S30.3: Proximate and ultimate aspects of photoperiodic sensitivity in equatorial Stonechats Saxicola torquata axillaris
Alexander Scheuerlein & Eberhard Gwinner
Research Centre for Ornithology of the Max-Planck Gesellschaft, Von-der- Tannstrasse 7, 82346 Andechs, Germany, fax 0049 8152 37333, e-mail scheuerlein@erl.ornithol.mpg.de
Scheuerlein, A. & Gwinner, E. 1999. Proximate and ultimate aspects of photoperiodic sensitivity in equatorial Stonechats Saxicola torquata axillaris. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1756-1766. Johannesburg: BirdLife South AfricaEquatorial Stonechats of East Africa S.t. axillaris are seasonal breeders. Their gonads develop in anticipation of the short rains during which reproduction occurs. Subsequently gonads regress and the birds moult. Changes in gonadal size and moult are primarily controlled by an endogenous rhythm. Under seasonally constant conditions this rhythm persists with a period deviating from 12 months, indicating that under natural conditions it is usually synchronised by seasonal environmental cues. The nature of these Zeitgeber stimuli has not yet been identified. In the laboratory equatorial Stonechats were found to respond to photoperiodic changes. However, it is unclear whether the small day-length changes that occur at the equator are sufficient for circannual synchronisation. It appears conceivable that the effective photoperiod caused by seasonal variations in weather conditions may suffice to synchronise reproduction appropriately with local precipitation cycles. Alternatively, seasonal changes of light intensity resulting from changes in cloud cover or the clearness of the atmosphere may provide synchronising cues; these may be perceived by the same mechanism that measures photoperiod. It is also possible that the photoperiodic responsiveness of equatorial Stonechats evolved in populations inhabiting higher latitudes and was transmitted to equatorial populations through gene-flow resulting from juvenile dispersal. Finally, the photoperiodic responsiveness may be a phylogenetic relic dating back to eras when ancestors of the equatorial birds inhabited the temperate zones.
INTRODUCTION
Equatorial Stonechats Saxicola torquata axillaris in Africa are seasonal breeders, like their temperate-zone conspecifics (S.t. rubicola) in Europe. At Lake Nakuru, Kenya, which is situated at the equator, Stonechats start developing their gonads in November, the beginning of the main dry season and initiate breeding at the onset of the rains in March or April (Dittami & Gwinner, 1985). On Mount Meru in Tanzania, about 250 km south of Lake Nakuru, the birds begin breeding as early as October, concomitant with the much earlier onset of the drought-breaking rains in this area. Which proximate cues are the basis for the birds’ seasonality of breeding and the timing of their reproduction?
Investigations on the timing of breeding in birds have suggested that there are two sets of proximate factors (Wingfield & Farner, 1980; Wingfield, 1983):
(1) Initial predictive factors (e.g. endogenous circannual rhythms and photoperiod).
(2) Modifying factors.
In this paper we focus on initial predictive factors and the way in which they orchestrate the succession of the various stages in the annual cycle of Stonechats.
Initial predictive factors dictate the general timing of breeding as well as other processes such as moult and migration (Wingfield & Farner, 1980). It is well-known that photoperiod can provide this information at least in temperate zones. In some avian species photoperiod acts as a Zeitgeber on an underlying endogenous circannual rhythm (Gwinner, 1986). In the following we present evidence that an endogenous circannual rhythmicity may make a greater contribution to the control of seasonal activities in the equatorial subspecies of the Stonechats than in the temperate-zone subspecies. Then we discuss the possible role of photoperiod and light intensity as potential synchronisers of circannual rhythms of both subspecies. Our data demonstrate that in addition to being a potential Zeitgeber, photoperiod also exerts a variety of other effects on several life-history traits of these birds. Finally we provide evidence that the two subspecies may differ in their reaction norms to photoperiodic cues.
