S30.1: Environmental control of reproduction in a neotropical rainforest bird
Michaela Hau1*, Martin Wikelski 2 & John C. Wingfield1
1Dept. Zoology, Box 351 800, University of Washington, Seattle, WA 98195, USA, fax 206 543 3041, e-mail Hau@pop.life.uiuc.edu; 2Smithsonian Tropical Research Institute, Apartado 2072, Balboa, Republic of Panama; *present address: Department of Ecology, Ethology and Evolution, 515 Morrill Hall, 505 S. Goodwin Ave., University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA, fax 217 244 4565, e-mail Hau@pop.life.uiuc.edu
Michaela Hau, Martin Wikelski & John C. Wingfield. 1999. Environmental control of reproduction in neotropical rainforest birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1720-1739. Johannesburg: BirdLife South Africa.Many species of tropical birds show a seasonal pattern in breeding activity. Yet it is still unclear how near-equatorial rainforest birds regulate breeding. Using Spotted Antbirds Hylophylax n. naevioides from a moist forest in central Panama (9°N) as a model system, we asked: (1) What environmental changes are these birds exposed to? (2) Are rainforest understory birds opportunistic or regular seasonal breeders? (3) What environmental cues do Spotted Antbirds use to initiate reproductive activity? During a two-year combined field and laboratory study we obtained the following results: (1) There were seasonal changes in rainfall, photoperiod and light intensity in the understory of the forest. (2) In both years, individual free-living Spotted Antbirds underwent regular seasonal changes in gonad size. Gonad sizes always increased in anticipation of the rainy (= nesting) season. This suggested that these birds employ a regular seasonal breeding strategy and may use long-term environmental cues to regulate breeding. (3) In experiments, Spotted Antbirds responded to an abrupt increase in photoperiod of either 60 min. or only 17 min. with gonadal growth. This indicated that these birds possess the capability to exploit the slight photoperiodic changes in their natural habitat as long-term seasonal information. The additional use of short-term environmental stimuli such as food availability was suggested from delayed gonadal growth in a year with a pronounced dry season (rainfall and food availability are strongly correlated). Experiments confirmed that food availability affects gonadal development and song activity in captive Spotted Antbirds. Our data suggest that near-equatorial tropical birds experience seasonality even in the rainforest and possess the physiological machinery to employ a regular seasonal breeding strategy.
INTRODUCTION
In past decades information has accumulated on how birds time reproduction relative to seasonal changes in their environment (summaries in e.g., Lack 1968; Perrins 1970; Murton & Westwood 1977; Farner & Follett 1979; Wingfield 1983). Ultimate factors such as food availability and predation pressure may act as selective forces that limit reproduction to certain parts of the year (Immelmann 1971; Drent & Daan 1980; Martin 1987; Nager & Nordwijk 1995; Svensson 1995). Other factors such as photoperiod, food, and temperature act as proximate cues and, together with endogenous rhythms, regulate reproductive activity on the physiological level (Farner & Lewis 1971; Immelmann 1971, 1973; Wingfield 1980; Follett 1984; Gwinner 1986; Ball 1993; Cockrem 1995).
So far, most studies have concentrated on species that breed in mid or high latitudes of the northern hemisphere. Consequently, our current understanding of reproduction in birds is biased towards these particular environments. In contrast, our knowledge on breeding strategies in the tropics - where the majority of bird species live - is scarce. Therefore it is questionable whether we can extrapolate our insights gained from studies in north temperate zones to tropical species, or even birds breeding in south temperate habitats, because environmental and ecological circumstances are so different.
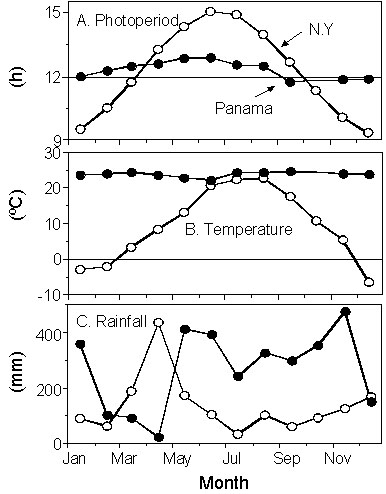
Tropical habitats are exposed to much slighter seasonal changes in environmental conditions than temperate zones (e.g., Fig. 1). Nevertheless, many tropical birds have specific breeding seasons (e.g., Baker 1938, 1940; Moreau 1936, 1950; Skutch 1950, 1966; Voous 1950; Serventy & Marshall 1957; Miller 1961; Snow & Snow 1964; Immelmann 1971; Snow 1976; Murton & Westwood 1977; Sinclair 1978; Brown & Britton 1980; Bell 1982; Gwinner 1986; Tye 1991; Wrege & Emlen 1991; Chapman 1995). There is good evidence that similar ultimate factors as in temperate zones, namely food availability and predation pressure, select for specific breeding seasons in the tropics (e.g., Skutch 1950; Snow 1976; Ward 1969; Morton 1971; Fogden 1972; Sinclair 1978; Worthington 1982; Boag & Grant 1984; Millington & Grant 1984; Poulin et al. 1992; Young 1994; Grant 1996; Komdeur 1996). In contrast, on the proximate level we have only very limited knowledge about how tropical birds achieve a seasonal timing of breeding. Yet the quest for proximate environmental factors is especially intriguing in tropical birds, in light of the low-amplitude seasonality in their environment.
In this paper, we focus on three questions concerning the proximate regulation of breeding in tropical birds: (1) What environmental changes are tropical rainforest birds exposed to in the rainforest understory? (2) What breeding strategy do tropical rainforest birds use to adjust to environmental fluctuations in their environment? (3) What stimuli do these birds use to optimise reproduction in a fluctuating environment?
We chose a small neotropical bird from the understory of a moist forest in Central America as a study species. To answer the first question, we monitored seasonal changes in environmental parameters at our study site. We approached the second question by measuring gonad sizes in free-living birds; in order to distinguish between opportunistic and regular seasonal breeding. For the third question, we correlated seasonal changes in environmental conditions with the breeding pattern shown by these birds. More importantly, we experimentally tested the effectiveness of single environmental factors that could potentially act as cues for the birds in the wild.
In the following paragraphs, we first briefly review the relevant information on the proximate regulation of avian reproduction. Next, we summarise our results of a two-year field and laboratory study. In a final section we discuss the generality of our results for tropical organisms and highlight open questions and future research directions.
Proximate regulation of seasonal breeding in birds
Breeding strategies
Most birds breeding in temperate zone habitats show distinct seasonal changes in gonadal development (summaries in, e.g., Murton & Westwood 1977; Farner 1985; Wingfield & