateral laparotomy under methoxyflurane (PenthraneÒ ) anesthesia. Left testis length and width were measured and ovaries were scored 0-5 based on follicular activity. The incision was closed with medical grade cyanoacrylate glue (VetbondÔ ); birds were held in cloth bags until completely recovered and then released. The entire handling, including laparotomy and recovery, typically required 30 to 60 min. Females with active brood patches were not laparotomised.
GnRH ChallengeTo examine the competence of the hypothalamic-pituitary-gonadal (HPG) axis at different times of the year, we challenged adult male White-plumed Honeyeaters with either an intrajugular injection of gonadotropin releasing hormone (GnRH-I or chicken LHRH, Auspep; 500 ng in 10 ul saline) or an injection of saline (controls). Birds were selected on the basis of winglengths exceeding 80 mm, a measure we had previously determined would exclude 95% of the females. Free-living birds were injected within 10 minutes of capture; small blood samples (about 120 ul each) were taken at 5 and 15 minutes after the injection for LH and testosterone, respectively, as per Wingfield et al. (1991). Initially, a pre-injection sample was to be taken from each bird to provide background hormone levels, but this protocol was abandoned as it requiredexcessive sampling from these small (18 g) birds. Instead, background levels were obtained from sampling non-experimental adult males captured during the same period. Eight control and 8 GnRH-challenged experimental males were sampled during each of the five 2-week sampling periods during 1996 (in January, April, July, October and November). Testes size was determined by unilateral laparotomy after blood samples were completed.
Hormone AssaysTestosterone (T) was measured using a Pantex Direct I125 radioimmunoassay kit (# 135, Santa Monica CA) adapted for small plasma volumes. The lowest detectable mass of T in this assay is 2 pg. The intra-assay and interassay coefficients of variability at the low end of the standard curve (0.4 ng ml-1) are 10% and 12%, respectively. This assay was selected after earlier trials using the assay described by Wingfield and Farner (1975, 1978) and Ball and Wingfield (1987), an assay developed for detecting steroid levels in the small plasma samples obtained from passerine birds. Unfortunately, the latter assay, which employs a tritiated label, lacked adequate sensitivity to differentiate the very low testosterone levels in many of our samples.
Immunoreactive luteinizing hormone (LH) was measured using heterologous double antibody method described by Follett et al. (1972, 1975) and adapted according to J. Proudman (pers. comm). Chicken LH and specific LH antisera were a gift from the USDA Animal Hormone Program (J. Proudman; Bethesda, MD).
Testosterone Implant StudiesThe results of experiments (to be published independently) in which T was experimentally elevated using subcutaneous implants are briefly addressed. Implants were made of Silastic tubing (1.47 mm I.D., 1.96 mm O.D.; Dow-Corning) which were either packed with T (Sigma) or empty (control groups) and sealed at both ends with Silastic sealant. The implant length used, 5.5 mm, was based on values in the literature (Hegner & Wingfield 1987; Morton et al. 1990). Birds were lightly anaesthetised with methoxyflurane and implants were inserted through a small incision made in the body wall along the flank and placed under the thigh. The incision was closed with VetBond. Rufous Whistlers were given single implants while House Sparrows and White-plumed Honeyeaters were given bilateral implants.
RESULTSGonadal Phenology
The influence of photoperiodic cues on reproductive timing in Australian species with flexible breeding schedules has received little attention. However, our studies of the White-plumed Honeyeater indicate that photoperiod has little importance in entraining gonadal cycling, particularly in males.
Male White-plumed Honeyeaters do not undergo an annual pattern of gonadal regression and recrudescence, either as individuals (Fig. 2) or as a population (Fig. 3), although somewhat smaller testes were observed during the drought years 1994-95 and 1998. With few exceptions, adult males appear to maintain testes mass above two-thirds maximal size all year. We have yet to determine the relation between gonadal size and spermatogenic activity in this species. In contrast, female White-plumed Honeyeaters in this population tend to have reduced follicular activity during winter compared to other seasons (Fig. 3), particularly during protracted droughts. Despite this pattern, we found some females actively producing eggs (having a pronounced follicular hierarchy) and/or incubating at all times of the year, including a few days before the winter solstice.