ENDOGENOUS CIRCANNUAL RHYTHMS
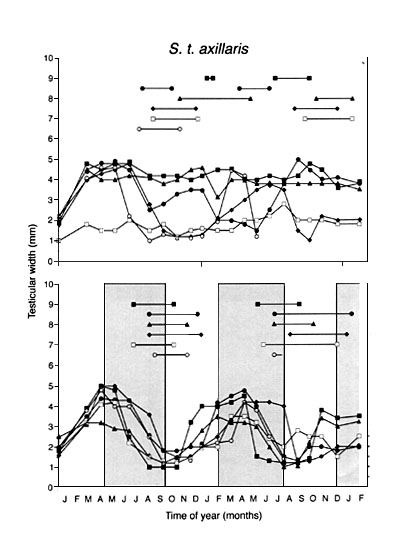
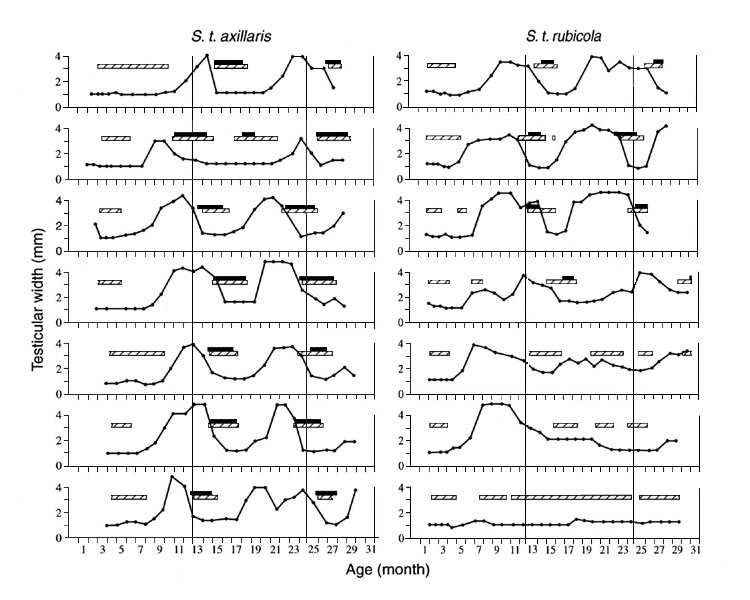
In the field in Kenya, gonads of the equatorial subspecies of Stonechats start to develop months ahead of the breeding season. At the end of the reproductive period, gonads regress and a postnuptial moult ensues. The interval between successive onsets of gonadal growth is, on average, one year (Dittami & Gwinner, 1985). Under a constant light-dark cycle of 12.25h L, 11.75h D in the laboratory a rhythm in gonadal size and moult also persists but its period now deviates from one year. A continuing circannual rhythm in gonadal size and moult can be observed in birds of both subspecies. However, as seen in Fig. 1, rhythmicity was abolished in a high proportion of birds of the European subspecies before the experiment was over, whereas most of the equatorial birds retained rhythmicity throughout the experiment. Up to 12 successive cycles could be observed in equatorial Stonechats kept in a constant photoperiod (Gwinner, 1996 b). This finding suggests that endogenous circannual rhythms are more significantly involved in the control of annual cycles in equatorial than in temperate-zone Stonechats. Possibly selection pressure favouring the evolution of circannual rhythms is stronger in the equatorial than in the temperate zone subspecies.
