S30.2: Factors influencing the reproduction of Asian Hornbills
Pilai Poonswad1, Vijak Chimchome2, Kamol Plongmai1 & Phitaya Chuilua1
1 Faculty of Science, Mahidol Univ., Rama 6, Bangkok 10400, Thailand, fax 662 644 5411, e-mail scpps@mahidol.ac.th; 2 Faculty of Forestry, Kasetsart Univ., Bangkhen, Bangkok 10900, Thailand
Poonswad, P., Chimchome, V., Plongmai, K. & Chuilua, P. 1999. Factors influencing the reproduction of Asian hornbills. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1740-1755. Johannesburg: BirdLife South Africa.Asian hornbills are single-brooded, conditional breeders. The trigger of breeding onset is still uncertain. Between 1981 and 1996, we collected data on nesting behaviours and food diversity by following males to their nests. Hornbills started to nest in the dry season, the onset of which varied with geographical area. Breeding pairs were limited by the availability of suitable nest cavities. Intra- and interspecific competitive interactions occurred at 45.5% of the nest sites observed in Khao Yai National Park. As a result, nest abandonment was as high as 53.3%. Our data show that greater food diversity may enhance breeding success by as much as 91% in the Oriental Pied Hornbill Anthracoceros albirostris. Hornbill chick survival was also affected by predators and/or invaders. The Yellow-throated Marten Martes flavigula and the Binturong Arctictis binturong were the most important predators of Great, Wreathed, Rufous-necked and Brown Hornbills in certain areas. In addition, disturbance by humans, particularly chick collecting activity, reduced the reproductive output of hornbills. Therefore, we conclude that reproduction of Asian hornbills is triggered by drought after heavy rain and is also affected by the availability of nest cavities and food, the presence of predators and/or invaders as well as by human disturbance.
INTRODUCTION
Breeding is the part of the life cycle of birds which relates most intimately to the environment. In other organisms, conditions may favour breeding for only brief periods; accordingly, for example, cold-blooded animals are capable of interrupting their reproductive cycles at different stages of egg, embryonic or larval development by inserting states of rest or encystment (Immelmann 1971). These animals are therefore capable of distributing the whole cycle among periods when the conditions are appropriate, allowing the adjustment of physiologically important functions to favourable seasons. Because of its severe physiological demands, reproduction must occur when stress is minimal and survival of parents and young is maximal. Birds, however, have developed remarkable adaptations to the changes in environmental conditions.
The majority of birds have definite breeding seasons with almost regular intervals, at approximately the same time every year (Immelmann 1971). Lack (1968) pointed out that the period of egg laying depends more on geographical area than on the particular species or family. In temperate zones, most species start laying eggs in spring and have young in late spring to early summer (Lack 1950). In contrast, in tropical regions there is no particular pattern of breeding season due to the greater heterogeneity of the ecological complexes there; breeding activity of various species may be observed throughout the year. The annual cycle of birds that reside in the tropical rainforest, including the periods of breeding and moulting, is intricately related to the variable seasonal cycle. Although the breeding season of birds in tropical regions may be protracted, even lasting all year, it tends to peak in a certain period. Furthermore, geographical variation in the period of reproduction tends to be much greater than in the temperate zone (Baker 1938; Lack 1954; 1968; Miller 1960).
At Khao Yai National Park, two species of relatively large hornbills, the Great Hornbill, Buceros bicornis and Wreathed Hornbill, Aceros (Rhyticeros) undulatus, start their breeding in January and finish in May or June, whereas smaller-sized hornbills, the Oriental Pied Hornbill, Anthracoceros albirostris and Brown Hornbill, Anorrhinus austeni breed from February to May (Poonswad et al. 1987). Similarly, Rufous-necked Hornbill, Aceros nipalensis and Plain-pouched Hornbill, Aceros (Rhyticeros) subruficollis at Huai Kha Khaeng, in western Thailand, begin to breed in January and finish in May or June (Chimchome et al.1998). Most hornbill species found in Khao Yai National Park and western Thailand, like most of the hornbills found in India, breed between January and June, the period during which it is dry (Ali & Ripley 1987; Lauer 1989). The breeding season of hornbills in peninsular Thailand and Malaysia is delayed to between March and July, the driest months, or even until August and September (present study; Kemp 1995). It is likely that the breeding season of hornbills in the Indian subcontinent and the Sunda Islands region is during the dry season, i.e. between January and May or extended to June (Ali & Ripley 1987; Poonswad 1993; Kemp 1995). The island species, such as the Sumba Hornbill, Aceros (Rhyticeros) everetti and Red-knobbed Hornbill, Aceros (Rhyticeros) cassidix, breed from July to September and June to early September respectively (Kinnaird & O’Brien 1993; O’Brien et al. 1998). Those recorded periods fall within either the wet or the dry season depending on geographical area. The differences in breeding months may indicate the availability of particular stimuli at a certain period. Thus, a question addressed here is, what is the trigger for the onset of breeding in these hornbills?
Hornbills are well known for their nesting habits, in that the females seal themselves inside an existing tree cavity. Hornbills are monogamous, seasonal single-brooded species. Like other tropical birds, hornbills produce relatively small clutches of 1 to 4 eggs (Kemp 1995). The number of nestlings that fledge per season per pair is low: on average, the Great Hornbill fledges one chick; Wreathed Hornbill fledges one; Oriental Pied Hornbill fledges 1.5; and Brown Hornbill fledges 2.5. The survival of the nestlings seems to depend on the ability of the breeding male to find food and protect the brood from predators. At all stages in the breeding process, internal as well as external factors determine the reproductive success of hornbills (Poonswad 1993).
Therefore, this paper addresses and discusses roles of basic external or ecological factors which influence the success or failure of reproduction in some Asian hornbill species.
METHODOLOGY
Study area
The study was carried out mainly in Khao Yai National Park between 1981 and 1996, except in 1986 and 1987. In two other protected areas particular aspects of some species were studied.
Khao Yai National Park
The park is situated in the north-central region of Thailand, approximately between 14° 15' to 14° 35' N and 101° 5' to 101° 52' E. With an area of 2168 km2, the park is generally mountainous, varying from 250 to 1351 m altitude. The study area measures 70 km2 and is covered with highly diverse forest types including dry mixed deciduous, dry evergreen, wet evergreen, hill evergreen, savanna and secondary growth forests. Hornbills found in the park and in the study area mainly inhabit dry evergreen and wet evergreen zones. These were the Great Hornbill, Wreathed Hornbill, Oriental Pied Hornbill and Brown Hornbill.
Huai Kha Khaeng Wildlife Sanctuary
The sanctuary is situated in the west of Thailand, covering an area of 2575 km2, approximately between 15° 10' to 15° 49' N and 90° 2' to 90° 22' E. This is a mountainous regions, a mosaic of evergreen and deciduous forest formation. The hornbills inhabiting the sanctuary are the Great Hornbill, Wreathed Hornbill, Oriental Pied Hornbill, Plain-pouched Hornbill, Rufous-necked Hornbill and White-crowned Hornbill, Aceros (Berenicornis) comatus.
Budo Su-Ngai Padee National Park
The park has not yet been officially designated as a national park. It is situated in peninsular Thailand, approximately between 6° 0' to 6° 40' N and 101° 30' to 101° 55' E. With an area of 293 km2, this prospective national park is mountainous. The highest peak reaches an altitude of 1800 m. The vegetation is tropical rainforest dominated by Hopea and Dipterocarpus. Hornbills found in this park are the Great Hornbill, Rhinoceros Hornbill, Buceros rhinoceros, Helmeted Hornbill, Buceros (Rhinoplax) vigil, White-crowned Hornbill, Bushy-crested Hornbill, Anorrhinus galeritus, and Wreathed Hornbill.
Data collection
Climate
Climate data was obtained from weather stations of the Meteorological Department closest to the study areas. For Khao Yai National Park and Budo Su-Ngai Padee, the weather data was obtained from Nakhon Ratchasima and Narathiwat stations, respectively.
Nests
In order to locate hornbill nests in these three study areas, lone males were followed on foot. At the nest, direct observations and data records were made regarding: (1) competition at the nest cavity; (2) dates of female imprisonment and chick fledging to determine breeding cycle and breeding success, (3) fruit species brought by the breeding male to feed the brood; these were identified, counted and weighed to quantify consumption rate (collected between 1982 and 1985), (4) seeds of non-fig fruit (identified and counted between 1993 and 1996); (5) predators and invaders, (6) nest reuse in the case of old nest cavities.
The above data were collected from 1981 to 1996 except in 1986 and 1987 and where otherwise stated.
Nest improvement
To increase the number of suitable nest cavities, marginal nests were improved, a suitable condition being defined according to Poonswad (1993) as: (1) size of nest entrance is approximately 12 cm ´ 25 cm, (2) depth from entrance to opposite wall is approximately 45 cm, (3) width of cavity is approximately 40 cm, (4) height of ceiling is over 100 cm and (5) floor level below lower edge of entrance can range from 5 to 15 cm. Based on accessibility and on long-term observations of nest reuse and abandonment for a period of time, the condition of some nest cavities found between 1981 and 1992 within the study area at Khao Yai National Park (Poonswad et al. 1987) was examined beginning in 1993. The improvement of marginal or unsuitable nests was started and carried out between 1993 and 1995. Thirteen nests in 1993 and 14 nests in 1994 were checked by direct climbing. Unsuitable nest conditions were identified as: (i) nest entrance narrowed or closed, (ii) sunken nest floor and (iii) lack of an appropriate perch in front of the nest for the male to use when delivering food. Criteria for nest improvement were based on the size range of the nest entrance and floor depth in suitable nests studied by Poonswad (1993).