Testosterone Levels and LHRH ChallengeAlthough male White-plumed Honeyeaters lack a distinctive anatomical gonadal regression, we expected measurement of reproductive hormones to show seasonal variation. This expectation was realised, but the amplitude in annual variation was very low. Further, plasma testosterone levels were surprisingly low at all times of year sampled, with levels attained during peak breeding an order of magnitude lower than those observed in north temperate species. In fact, T levels in many samples were below the detection threshold of our assay.
Male White-plumed Honeyeaters at our study areas show a consistent pituitary response to GnRH year-round (Fig. 4), with GnRH-injected birds having significantly higher levels of LH than comparable controls during the January, July and November sampling periods (t = 2.51, 3.97, and 2.1; P = 0.017, 0.003, and 0.03, respectively). During the April and October sampling periods, GnRH and Saline controls were not statistically different. Interestingly, maximal levels of LH in response to GnRH stimulation were achieved in mid-winter (July) suggesting the greatest pituitary sensitivity to GnRH at this time. Conversely, LH levels were lowest in GnRH- challenged birds in the spring (October) sample. Where background samples were available (April, October and November), these did not differ significantly from Saline controls.
Though there is a general trend for levels of T of GnRH-treated birds to be higher than Saline controls, this was not significant except in the April sampling period. Interestingly, the opposite trend existed for October when T levels of saline-treated controls appear to be higher than GnRH-treated birds (Fig. 5), although this difference did not reach statistical significance (t = -1.63, P = 0.08) . Testosterone levels in non-experimental birds (background) were in the same range or somewhat higher than Saline controls (although there were no background samples collected in April 1996), particularly during the spring (October/November).
Phenology of Moult and Effect of TestosteroneUnlike the temporal flexibility shown for breeding, White-plumed Honeyeaters at Fowler’s Gap display very consistent annual moult schedules. For example, primary moult scores measured at different stages of moult in 1995 and 1996 show a highly significant correlation between moult score and calendar date (r =0.99; P, 0.01). This in itself was not too surprising, however there were substantial differences in the timing of peak breeding in these two years. There was a drought-breaking rain in mid-summer of 1995 which stimulated summer breeding at a time when moult was at its peak in 1995. By contrast, breeding took place predominantly in late-winter to early spring in 1996, before moult was underway.
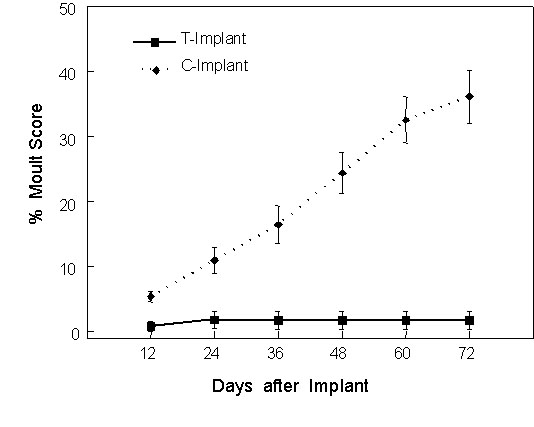
Male White-plumed Honeyeaters receiving testosterone implants had T levels similar to the endogenous levels of in north-temperate passerines during breeding (ca. 4 ng ml-1), but an order of magnitude higher than mean (average) endogenous levels found in breeding populations of White-plumed Honeyeaters. These T-implanted honeyeaters showed a 40% reduction in the rate of moult compared to control birds receiving empty implants (Fig. 6). By contrast, male House Sparrows receiving similar T-implants attained plasma T levels about twice as high as their usual peak breeding values (Hegner & Wingfield 1986), yet this treatment resulted in a dramatic complete arrest of moult within 12 days after implantation (Fig. 7; Fildes 1997).
DISCUSSIONGonadal Phenology
Partial testicular regression has been described in Zebra Finches (Farner and Serventy 1960) who suggested that it assured reproductive readiness in males of this opportunistically breeding species. Although White-plumed Honeyeaters do not share the nomadic, eruptive lifestyle found in Zebra Finches or north temperate crossbills (Loxia spp.), the most frequently cited examples of opportunistic breeders (e.g., Zann 1996; Newton 1973), we believe breeding in these birds is also centred around unpredictable resource availability. One of the dietary staples of these short-billed honeyeaters is honeydew produced by phytophagous lerps (psyllid insects) as well as the lerps themselves (pers. obs.). The abundance of these lerps, which show a preference for River Red Gum infestation, varies markedly in time and space and does not follow a predictable pattern. However, honeydew production often occurs in midwinter and birds use this resource extensively in the absence of nectar sources. We suspect that an individual female White-plumed Honeyeater’s reproductive capability is largely dictated by her relative energy balance, which means that breeding in mid-winter is possible for those females able to secure sufficient food to meet the added demands of egg production and winter thermoregulation.
Although we have not yet conducted experiments to evaluate the importance of photoperiod in the reproductive timing in this species, we have noticed that first-year males remain regressed, at least until the spring or summer after their first year. In contrast, adult males rarely show substantial declines in gonadal size once they become sexually mature. We are not certain what stimulates the persistence of gonadal size, but we have noticed that males do show gonadal regression when held in cages in isolation from females. In an experiment to examine the effect of testosterone on basal metabolic rates, we housed adult males separately, in visually isolated cages for an 8-week period. Those receiving empty silastic implants (control birds) showed more significant testicular regression in testes size than we have ever observed in the field, from an initial size of 6 x 4 mm down to 2 x 1.5 mm after 8 weeks. Those males receiving T-implants showed less reduction, completing the experiment with a mean testes size of 4 x 3 mm after the same period of confinement. It is important to note that birds with T-implants had much higher levels of T than we have measured in the field, so the natural testes maintenance we have observed in free-living birds cannot depend upon elevated T levels alone. We are now planning experiments to determine whether the presence of females or other social cues are important for year-round testes maintenance in adult males of this species.
Life history traits may also influence the annual pattern of male testicular size in old endemic species. For example, we have noted persistence of enlarged testes among some male White-browed Babblers throughout the year, a sedentary, co-operatively breeding species (Oppenheimer, Astheimer, & Buttemer, unpubl. data). In contrast, we have seen uniform testicular regression at the end of the breeding season in male Rufous Whistlers, a migratory, seasonally breeding passerine (McDonald 1998). Clearly, more comparative data are needed before patterns of gonadal activity can be correlated with life history characteristics in this phyletic group.
The persistence of enlarged testes in male White-plumed Honeyeaters suggests that these birds do not undergo an absolute refractory period as found in passerines studied to date (Hahn et al. 1998). In this context, the large number of Australian passerines reported to breed year-round suggests that the Australian habitat has favoured flexible breeding strategies and perhaps has favoured relative rather than absolute photorefractory mechanisms in these birds. Such flexibility in breeding readiness would be favoured in environments that lack highly predictable variation in resource availability, a situation that applies to much of Australia’s landmass (Nix 1976).
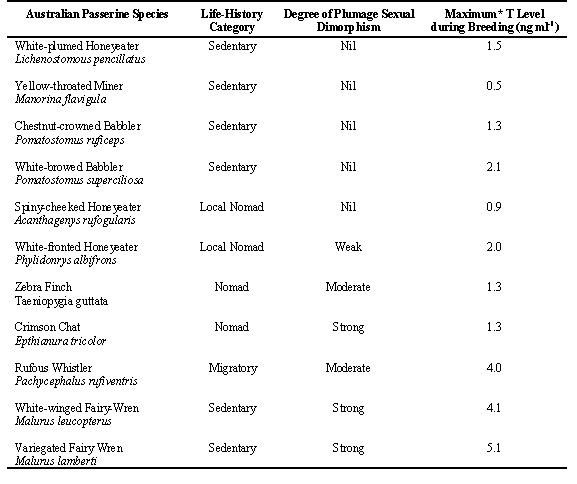
Testosterone Levels and LHRH ChallengeTestosterone levels in male White-plumed Honeyeaters are low, typically ranging between 0.3 and 1.0 ng ml during the spring and summer months (August through February) and are frequently undetectable during autumn and winter. These low levels are similar to those reported for several tropical species (e.g. Dittami 1986, Levin , pers. comm.). Our data for T secretion in other old endemic Australian passerines are less complete, but peak T levels in males sampled during periods of active breeding also show relatively low levels (Table 1). When we compare the highest T levels during breeding that we have measured in a range of Australian passerines, there does appear to be a general correlation with certain life-history characteristics (Table 1). We emphasise that these values were collected on birds associated with a breeding population, but we did not verify the breeding activities of each individual sampled. Nonetheless, birds with sedentary habits and little or no plumage sexual dimorphism have the lowest peak T values, species with strongly nomadic or migratory habits have intermediate levels, and species with dramatic plumage differences have the highest levels (Table 1). It is interesting that this relationship appears despite the very great difference in breeding strategy amongst the species listed (e.g. territorial pairs to co-operatively breeding groups).
The low T levels in combination with the absence of testicular regression observed in White-plumed Honeyeaters prompted us to examine whether the reproductive axis remained physiologically responsive all year. The GnRH-challenge experiment demonstrates that the flexible breeding of White-plumed Honeyeaters observed in the field is based upon flexibility of the HPG axis, the downstream elements of which remain sensitive to GnRH stimulation all year. Although responses to a range of GnRH doses were not examined, our data also suggest that the pituitary and testes are most sensitive to GnRH in autumn and winter. We would expect that lower levels of GnRH would be needed to stimlate HPG activity then than at other times of the year. Interestingly, the period we found lowest testicular senstivity to GnRH sensitivity—spring—is also when there is greatest breeding activity within this population. By October, most adults would have initiated at least one clutch and the HPG axis would have received ample stimulation to allow peak LH and T levels to be achieved during the course of a breeding event. At this phase of the annual cycle, pituitary and testicular sensitivity may fall as a result of inhibitory feedback from the relatively high circulating levels of LH and T, an idea supported by the lack of effect of GnRH on increasing T over the levels observed in the saline controls (October in Fig. 5). This seasonal change in testicular sensitivity is reminiscent of the reduction in sensitivity experienced in northern hemisphere breeders in the late breeding season where natural LH levels remain elevated, but the testes begin to regress, apparently insensitive to continued stimulation.
Phenology of Moult and Effect of TestosteroneThe Australian honeyeaters studied to date show a relatively slow rate of moult compared to north-temperate passerines from similar latitudes. For example, both coastal and arid-zone Honeyeaters take between 14 and 18 weeks to complete their moult (Keast 1968; Paton 1982), whereas many temperate Cardueline and Emberizid finches require between seven to 12 weeks to complete theirs (DeGraw & Kern 1990; Morton & Morton 1990; Newton 1972). Despite these differences in moult rate, all these species share the tendency to schedule moult at particular times each year (Keast 1968; Newton 1972). Because of the relatively protracted moult shown by the Melaphagidae from late spring to early summer, there is more opportunity for moult/breeding overlap to occur in these species than in those with more abbreviated moult schedules. In a three year study of Australian birds from arid regions, Keast (1968) noticed the co-occurrence of these two processes in many of the species that he studied.
The nearly identical moult schedule in years with and without moult/breeding overlap suggests that moult in White-plumed Honeyeaters is not inhibited by breeding as has been noted in some north-temperate passerines (Payne 1972). The low levels of T measured in White-plumed Honeyeaters may account for part of the apparent lack of inhibition that breeding activities have on moult in this species. However, there is also evidence for a reduced sensitivity to T in these male honeyeaters. The moult of male House Sparrows showed a much greater relative sensitivity to T (moult was suspended when T was increased to double the typical breeding level) compared to that of male White-plumed Honeyeaters (moult was 40% slower but persisted at T levels over 10-fold higher than their usual breeding levels). In addition, Schleussner et al. (1985) found that T doses as low as 1.5 ngml-1 reduced the moult rate of Starlings Sturnus vulgaris by more than 50%. Thus, moult in these north-temperate species is more sensitive to the inhibitory effects of T than is that of White-plumed Honeyeaters. Furthermore, because T is known to inhibit moult in a dose-dependent manner (Schleussner et al. 1985), the very low levels of T normally found in breeding White-plumed Honeyeaters would probably have minimal effect on moult rate of birds moulting and breeding simultaneously.
Moult/breeding overlap is more commonly reported in tropical than in temperate species (Foster 1975). Like the honeyeaters, moult rates in most tropical birds are relatively protracted compared to those of many north-temperate passerines. The slow rate of moult will reduce daily costs of moult in two ways; it reduces the amount of energy needed to overcome aerodynamic inefficiencies induced by rapid feather loss and it also reduces the daily increment of energy and nutrients required to sustain feather growth and replacement. These reductions in daily moult costs make moult/breeding overlap an energetically and nutritionally affordable option for these birds. By contrast, birds using a moult strategy that is confined to very short duration must partition much of their daily intake to this effort alone. Accordingly, such species with demanding moult schedules would be more compromised energetically by simultaneous moult and breeding. There is ample evidence in the literature demonstrating the mutual exclusion of these two energetically demanding processes in north temperate birds. We suggest that north temperate birds have evolutionarily avoided this overload by co-opting the use of hormonal signals that are intimately associated with the competing process of reproduction to inhibit moult. For males, this signal is likely to be T; females have not been as well-studied, but oestradiol, progesterone and even T (via central aromatisation) are possibilities. In species taking a more leisurely approach to the energy demands of moult and reproduction—such as tropical and Australian passerines, not only may the response to hormones be attenuated, but the hormonal signals themselves may be diminished in amplitude.
Evolutionary ImplicationsSome of the life history and physiological characteristics we have identified for the south-temperate population of White-plumed Honeyeaters are more similar to those of tropical passerines than to north-temperate birds. The obvious question that arises is: To what extent do the physiological characteristics of species reflect their phylogenetic constraints versus adaptive responses to variations in life history and environmental conditions? For example, are the very low peak levels of T in the male White-plumed Honeyeaters related mainly to their lineage?, to their sedentary life style?, or to their lack of abrupt phenotypic changes in plumage and/or behaviour throughout the year? Resolution of these questions would require a very elaborate experimental design, but the current literature permits some reasonable speculation.
It has long been appreciated that avian T levels vary markedly both intra- and interspecifically. An insightful analysis of the available data by Wingfield et al. (1990) revealed an important relationship between T levels and breeding systems. These authors showed that within breeding season variation in T levels was highest in species showing biparental care and least variant in polygynous species. They explained this pattern as a result of the need for biparental males to make a dramatic behavioural shift from territorial and mating behaviours associated with peak T levels to incubation and/or chick provisioning associated with low T levels. Wingfield et al. (1990) further suggested that the peak T levels reached by individual males in biparental species depended on their history of confrontation with conspecifics, which is the basis of the ‘challenge hypothesis’. Field studies of Song Sparrows strongly support this hypothesis; males living in high density habitats have higher T levels than conspecifics living in lower density (Wingfield and Hahn 1994) and males exposed to ‘new neighbours’ have higher T levels than birds in a socially stable population (Wingfield 1985). Furthermore, males breeding in highly synchronous populations have some of the highest average T levels known among passerines (Hunt et al. 1995 ).
Given that the challenge hypothesis is based on seasonally breeding birds of similar phylogenetic history, can it account for the very low levels of T we have found in flexibly breeding birds from a different lineage? The White-plumed Honeyeaters in our study populations are extremely sedentary, as evidenced by all repeated captures occurring within 200 metres of previous ones. The high incidence of repeated captures at these sites indicates that the adult composition is relatively stable throughout the year. According to the challenge hypothesis, this should lead to reduced T levels in this population; but phylogenetic constraints on T secretion might also be responsible for the extremely low levels we measured (typically less than 0.3 ng ml-1). We have recently measured T levels in a migratory population of Rufous Whistlers, an Australian old endemic with seasonal breeding in eastern New South Wales. According to the challenge hypothesis, we would predict that T levels should be higher in this species than in White-plumed Honeyeaters due to the whistler’s need to establish and defend territories against new neighbours each year. The T values are, in fact, much higher in the Rufous Whistlers, but their average peak breeding T level (1.8 ng ml-1) is still quite low compared to values measured in north-temperate males undergoing similar transitions in life history (e.g., Hunt et al. 1995; Wingfield & Farner 1978). Thus, the low T values in White-plumed Honeyeaters may reflect both their phyletic heritage as well as their sedentary life history.
We suggest that birds undergoing dramatic phenotypic changes in their annual cycle are more likely to exhibit large excursions in T than are those exhibiting more similar behaviour and appearance throughout the year. Thus, species undergoing dramatic behavioural changes in their annual cycle from flocking to territorial behaviour to being a parent (as in migratory, biparental species) can most effectively make transitions from one phenotype to the next by using an internal cue with a high signal to noise ratio. We believe that testosterone is used as such an endogenous cue. Thus, in species having the potential for high secretory rates of T in combination with high densities of T receptors at appropriate target tissues, rapid shifts in behaviour would be assured with changes in T-secretion. By contrast, sedentary species undergoing little annual change in behaviour or appearance would not need such signalling and thus, would rely on T primarily for its role in maintaining reproductive competence (e.g. spermatogenesis), processes requiring much lower plasma T levels. The latter pattern has been reported in several species of tropical passerines (Dittami 1986; Wingfield et al. 1991; Levin pers. comm.) and now in several old endemic sedentary Australian passerines. Thus, life history appears to strongly affect the extent to which birds rely on hormonal signalling in the transitional periods between reproductive and non-reproductive phases of their life cycle.
ACKNOWLEDGEMENTSWe gratefully acknowledge the assistance of the personnel of Fowler’s Gap Research Station, University of New South Wales and especially the logistical support provided by Paul and Chris Adams and John Beck. Some of the research described is from the yet unpublished work of post-graduate students Karen Fildes, Paul McDonald and Suzanne Oppenheimer. Excellent laboratory assistance was provided by Fiona Edwell and Karen Fildes. LH standard and antisera were a generous gift from John Proudman, USDA, USA. This research was supported by a grant from the Australian Research Council.
REFERENCESAshmole, N.P. 1963. The biology of the Wideawake or Sooty Tern Sterna fuscata on Ascension Island. Ibis 103: 297-364.
Ball, G. & Wingfield, J.C. 1987. Changes in plasma levels of luteinizing hormone and sex steroid hormones in relation to multiple-broodness and nest-site density in male starlings. Physiol. Zool. 60: 191-199.
Christidis, L. 1987. Phylogeny and systematics of estrildine finches and their relationships to other seed-eating passerines. Emu 87: 119-123.
Christidis, L. & Boles, W.E. 1994. The taxonomy and species of Australia and its territories. Royal Australasian Ornithologists Union, Melbourne.
DeGraw, W.A. & Kern, M.D. 1990. Postnuptial molt in Harris Sparrows. Condor 92: 829-838.
Dittami, J.P. 1986. Seasonal reproduction, moult and their endocrine correlates in two tropical Ploceidae species. J. Comparative Physiol. B. 156: 641-647.
Farner, D.S. & Serventy, D.L. 1960. The timing of reproduction in birds in the arid regions of Australia. Anatomical Record 137: 354.
Fildes, K. 1997. Avian moult and its relations in the White-plumed Honeyeater. Hons. Thesis. University of Wollongong, Australia.
Follett, B.K., Scanes, C.G., & Cunningham, F.J. 1972. A radioimmunoassay for avian luteinizing hormone. J. Endocrinology 52: 359-378.
Follett, B.K., Farner, D.S., & Mattocks, P.W. 1975. Luteinizing hormone in the plasma of whit-crowned sparrows (Zonotrichia leucophyrs gambelii) during artificial photostimulation. Gen. & Comp. Endo. 26: 126-134.
Ford, H.J. 1989. Ecology of birds: An Australian perspective. Surrey Beatty & Sons, Chipping Norton.
Foster, M.S. 1975. The overlap of molting and breeding in tropical birds. Condor 77: 304-314.
Hahn, T.P., Boswell, T., Wingfield, J.C., & Ball, G.F. 1997. Temporal flexibility in avian reproduction: Patterns and mechanisms. In: Nolan, V & Ketterson, E.D. (eds) Current Ornithology 14. Plenum Press; NY: 39-80
Hegner, R.E. & Wingfield, J.C. 1986. Behavioral and endocrine correlates of multiple-brooding in the semicolonial House Sparrow Passer domesticus. 1 Males. Hormones and Behavior 20: 294-312.
Hegner, R.E. & Wingfield, J.C. 1987. Effects of experimental manipulation of testosterone levels on parental investment and breeding success in male house sparrows. Auk 104:462-469.