THE INFLUENCE OF PHOTOPERIOD ON THE REPRODUCTIVE SYSTEM OF STONECHATS: LABORATORY STUDIES
Photoperiod as a Zeitgeber
Photoperiod acts as a reliable and strong Zeitgeber in temperate-zone birds, which so far have been the focus of research (Murton & Westwood, 1977; Farner & Follett, 1979; Farner & Gwinner, 1980; Follett, 1984; Gwinner, 1986; Wingfield & Kenagy, 1991; Wingfield & Farner, 1993). When European Stonechats were kept in cages under a photoperiodic cycle with a period of one year simulating conditions at 47.5 ° N in summer (breeding site) and respectively 40 ° N in winter (wintering site) their gonadal and moult cycles became synchronised with it (Fig. 2, upper left) (Gwinner & Scheuerlein, 1999). Birds of the equatorial subspecies were also able to synchronise their gonadal rhythms with this temperate-zone photoperiodic cycle, although the birds’ rhythms showed more inter-individual variability than those of the temperate-zone birds (Fig. 2, lower left). To further test whether photoperiod indeed functions as a Zeitgeber, the period of the photoperiodic cycle was reduced to 6 months. Birds of both subspecies shortened the period of their gonadal and moult cycles accordingly to the shortened photoperiodic cycle (Fig. 2, upper and lower right), indicating that a high-amplitude temperate-zone photoperiodic cycle can act as a Zeitgeber on the endogenous rhythms of both temperate-zone and equatorial birds (Gwinner & Scheuerlein, 1999). Photoperiodic effects on annual reproductive cycles have also been documented in other tropical, partly equatorial, passerines (Rollo & Domm, 1943; Miller, 1959; Miller, 1965; Wolfson & Winchester, 1959; Disney, Lofts, & Marshall, 1961; Epple, Orians, Farner, & Lewis, 1972; Chandola & Chakravorty, 1982; Gwinner & Dittami, 1985; Tewary & Dixit, 1986; Hau, Wikelski, & Wingfield, 1998). Even the domestic Bantam chicken, a bird with tropical ancestry, responds to photoperiod (Sharp, 1984).
When Stonechats were kept in a low-amplitude photoperiodic cycle simulating tropical conditions at 10° latitude (70 min amplitude) but with a period of only 6 months, birds of the two subspecies behaved differently: none of them synchronised its rhythm in a 1:1 fashion to the photoperiodic cycle (Fig 3.). However, most of the equatorial birds eventually regressed their gonads, whereas most of the temperate-zone birds retained large gonads for almost one year (Gwinner & Scheuerlein, 1999). The most likely interpretation of this difference is that the critical photoperiod for the induction of photorefractoriness (for which most avian species require long days; (Nicholls, Goldsmith, & Dawson, 1988) is longer in the temperate-zone birds than in the equatorial birds. This is consistent with the fact that during the time at which photorefractoriness is initiated, temperate-zone birds are exposed to long photoperiods, whereas equatorial birds are exposed to relatively shorter photoperiods. This interpretation implies that the photoperiodic system of equatorial Stonechats has been subject to selection specific for tropical conditions, resulting in a shortening of the critical daylength required for the induction of refractoriness. Evolutionary adjustments of critical photoperiods controlling refractoriness have also been shown in long-distance migrants, e.g. flycatchers (Gwinner, 1996a).
Photoperiod as a modulating factor
Photoperiod, in addition to its synchronising action, exerts other effects on seasonal timing and life-history traits in both temperate-zone and equatorial Stonechats. Usually these effects have been found to be qualitatively similar but quantitatively different between the two subspecies, indicating subspecies-specific reaction norms.
A typical example is provided by postjuvenile moult, during which the nestling plumage is replaced by the adult plumage. Evidence from field studies suggests that postjuvenile moult is initiated earlier and completed sooner in the temperate-zone than in the equatorial subspecies ( Flinks, 1999). This difference is related to the fact that the temperate-zone birds are migrants and consequently have to moult earlier in life to be ready for migration to their wintering quarters with a fresh plumage. The equatorial birds, in contrast, are non-migratory and can therefore moult later. The subspecies difference was retained in birds raised and kept in identical conditions under both an equatorial or a temperate-zone photoperiod. F1-hybrids between the subspecies showed an intermediate pattern (Gwinner & Neusser, 1985; Helm & Gwinner, 1999), indicating a genetic basis for the subspecies difference. Furthermore, the subspecies differed in their reaction to the two photoperiods: the difference in moult onset and end under the short equatorial and the long temperate-zone photoperiod was larger in the temperate-zone than in the equatorial birds (Helm & Gwinner, unpubl. results). In the temperate-zone birds the advancing effect of short photoperiods may have evolved as a mechanism that allows birds from late broods (which experience shorter photoperiods than birds from early broods) to complete moult at an earlier age. This enables birds from late broods to be ready for autumn migration at about the same time as birds from earlier broods. Similar effects are also known from other temperate-zone migrants (Gwinner & Neusser, 1985; Berthold, 1988). The significance of the effect of photoperiod on moult in the equatorial birds is yet unknown.
Another example of a photoperiod-dependent trait is the interval between successive broods, measured as the time span between the laying date of the first egg of a second or third clutch and the fledging date of the preceding clutch. It was found to be larger in the equatorial than in the temperate-zone birds kept in a 47.5° N photoperiod (Koenig & Gwinner, 1995). At least in the equatorial birds this interval seems to depend on photoperiod: it tended to be longer in birds kept in an equatorial photoperiod than in birds kept in a temperate-zone photoperiod. The apparent subspecies-specific fixation of the trait is consistent with the results from a between-species comparison, which revealed that tropical birds generally tend to require more time for renesting than temperate-zone species (Ricklefs, 1969).
While photoperiod has clear effects on the synchronisation of the circannual cycle as a whole and on particular aspects of the circannual rhythmicity, other parameters characteristic for the life cycles of these birds appear to be more resistant to photoperiodic modulation. Examples include clutch size (Gwinner, Koenig, & Haley C, 1995), the incubation and nestling periods (Koenig & Gwinner, 1995) and the growth rate of nestlings (Starck, König, & Gwinner, 1995), for which experimental studies failed to reveal any photoperiodic effects. It is likely that these traits are more susceptible to non-photic proximate environmental factors. Nestling growth rates, for instance, were affected by factors such as holding conditions in laboratory studies (Starck, et al., 1995) and by predator presence in the parents’ territory in the field (Scheuerlein, Gwinner unpublished data).
WHAT IS THE ORIGIN AND FUNCTION OF THE PHOTOPERIODIC RESPONSIVENESS OF THE EQUATORIAL STONECHATS?
The above-mentioned laboratory studies have shown that some basic features of the annual cycles of equatorial Stonechats can be affected by photoperiod, usually in the same way as those of their temperate-zone conspecifics. How can this sensitivity to photoperiodic changes, which are essentially absent in the natural environment of the equatorial birds, be explained? Several hypotheses have been proposed (Gwinner & Dittami, 1985), of which the three most important ones are briefly summarised.
Birds perceive weather-dependent changes in light intensity.
Changes in cloud cover or in the clearness of the atmosphere associated with the onset and offset of the rainy seasons generate conspicuous differences in light intensity. Theoretically these changes might be used as seasonal cues. Two modes of action are conceivable:
The changes in light intensity result in changes of effective photoperiod: during days with low daytime light intensity any threshold level of light intensity is reached later in the morning and earlier in the evening than during days with high daytime light intensity. The magnitude of the variations in effective daylength thus generated is unknown. It depends on the minimal light intensity required for photoperiodic reactions, which has not yet been studied in any tropical bird.
The changes in light intensity itself are perceived by the photoperiodic response system, supplying birds with systematically changing photic information; this information is independent of day length but may be measured by the same mechanism. Fig. 4 summarises the results of an experiment in which male equatorial Stonechats were kept for two years in a constant 12.25h photoperiod in the absence of any twilight phases (Gwinner & Scheuerlein, 1998). For the control group (upper diagram) light intensity during the light phase (measured as illuminance) was between 2000 lux and 12000 lux (depending on the position of the birds inside the cage) throughout the experiment. For the experimental group light intensity alternated, on the basis of a 300-day cycle, between 2000 and 12000 lux during one part of the cycle and 250 and 1,600 lux during the other. In all experimental birds testicular size and moult were clearly rhythmic with a period close to the period of the 300-day light intensity cycle. Among the control birds only two showed a rhythm in testicular size and five exhibited a rhythm in moult; however, inter-individual variability in gonadal and moult period was high and most periods differed considerably from 300 days. Four birds showed no obvious testicular rhythmicity. While the performance of the experimental birds indicates that the light intensity cycle is capable of synchronising circannual rhythms, the variable and mainly arrhythmic performance of the control birds (contrasting with the rhythmic behaviour in constant low-light intensity photoperiodic conditions) suggests caution in drawing too far-reaching conclusions from this study. Nevertheless, the results provide an incentive for future research aimed at answering the question whether light intensity changes may provide seasonal cues for synchronisation.
Sensitivity to photoperiod is adaptive on a population level
The East African Stonechat subspecies S.t. axillaris breeds from 5° north to 10° degrees south of the equator (Keith, Urban, & Fry, 1992); in the southernmost areas of the range the amplitude of the photoperiodic cycle may be as large as about 70 min. It is possible that the birds are able to synchronise to a day-length change of this magnitude when it has an annual periodicity. Depending on the still unknown distance and direction of dispersal, juvenile Stonechats that leave equatorial regions may reach areas where the amplitude of the photoperiodic cycle is large enough to be effective; as a consequence, a photoperiodic timing mechanism may have evolved in these regions. Conversely there may be dispersal from higher latitudes in the north or south into regions close to the equator with its minute photoperiodic changes. Provided that the photoperiodic responsiveness acquired in higher latitudes is not disadvantageous at the equator, equatorial birds would retain their photoperiodic responsiveness and thus be equipped with a mechanism that exerts its function only in other regions. Hence, sensitivity to photoperiod could be the result of gene-flow benefiting some subpopulations but not others. Such a system would also work, if the dispersal of birds across the whole range of the subspecies took many successive generations. A possible approach to test this hypothesis is to investigate the genetic diversity of the population as well as the dynamics of juvenile dispersal throughout the range of the equatorial subspecies.
Sensitivity to photoperiod is retained as a phylogenetic relic
It is likely that the genus Saxicola originated in the temperate zones with their pronounced photoperiodic changes (Voous, 1960; Hall & Moreau, 1970). Under these conditions the evolution of a photoperiodic response system is adaptive. At some later stage the birds of this genus colonised the African continent and established themselves successfully in regions near the equator. Since in these regions photoperiodic variations are too small to be useful, the birds selected other, as yet unknown environmental cues. In the absence of selection pressures against it, however, the photoperiodic response system was maintained.
Conclusions
It is impossible on the basis of the available results to reject any one of the above hypotheses, and each of them is supported by some data.
The possibility that some aspect of light may actually play a role in synchronisation at the equator is supported by the results, shown in Fig. 2 and Fig. 4, indicating that equatorial birds are not only highly responsive to photoperiod but also may respond to light intensity changes. Changes in photoperiod are not entirely absent at the equator and - given that the effective photoperiod is modified by cloud cover and atmospheric conditions - may indeed be larger than hitherto assumed. Therefore selection may have favoured the evolution of a photo-sensitive system even at the equator. Also, as has recently been shown for the neotropical Spotted Antbird (Hylophylax naevioides), the photoperiodic discrimination threshold may be as low as 17 min or even smaller (Hau, et al., 1998).The magnitude of seasonal light intensity changes in the tropics and their effectiveness in seasonal timing have barely been investigated yet. However, it has been shown in several studies that an increase in light intensity may have the same effect as a lengthening of photoperiod (Bissonette, 1937; Burger, 1949; Bentley, Goldsmith, Dawson, Briggs, & Pemberton, 1998). Although in these studies much lower light intensity levels were used than in our study on Stonechats, these results suggest that the measurement of light intensity and photoperiod may be accomplished by the same mechanism. Such a common mechanism may facilitate the exchange of individuals from equatorial regions (where the range of light intensity variation is greater than the photoperiodic amplitude) with individuals from higher latitudes (where changes in photoperiod override those of light intensity). Light intensity control of the annual cycles in tropical birds may explain the close and population-specific association of breeding seasons with rainy seasons, because seasonal light intensity changes are likely to result from changing weather conditions. Regarding the high variability of rainfall patterns between years, seasonal light intensity changes appear to be better indicators of the rainfall pattern in a given year than photoperiod itself.