Narrowed or closed nest entrances were enlarged with a chisel. In nests with deep floors the level was raised with soil and the weight of the soil was recorded. An additional perch was nailed at an appropriate position to assist the male while feeding the brood. The nest improvement was done prior to the new breeding season and during dry conditions (October, November and December).
Reuse of improved nest cavity
The improved nests were checked for reuse and if they were being used, data were recorded as in (2).
Human disturbance
To study the effects of disturbance by human on hornbill breeding, we extended our research to known locations of nests in Budo Su-Ngai Padee where local people collected hornbill chicks for sale as pets. Since the collectors would not care whether they disturbed the birds, they had never built a blind. After we succeeded in convincing them to stop collecting chicks and tell us the locations of the hornbill nests, we taught them to build a blind at every nest and how to properly approach the blind, so that we could test the effect of minimising disturbance. At the blind, the local people were assigned to check for signs of nest visiting by looking for new faeces dropped under the nest. They recorded dates of nest visiting, nest sealing, nest imprisonment and chick fledging.
RESULTS
Climate
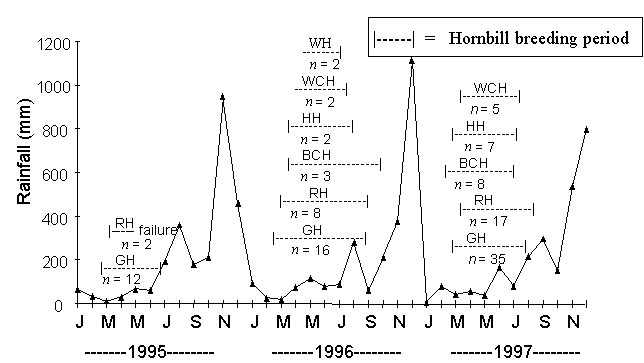
In Thailand, drought seemed to be an important trigger of the breeding onset of hornbills (Fig. 1 and Fig. 2). Hornbills nested during the relatively driest months. Dry conditions in a nest cavity are evidently preferred. We have never seen hornbills bringing any material for nest lining. At least three known nests of Oriental Pied Hornbills and one of the Brown Hornbill were abandoned due to floods. The breeding cycle of hornbills in this study ended before the heavy rain (monsoon) came.
Availability of nest cavities and nest loss
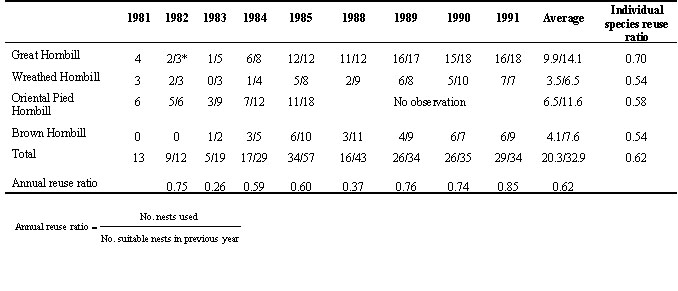
From this study, it is clear that the most important factor, an independent factor, limiting the reproduction of hornbills is the availability of nest cavities, which they cannot excavate by themselves. If nest conditions remained suitable and there was no competition or disturbance, hornbills tended to reuse old nest cavities year after year. Four sympatric hornbill species at Khao Yai National Park were observed to reuse nest cavities as shown in Table 1. Great Hornbills reused the old nest cavities most (0.70), whereas the remaining species reused in similar, lower ratios regardless of successful breeding (Table 1).
There were a number of causes of reduced availability of suitable nest cavities. The most serious cause was the breakage of nest trees by storms; this irreversible loss accounted for as much as 53.8% of the total nest loss (Table 2). Losses of nest cavities would directly affect the reproduction of hornbills. However, the loss of nests caused by nest-entrance closure due to growing tissue and nest floor sinking due to rotting can be reversed. Another irreversible loss, nest trees cut by poachers, was found occasionally.
Marginal nests and improvement
The evidence which confirmed the effect of nest availability on hornbill reproduction is shown in Table 3. Nineteen marginal nests were improved by adding soil to bring the nest floor up to 5 to 15 cm below the lower edge of the nest entrance. Weight of the added soil varied from 2 to 340 kg (x = 59.2, S.D. = 81.1 kg, n = 19). Hence, as time passes, the rotting process becomes progessively more serious and causes nest floor sinking. The bark tissue continues to grow, reducing the size of the entrance. However, in three years after improvement, there were as many as 31 pairs using 15 improved cavities, not necessarily the same species or the same pair as formerly (Table 3). Total breeding success of the birds using the improved nests was high (90.3%) (Table 3).
Competition
The number of suitable cavities may be limited, resulting in intra- and interspecific competition for nest cavities among these four sympatric hornbill species (Table 4). As a consequence of such competitions, 53.3% of the observed nests were abandoned (Table 4). If the competition is severe enough, pairs may not have a chance to breed at all in that breeding season before the suitable time has passed. Unfortunately, there has been no study to determine whether competition is decreased after unsuitable nests have been improved.