Hunt, K.E., Wingfield, J.C., Astheimer, L.B, Buttemer, W.A., & Hahn, T.P. 1995. Temporal patterns of territorial behavior and circulating testosterone in the Lapland Longspur and other Arctic passerines. Amer. Zool. 35: 274-284.
Keast, A. 1968. Moult in birds of the Australian dry country relative to rainfall and breeding. Journal of the Zoological Society of London 155: 185-200.
McDonald, P. 1998. Breeding biology and role of testosterone in Rufous Whistlers (Pachycephala rufiventris). Hons. Thesis. Univ. of Wollongong, Australia.
Morton, G.A. & Morton, M.L. 1990. Dynamics of the postnuptial moult in free-living Mountain White-crowned sparrows. Condor 92: 813-828.
Newton, I. 1972. Finches. William Collins Sons & Co. Ltd., London.
Nix, H.A. 1976. Environmental control of breeding, post-breeding dispersal, and migration of birds in the Australian region. Proceedings of the XVI International Ornithological Congress (Canberra): 272-305.
Paton, D.C. 1982. Moult of New Holland honeyeaters in Victoria, I. Moult of adults. Australian Wildlife Research 9: 345-356.
Payne, R.B. 1972. Mechanisms and control of moult. In: Farner, D.S. & King, J.R. (eds) Avian Biology 2. Academic Press: 104-157.
Schleussner, G., Dittami, J.P., & Gwinner, E. 1985. Testosterone implants affect molt in European Starlings, Sturnus vulgaris. Physiol. Zool. 58:597-604.
Sibley, C.G. & Ahlquist, J.E. 1985. The phylogeny and classification of birds of the Australo-Papuan passerine birds. Emu 85: 1-14.
Simpson, K. & Day, N. 1993. Field guide to the birds of Australia. (4th Ed.). Viking O’Neil, Ringwood, Victoria.
Wingfield, J.C. 1985. Short-term changes in the plasma levels of hormones during establishment and defence of a breeding territory in male song sparrows, Melospiza melodia . Horm. Behav. 19: 174-187.
Wingfield, J.C. & Farner, D.S. 1975. The determination of five steroids in avian plasma by radioimmunoassay and competitive protein binding. Steroids 26: 311-327.
Wingfield, J.C. & Farner, D.S. 1978. The endocrinology of a natural breeding population of the white-crowned sparrow (Zonotrichia leucophrys pugetensis). Physiol. Zool. 51:188-205.
Wingfield, J.C., Hegner, R.E., Dufty, A.M., & Ball, G.F. 1990. The ‘Challenge Hypothesis’: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136: 829-846.
Wingfield, J.C., Hegner, R.E., & Lewis, D.M. 1991. Circulating levels of luteinizing hormone and steroid hormones in relation to social levels in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. J. Zool. Lond. 225: 43-58.
Wingfield, J.C. & Hahn, T.P. 1994. Testosterone and territorial behaviour in sedentary and migratory sparrows. Anim. Behav. 47: 77-89.
Yom-Tov, Y. 1987. The reproductive rates of Austrailan passerines. Austr. Wildl. Res. 14: 319-330.
Zann, R.H. 1996. The Zebra Finch: A synthesis of field and laboratory studies. Oxford Univ. Press, New York.
Table 1. Maximum testosterone levels measured during the breeding season in a variety of ‘old endemic’ Australian passerines (excepting Zebra Finch). Values are based on highest T levels measured in at least 1 adult male captured during a period when conspecifics were breeding and gonads were enlarged. Note that mean values (where available0 are considerably lower.

Fig. 1. Life history and habitat associations of 53 species of old-endemic Australian passerines reported to breed year round (from Simpson & Day 1993).
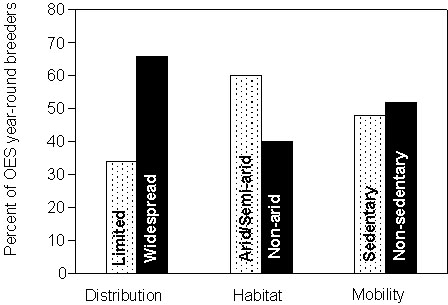
Fig. 2. Length of left testis (mm) of adult White-plumed Honeyeaters recaptured at Fowler’s Gap Station, NSW in relation to calendar date. Each point represents a single capture; points with the same symbol indicate recapture and re-measurement of the same individual.