The remaining two hypotheses discussed above state that the sensitivity of equatorial birds to photoperiod has evolved as a result of selection pressures in more northerly or southerly regions of the population range and that it has manifested itself in the equatorial subpopulation through gene-flow which is either still going on (first hypothesis one) or has occurred in the past (second hypothesis). Consistent with these hypotheses is the higher inter-individual variability of seasonal timing among equatorial Stonechats exposed experimentally to photoperiodic cycles (Fig. 2). On the other hand, equatorial Stonechats were found to give qualitatively different responses to the low-amplitude photoperiodic cycle of Experiment 3, suggesting that selection may have resulted in different properties of the photoperiodic response system in the equatorial than in the temperate zone-birds.
ACKNOWLEDGEMENTS
We appreciate the financial support of the Max-Planck Society to conduct the studies. U. Abraham, W. Goymann, M. Hau, S. Heigl and M. Wikelski made valuable comments on the manuscript.
REFERENCES
Bentley, G.E., Goldsmith, A.R., Dawson, A., Briggs, C. & Pemberton, M. 1998. Decreased light intensity alters the perception of day length by male European Starlings (Sturnus vulgaris). J. Biol. Rhythms, 13, 140-150.
Berthold, P. 1988. The control of migration in European Warblers. In: XIX Congr. Int. Orn., Ottawa: 215-249.
Bissonette, T.H. 1937. Photoperiodicity in birds. Wilson Bull., 49, 241-270.
Burger, J.W. 1949. A review of investigations on seasonal reproduction in birds. Wilson Bull., 61, 211-230.
Chandola, A. & Chakravorty, K. 1982. Termination of seasonal breeding in the photoperiodic weaver bird. Journal of Experimental Zoology, 222, 169-172.
Disney, H.J.d.S., Lofts, B. & Marshall, A.J. 1961. An experimental study of the internal rhythm of reproduction in the red-billed dioch Quelea quelea by means of photostimulation, with a note on melanism induced in captivity. Proceedings of the Zoological Society London, 136, 123-129.
Dittami, J. & Gwinner, E. 1985. Annual cycles in the African stonechat Saxicola torquata axillaris and their relationship to environmental factors. Journal of Zoology, London, 207, 357-370.
Epple, A., Orians, G.H., Farner, D.S. & Lewis, R.A. 1972. The photoperiodic testicular response of a tropical finch, Zonotrichia capensis costaricensis. Condor, 74, 1-4.
Farner, D.S. & Follett, B.K. 1979. Reproductive periodicity in birds. In: Barrington, J.W. (ed.) Hormones and Evolution: 829-872. New York: Academic Press.
Farner, D.S. & Gwinner, E. 1980. Photoperiodicity, circannual and reproductive cycles. In: Epple, A. & Stetson, M.H. (eds) Avian Endocrinology: 331-366. New York: Academic Press.
Flinks, H. 1999. Pattern, intensity and duration of the post-juvenile moult in stonechats. Vogelwarte, in press.
Follett, B.K. 1984. Birds. In: Lamming, G.E. (ed.) Marshall's physiology of reproduction: 283-350. Edinburgh: Churchill Livingston.
Gwinner, E. 1986. Circannual Rhythms. Heidelberg: Springer.
Gwinner, E. 1996a. Circannual clocks in avian reproduction and migration. Ibis, 138(1), 47-63.
Gwinner, E. 1996b. Der innere Kalender tropischer Vögel. Biologie in unserer Zeit, 26(3), 153-162.