Animals other than the hornbills themselves also competed for cavities at Khao Yai National Park; these included bees (Apis sp.), resinous bees (Trigona sp.), wasps (Vespa sp.) monitor lizards (Varanus sp.), Red Giant Flying Squirrels (Petaurista petaurista) and King Cobra (Ophiophaga hannah). These animals took over the hornbill nest cavities after the breeding season finished and stayed until the next breeding season. Most of these nests were abandoned after the hornbills found the competitors, except for one nest which was occupied by a monitor lizard: the breeding pair chased it away and nested successfully.
Food
Hornbills are omnivorous, feeding on a wide variety of fruit including figs (Ficus spp), non-fig fruit and animals (Table 5). Food seemed to be a climate-linked factor. Favourable weather may trigger the onset of flowering which subsequently produced fruit. Although the linkage between climate and food is clearly important in limiting reproduction of hornbills, it is difficult to obtain accurate data in natural conditions to substantiate such a statement. The data obtained in this study indicate that a great diversity of food may enhance breeding success (Table 5). That is, we observed several females rejecting certain food items which were offered repeatedly for several days during the chick-raising period. Once this happened, the males changed the menu. Greater diversity of food may also imply an abundance of food in terms of productivity within the habitat. However, even with less diversity of food species productivity could still be sufficient for nesting hornbills to succeed in raising young. There was a notable size-dependent difference of consumption rate between the two sizes of hornbill species (Table 5).
Table 6 shows the number of non-fig fruit species found under the nests and the total number of seeds counted in relation to number of breeding pairs and percentage of breeding success. There was no statistical correlation between number of seeds counted and percentage of breeding success, between number of fruit species and percentage of breeding success or between number of fruit species and number of seeds counted (Spearman correlation, P > 0.05). During 1993 to 1996, annual breeding success was high except in 1995, when breeding success was markedly low in all species, particularly the Great Hornbills (Table 6). The number of seeds counted per nest per day was lowest in 1995. Hence, it might be that although the number of fruit species was similar to that in other years, the productivity of the individual trees was low. At Huai Kha Khaeng in 1993, we found 12 nests of Rufous-necked Hornbills. In 1994, nine females of this species imprisoned themselves and in only 6 nests chicks successfully fledged. In 1995, no female became imprisoned in any of these 12 nests. During regular hikes within these two study areas in the 1995 breeding season, we observed that fruiting trees were few and fruit was scarce, both on the ground and on the trees. Unfortunately, we were unable to quantify the abundance of food within the study areas. It is interesting to note that during 1993 to 1996, the Wreathed Hornbill consumed the greatest diversity of non-fig species (Table 6) and its breeding success increased from 78% to 86% when compared with the breeding success during 1982 to 1985 (Table 5 and Table 6).
The data in Table 6 also suggest that the number of breeding pairs was within the carrying capacity of the area, where it provided food and nesting sites. At Khao Yai, within 70 km2, we have never observed more than 50 breeding pairs (the maximum number was 47 pairs in 1997). This carrying capacity was, perhaps limited by the nesting site and food supply.
Predation
Hornbills are very large to moderately large birds. They live mostly in the high canopy and their nesting habit is relatively safe. Chicks are raised inside a protective tree cavity. The predation rate was quite low. However, hornbills were known to be preyed upon by some arboreal mammals. The most dangerous one was the Yellow-throated Marten Martes flavigula. In Khao Yai National Park, the martens were observed climbing to the nests of three species of hornbills and killing nestlings (Table 7). In 1994, at Huai Kha Khaeng, before fledging, one female Rufous-necked Hornbill and three chicks were eaten by Yellow-throated Martens and another female of the same species was eaten by a Binturong (Arctictis binturong). Besides preying on hornbills, Yellow-throated Martens also caused the birds to abandon their nests. This was observed for at least one nest of the Wreathed Hornbill.
Human disturbance
Disturbances by humans can seriously affect hornbill breeding. In 1993, at Budo Su-Ngai Padee, at least nine villagers were known to collect chicks from 38 nests of hornbill species listed in Table 8. It was obvious that when the villagers stopped disturbing them, the hornbills gradually came to nest with high success ratios (Table 8). Continuous collection of hornbill chicks will affect not only a wild population but also reproductive generations.
DISCUSSION
Climate
Breeding peaks of birds studied in Trinidad and Sarawak is coincided with the wet season, i.e. June and January to June respectively (Keast 1995). The coincidence of the breeding peaks and the wet season may have been related to the abundance of food, such as insects in the case of insectivorous birds. In a study of Tockus hornbills, for example, Kemp (1973) observed that rainfall appears to be correlated with an increase in their insect prey, thus triggering the onset of breeding. Besides affecting food supply, rainfall makes mud available as plaster material for African hornbills (North 1942).
In contrast, our study shows that low rainfall or a dry period after a rainy season was an important stimulus for the breeding onset and subsequent imprisonment of the female hornbills. These dry months before imprisonment presumbly improve the condition of the nest cavities; if a nest cavity is too damp or becomes flooded, it may be abandoned (Poonswad 1993).