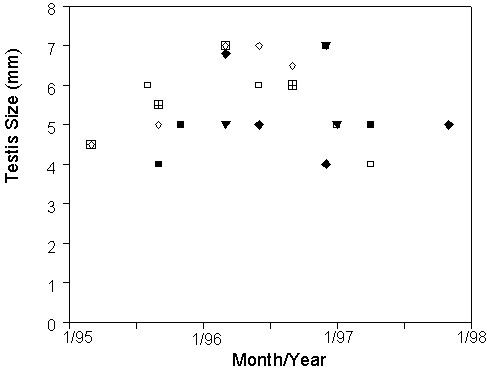
Fig. 3. Phenology of gonadal condition in adult White-plumed Honeyeaters; left testis length, mm, in males and ovarian score (0-5) in females at Fowler’s Gap Station over 3 years. Points represent Means +/- S.E. N varies from 2-15 individuals per symbol.
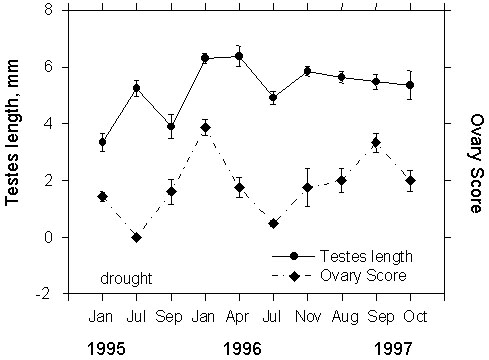
Fig. 4. Effect of GnRH and Saline (Control) injections on LH release in adult male White-plumed Honeyeaters in relation to time of year. Asterisks represents statistically significant (P < 0.05) differences between GnRH- and Saline-injected birds. During each sample period at least 5 Saline- and 8 GnRH-injected birds are represented. Background samples (not available for January and July) represent samples taken from non-injected, free-living birds and vary between 3-7 birds.
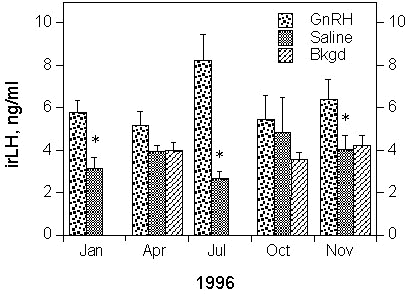
Fig. 5. Effect of GnRH and Saline (Control) injections on T release in adult male White-plumed Honeyeaters in relation to time of year. Asterisks represents statistically significant (P < 0.05) differences between GnRH- and Saline-injected birds. During each sample period at least 5 Saline- and 6 GnRH-injected birds are represented. Background samples (not available for January and July) represent samples taken from non-injected, free-living birds and vary between 3-7 birds per sampling period.
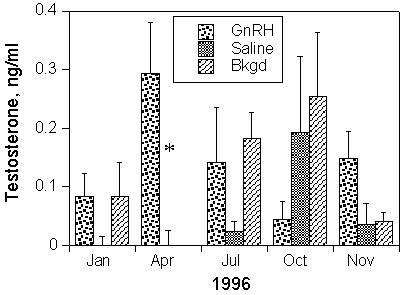
Fig. 6. Moult scores in relation to time following implant of either testosterone-filled or empty silastic implants in male White-plumed Honeyeaters. Points represent Mean +/- S.E.; n = 11.
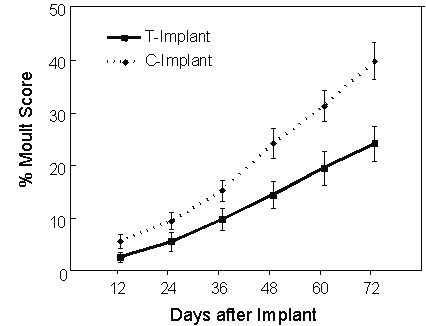
Fig. 7. Moult scores in relation to time following implant of either testosterone-filled or empty silastic implants in male House Sparrows. Points represent Mean +/- S.E.; n decreased from 12 to 7 as a result of mortality during the course of the experiment.