Gwinner, E. & Dittami, J. 1985. Photoperiodic responses in Temperate Zone and Equatorial Stonechats: a Contribution to the Problem of Photoperiodism in tropical Organisms. In: Follet, B.K., Ishii, S. & Chandola, A. (eds) The Endocrine System and the Environment: 279-294. Berlin: Springer-Verlag.
Gwinner, E. & Neusser, V. 1985. Postjuvenile molt of European and African Stonechats Saxicola torquata rubicola and Saxicola torquata axillaris and their F-1 Hybrids. Journal für Ornithologie, 126(2), 219-220.
Gwinner, E., Koenig, S. & Haley C.S. 1995. Genetic and environmental factors influencing clutch size in equatorial and temperature zone stonechats (Saxicola torquata axillaris and S. t. rubicola): An experimental study. Auk, 112(3), 748-755.
Gwinner, E. & Scheuerlein, A. 1998. Seasonal changes in day-light intensity a a potential Zeitgeber of circannual rhythms in equatorial stonechats. Journal für Ornithologie, 139(4), 1-5.
Gwinner, E. & Scheuerlein, A. 1999. Photoperiodic responsiveness of equatorial and temperate zone Stonechats. Condor, in press.
Hall, B.P. & Moreau, R.E. 1970. An atlas of speciation in African passerine birds. London: British Mus. Nat. History.
Hau, M., Wikelski, M. & Wingfield, J.C. 1998. A neotropical forest bird can measure the slight changes in tropical photoperiod. Proceedings of the Royal Society London B, 265, 89-95.
Keith, S., Urban, E.K. & Fry, C.H. 1992. The birds of Africa. London: Academic Press.
Koenig, S. & Gwinner, E. 1995. Frequency and timing of successive broods in captive African and European Stonechats Saxicola torquata axillaris and S. t. rubicola. Journal of Avian Biology, 26(3), 247-254.
Miller, A.H. 1959. Reproductive cycles in an equatorial sparrow. Condor, 61, 344-347.
Miller, A.H. 1965. Capacity for photoperiodic response and endogenous factors in the reproductive cycles of an equatorial sparrow. Proceedings of the National Academy of Sciences USA, 54, 97-101.
Murton, R.K. & Westwood, N.J. 1977. Avian breeding cycles. Oxford: Clarendon Press.
Nicholls, T.J., Goldsmith, A.R. & Dawson, A. 1988. Photorefractoriness in birds and comparison with mammals. Physiol. Rev., 68, 133-176.
Ricklefs, R.E. 1969. The nesting cycle of songbirds in tropical and temperate regions. Living Bird, 8, 165-175.
Rollo, M. & Domm, L.V. 1943. Light requirements of the weaver finch. I. Light period and intensity. Auk, 60, 357-367.
Sharp, P.J. 1984. Seasonal breeding and sexual maturation. In: Cunningham, P., Lake, E. & Hewitt, D.E. (eds) Reproductive Biology of Poultry: 203-218. Harlow: Longman Group.
Starck, J.M., Koenig, S. & Gwinner, E. 1995. Growth of stonechats Saxicola torquata from Africa and Europe: An analysis of genetic and environmental components. Ibis, 137(4), 519-531.
Tewary, P.D. & Dixit, A.S. 1986. Photoperiodic regulation of reproduction in subtropical female yellow-throated sparrows. Condor, 88, 70-73.
Voous, K.H. 1960. Atlas of European birds. London: Nelson.
Wingfield, J.C. 1983. Environmental and endocrine control of reproduction: an ecological approach. In: Mikami, S.I., Honma, K. & Wada, M. (eds) Avian Endocrinology. Environmental and Ecological Perspectives: 265-288. Tokyo Berlin: Japan Scientific Society Press.
Wingfield, J.C. & Farner, D.C. 1980. Control of seasonal reproduction in temperate-zone birds. Progr. Reprod. Biol., 5(62-101).
Wingfield, J.C. & Farner, D.S. 1993. Endocrinology of reproduction in wild species. In: Farner, D.S., King, J.R. & Parkes, K.C. (eds) Avian Biology: 163-327. New York: Academic Press.