There may be another, more important consequence of drought: it could be related to the flowering of wild fruit trees, which eventually produce fruits. It has been suggested that drought, short day length and low temperature are important stimuli for flowering of many fruit trees in tropical forests (Garner & Allard 1923; Webb 1958). This combination of circumstances also occurs at Khao Yai National Park from November through February (pers. obsv.). Moreover, Smitinand (1977) recorded flowering of 22 species of trees and 23 species of shrubs at Khao Yai National Park in the dry season, i. e. February and March. This period falls within the hornbill breeding season, starting in January and ending in May and June. Poonswad (1993) recorded the highest number of fruit food species (non-fig fruit) in April and May, at a time when the hornbills are raising their chicks. Undoubtedly, the young must be produced during the period of most abundant fruit food.
The climate may not only trigger environmental factors, such as food supply, that affect the reproductive cycle but may also influence the endogenous breeding rhythm of adult hornbills. Marshall (1959) stated that among adult birds the internal rhythm is the seasonal initiator. He also comments that there is rarely evidence that birds breed in response to rainfall rather than the environmental effects that follow it. This interpretation is consistent with the fact that either a wet or a dry season could be a trigger for breeding onset, depending on which seasonal condition provides a greater food supply, which in turn depends on what is the main food (fruit or insects) of the species in that area. In our study, climate also seemed to be a factor determining the end of the breeding cycle of hornbills. Most hornbills at Khao Yai finish their breeding cycle before heavy rain comes. Hence, an individual hornbill species, especially one of the larger species, must begin its breeding cycle within a rather specific period; otherwise, it will be too late to breed.
Availability of a suitable nest cavity and competition
Our study clearly revealed that the nest cavity is the most important ultimate factor in the reproduction of hornbills. Loss of suitable cavities could delay or inhibit ovulatory processes (Marshall 1952). Unavailabity of nest cavities, in the case of hornbills, makes breeding absolutely impossible even though the breeding pair may have been prepared to do so by all internal factors. In this case, reproduction is frustrated (Marshall 1959). A suitable cavity does not serve only as an egg receptacle, but also provides a suitable microenvironment or microclimate, which is crucial to the successful incubation of the eggs and the health of the chicks. Cavity nests help buffer eggs, parent and young against fluctuations in external temperature (Gill 1989). This was confirmed by Poonswad (1993), who monitored temperatures inside a nest cavity used by a hornbill for 24 hours and showed that there was very little fluctuation in comparison with that outside the nest.
Reproductive success also depends heavily upon choice of habitat. If a species settles in a suboptimal habitat, the result may be reproductive failure. The area needs to be suitable and sufficient to protect the young and parents (Smith 1996). Cavity-nesting species such as hornbills thus require not merely a cavity but a location where the nest tree is located in a habitat that provides conditions to be suitable for nesting, as studied by Poonswad et al. (1997). They pointed out that 67% of hornbill nests were found in dense forest, the remaining nests were found in open and forest edge.
The competition for a nest cavity observed in this study was severe; there was clearly a shortage of suitable tree cavities for hornbills to nest, and competitors frequently caused nest abandonment. In Khao Yai National Park, the hornbill nest trees appeared to be vulnerable to natural phenomena, i.e. storms and rotting processes. (Poonswad 1993; Chuilua et al. 1998). Competition for nest cavities was more intense between hornbills of the same species and of similar sizes. This could be because they have similar basic requirements for nest sites and overlapping breeding cycles (Poonswad 1993). Competition between hornbills in different size groups may result in ‘asymmetric’ disturbances, in that the larger-sized hornbills affect the breeding of the smaller ones, but the smaller ones do not affect the breeding of the larger ones (Mc Farland 1981; Poonswad 1993).
Food and breeding success
The abundance of suitable food is regarded as an ‘ultimate factor’. A number of researchers have indicated that the young of a great variety of species are produced when food is abundant (Baker 1947; Davis 1933; Marshall 1950, 1959; Lack & Silva 1949; Thomson 1950). Leighton and Leighton (1983) indicated that hornbills do not breed when the fruit crop is small. Availability of food is also suggested as the proximate factor controlling the annual cycle of birds, including moulting and breeding cycles. The availability of food may have acted in conjunction with an innate endocrine cycle (Keast 1985). Marshall (1959) found that the appearance of certain food species may have triggered the breeding onset of some birds. Food abundance can limit clutch size by limiting the number of eggs produced or by limiting the number of young that parents can successfully feed (Martin 1986).
In the case of hornbills no matter how many eggs are laid, the Great and the Wreathed Hornbills raise only one chick (Poonswad 1993). Hence, it is the number of hornbill chicks raised that is limited by food. Food limitation during breeding can restrict the number of offspring produced or negatively influence the quality and condition of offspring and parents, such that future survival or reproductive success is reduced (Martin 1986). Clearer evidence of food abundance affecting the survival of the chick is shown in the case of the Brown Hornbill. The Brown Hornbill breeds cooperatively with one to five helpers, but two nest helpers are sufficient to reduce the feeding load of the breeding male by as much as 40%. Nests of this species showed a low chick survival rate (43 %, n = 7 pairs) if there were no helpers (Poonswad 1993). Besides helping the breeding male in feeding, nest helpers contribute to protecting the brood from invaders (Poonswad 1993).
ACKNOWLEDGEMENTS
We thank the Royal Forest Department for permission to conduct our studies in various protected areas. We also wish to extend our thanks to the superintendents of Khao Yai National Park, Huai Kha Khaeng Wildlife Sanctuary, Khao Nang Rum Wildlife Research Station and Budo Su-Ngai Padee National Park for their kind cooperation.
We are indebted to our research assistants, Boonmar Saengthong, Sudjai Nuttato, Narong Jirawatkavi, and to villagers in Pattani and Narathiwat Provinces for their field work. We highly appreciate Siriwan Nakkuntod and Sopha Sa-nguanchat, for their assistance in data processing and typing.
The studies have been supported by companies, United Communication Industry Co., Ltd. and Total Access Communication Public Company Limited, the Government Housing Bank and the Hornbill Research Foundation.
REFERENCES
Ali, S.& Ripley, D. 1987. Handbook of the birds of India and Pakistan. Vol 4; Delhi; Oxford University Press: 260pp.
Baker, J.R. 1947. The season in a tropical rain-forest (New Hebrides). Part 7. Summary and general conclusion. Zoological Journal of the Linnean Society 41: 248-258.
Chimchome, V., Vidhidharm, A., Simchareon, S., Bumrungsri, S. & Poonswad, P. 1998. Comparative study on breeding biology and ecology of two endangered hornbill species in Huai Kha Khaeng Wildlife Sanctuary, Thailand. In: Poonswad P. (ed.) The Asian hornbills: ecology and conservation; Bangkok; Thai Studies in Biodiversity No. 2: 1-336.
Chuilua, P., Plongmai, K. & Poonswad, P. Status of nest cavities of hornbills in Khao Yai National Park, Thailand. In: Poonswad, P. (ed.) The Asian Hornbills: ecology and conservation. Bangkok; Thai Studies in Biodiversity No. 2: 1-336.
Davis, W.B. 1933. The span of the nesting season of birds in Butte Country, California: in relation to their food. Condor 35: 151-154.
Garner, W.W. & Allard, H.A. 1923. Further studies in photoperiodism, the response of the plant to relative length of day and night. Journal of Agricultural Research 23: 871-920.
Gill, F.B. 1989. Ornithology. New York; W. H. Freeman and Company: 660pp.
Immelmann, K. 1971. Ecological aspects of periodic reproduction. In: Farner, D.S. & King, J.R. (eds) Avian biology. Vol 1; New York and London; Academic Press: 341-389.
Keast, A. 1985. Tropical rainforest avifaunas: an introductory conspectus. In: Diamon, A.W. & Lovejoy, T.E. (eds) Conservation of tropical forest birds. ICBP Technical Publication No. 4: 3 - 31.
Kemp, A. C. 1973. Environmental factors affecting the onset of breeding in some southern African hornbills, Tockus spp. Journal of Reproduction and Fertility, supplement 19: 319 - 331.
Kemp, A. C. 1979. A review of the hornbills: biology and radiation. Living bird 17: 105 - 136.
Kemp, A.C. 1995. The hornbills. New York; Oxford University Press: 302pp.
Kinnaird, M.F. & O’Brien, T.G. 1993. Preliminary observation on the breeding biology of the endemic Sulawesi Red-knobbed Hornbills (Rhyticeros cassidix). Tropical Biodiversity 1: 107-112.
Lack, D. & Silva, E.T. 1949. The weight of nestling Robins. Ibis 91: 64 - 78.
Lack, D. 1950. The breeding seasons of European birds. Ibis 92: 288 - 316.
Lack, D. 1954. The natural regulation of animal numbers. Oxford; Clarendon Press:
Lack, D. 1968. Ecological adaptation for breeding in birds. London; Methuen:
Lauer, W. 1989. Climate and weather. In: Lieth, H & Werger, M. J. A. (eds) Ecosystem of the world 14B: Tropical rain forest ecosystems. Oxford; Elsevier: 1 - 53.
Leighton, M. & Leighton, D.R. 1983. Vertebrates response to fruiting seasonality within a Bornean rainforest. In: Sutton, S.L., Whitmore, T.C. & Chadwick, A.C. (eds) Tropical rain forest: ecology and management. British Ecological Society; Special publication No. 2; Oxford; Blackwell Scientific Publications: 181- 196.
Marshall, A.J. 1950. The function of the bower of the Satin Bower-bird in the light of experimental modifications of the breeding cycle. Nature 165: 388 - 392.
Marshall, A.J. 1952. Non breeding among Arctic birds. Ibis 94: 310 - 333.