Wingfield, J.C. & Kenagy, G.J. 1991. Natural control of reproduction. In: Pang, P.K.T. & Schreibman, M.P. (eds) Handbook of Comparative Endocrinology: 181-241. New York: Academic Press.
Wolfson, A. & Winchester, D.P. 1959. Effect of photoperiod on the gonadal cycle in an equatorial bird, Quelea quelea. Nature, 184, 1658-1659.
Fig. 1. Changes in testicular width, as established by repeated laparotomies of seven equatorial S.t. axillaris and seven temperate-zone S.t. rubicola Stonechats kept in a photoperiod with 12.25 L. Hatched bars indicate body moult, filled bars indicate flight-feather moult. ( after Gwinner, 1996b).

Fig. 2. Changes in testicular width (solid lines) and occurrence of body moult (hatched bars: mean ± SE) and flight-feather moult (black bars) in temperate zone Stonechats S.t. rubicola (upper diagrams) and equatorial Stonechats S.t. axillari (lower diagrams), exposed to a photoperiodic cycle characteristic in its shape and amplitude of a latitude of 47,5° N in summer (breeding site) and 40° N in winter (wintering site). The period of this photoperiodic cycle was 12 months for the birds in the left-hand diagrams, but only 6 months for the birds in the right hand diagrams. Photoperiodic conditions are shown in the upper section of each graph (after Gwinner & Scheuerlein, 1999).
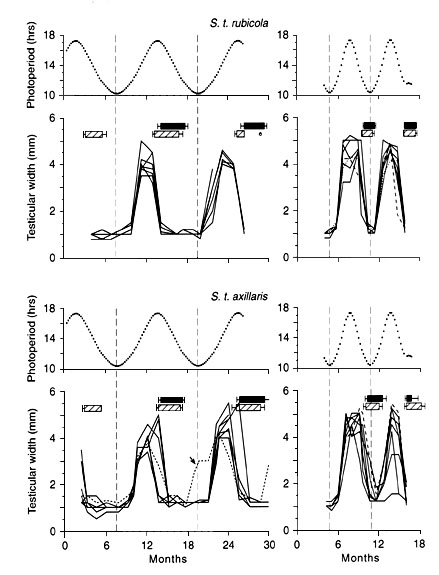
Fig. 3. Changes in testicular width (solid lines) and occurrence of body moult (hatched bars: mean ± S.E.) and flight-feather moult (black bars) in temperate-zone Stonechats S.t. rubicola (upper panel) and equatorial Stonechats S.t. axillaris (lower panel) exposed to a photoperiodic cycle characteristic in its shape and amplitude of a latitude of about 10° N, but with a period of only 6 months (as shown in the upper section of each graph). The dashed and dotted horizontal lines indicate the occurrence of body moult in the two individuals whose dashed and dotted testes curves are marked by arrows (after Gwinner & Scheuerlein, 1999).
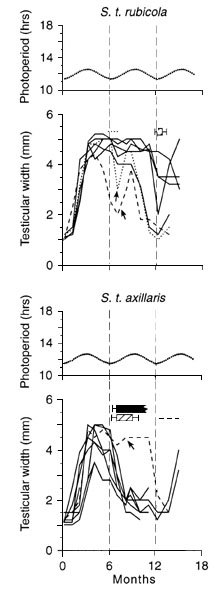
Fig. 4. Changes in testicular width (solid lines) and occurrence of moult (horizontal lines in the upper section) in equatorial Stonechats S.t. axillaris exposed to constant bright light (above; n = 6) and in birds exposed to a changing light intensity program (below; n = 6). During the phase of high-light intensity (measured as illuminance) birds were exposed to between 2000 and 12000 lux, depending on their position inside the cage. The changing-light intensity program alternated between high light intensity (2000 to 12000 lux) and low light intensity (250 to 1600 lux), the latter being indicated by the shaded bars in the lower diagram (Gwinner & Scheuerlein, 1998).