Marshall, A.J. 1959. Internal and environmental control of breeding. Ibis 101: 456 - 478.
Martin, T.E. 1986.Competition in breeding birds, on the importance of considering processes at the level of the individual. In: Johnston, R.F. (ed.) Current Ornithology. Vol. 4; New York and London; Plenum Press: 181 - 210.
McFarland, D. 1981. The Oxford companion to animal behaviour. Oxford; Oxford University press: 657pp.
Miller, A.H. 1960. Adaptation of breeding schedule to latitude. In: Proceedings: 12th International Ornithological Congress 1958: 513 - 522.
North, M.E.W. 1942. The nesting of some Kenya colony hornbills. Ibis 14 (6): 499 - 508.
O’Brien, T.G., Kinnaird, M.F., Jepson, P. & Setiawan, I. 1998. Effect of forest size and structure on the distribution of Sumba Wreathed Hornbill Aceros everetti. In: Poonswad, P. (ed.) The Asian Hornbills: ecology and conservation. Bangkok; Thai Studies in Biodiversity No. 2: 1-336.
Poonswad, P., Tsuji, A. & Ngampongsai, C. 1987. A comparative study on breeding biology of sympatric hornbill species (Bucerotidae) in Thailand with implication for breeding in captivity. In: Proceedings, Delacour/International Foundation for the Conservation of Birds; Symposium on breeding birds in captivity, February 11-15, 1987. North Hollywood: 250 - 315.
Poonswad, P. 1993. Comparative ecology of sympatric hornbills (Bucerotidae) in Thailand. D. Sc. Thesis, Osaka City University, Osaka, Japan.
Smith, R.L. 1996. Ecology and field biology. 5th ed; Harper Collins; College Publishers: 822pp.
Smitinand, T. 1977. Plants of Khao Yai National Park. Bangkok; New Thammada Press Ltd: 100pp.
Thomson, A.L. 1950. Factors determining the breeding seasons of birds: an introductory review. Ibis 92: 173 - 184.
Webb, L.J. 1958. Cyclone as an ecological factor in tropical lowland rain forest, north Queensland. Australian Journal of Botany 6: 220 - 228.
Table 1. The reuse of nest cavities by four sympatric hornbill species in Khao Yai National Park between the 1981 and 1991 breeding seasons (except in 1986 and 1987). * One nest tree broken.

Table 2. Cumulative number of nests found, number of nests lost and the known causes of nest loss at Khao Yai National Park. (Except in 1986 and 1987)
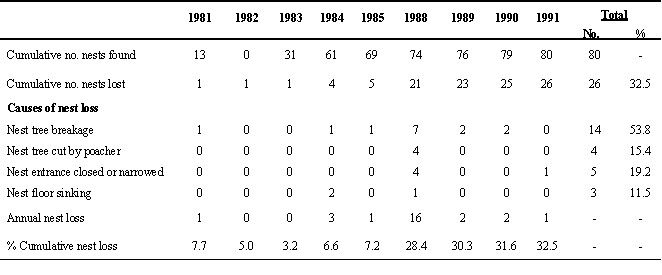
Table 3. Annual number of nests improved, nests reused and breeding success after improvement of hornbill nests in Khao Yai National park between 1993 and 1996. GH = Great Hornbill; WH = Wreathed Hornbill; PH = Oriental Pied Hornbill; BH = Brown Hornbill
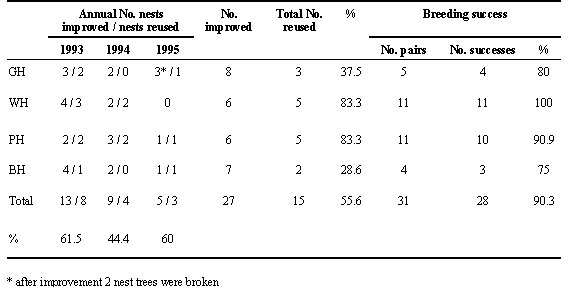
Table 4. Intra- and interspecific competition for nest cavities among four sympatric hornbill species and instances of nest abandonment observed in the 1983, 1984, 1985, 1988 and 1991 breeding seasons at Khao Yai National Park. GH = Great Hornbill; WH = Wreathed Hornbill; PH = Oriental Pied Hornbill; BH = Brown Hornbill
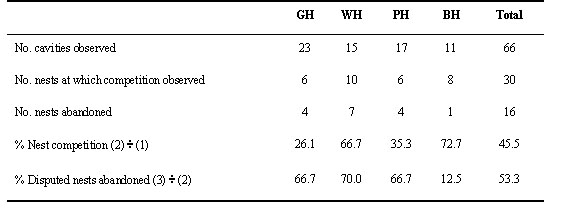
Table 5. Food diversity, average consumption rate and breeding success ratio of four sympatric hornbill species observed between 1982 and 1985 at Khao Yai National Park.
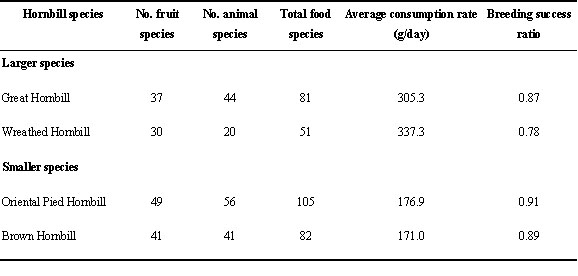
Table 6. Diversity of non-fig fruit species consumed, total seeds counted from under a nest, number of breeding pairs and percentage of breeding suceess of four sympatric hornbills in Khao Yai National Park.
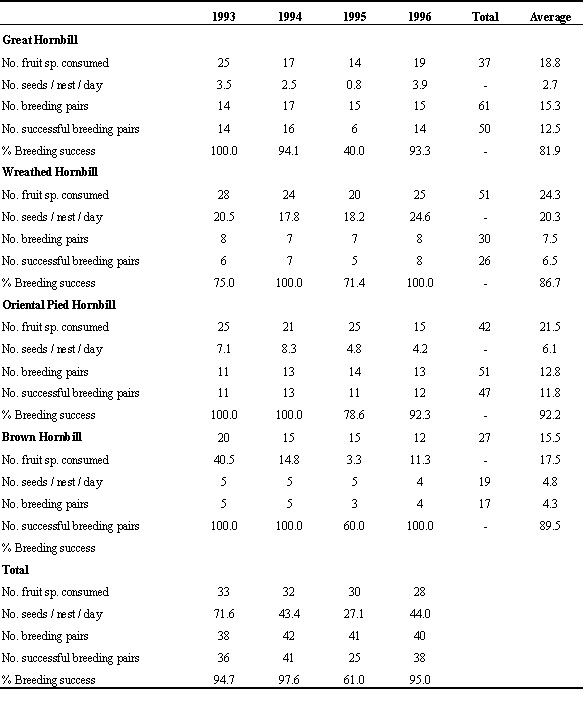
Table 7. Predation on hornbill breeding females and chicks by Yellow-throated Marten and Binturong.
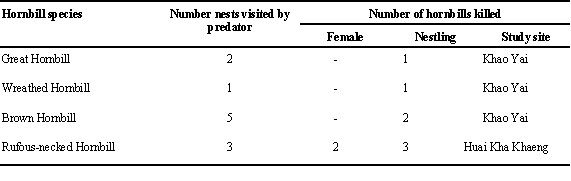
Table 8. Number of known nests in 1993 before the research was conducted, number of female hornbills entering nests and successful breeding pairs of six hornbill species studied in a forest of prospective national park in peninsular Thailand, where hornbill reproduction was clearly disturbed by human activities.
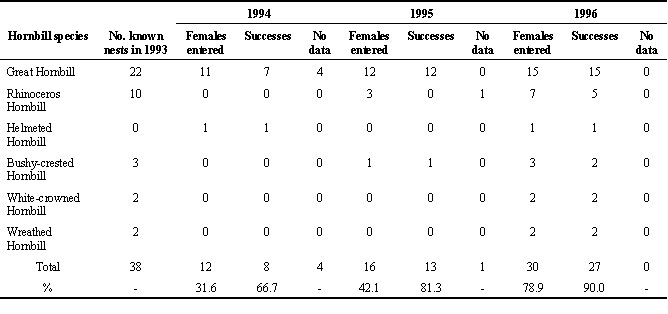
Fig. 1. Summary of periods during which various phases in the breeding cycle occurred in relation to rainfall (data derived from Meteorology Department at Nakhon Ratchasima Station between 1981 and 1990). GH = Great Hornbill, WH = Wreathed Hornbill, PH = Oriental Pied Hornbill, BH = Brown Hornbill.
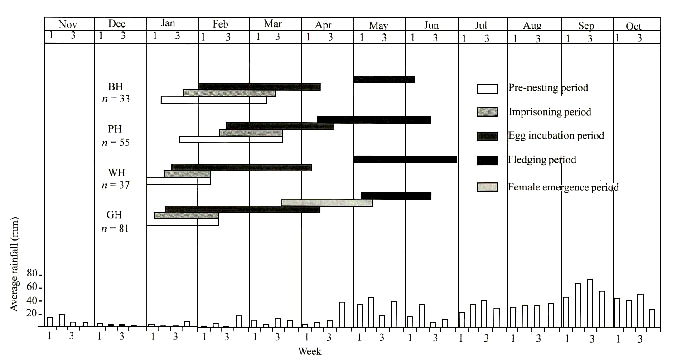
Fig. 2. Summary of breeding periods of various hornbill species found in Budo Su-Ngai Padee National Park, Thailand in relation to rainfall. (Rainfall data from Meteorological Department) GH = Great Hornbill, RH =Rhinoceros Hornbill, HH = Helmeted Hornbill, BCH = Bushy-crested Hornbill, WCH = White-crowned Hornbill, WH = Wreathed Hornbill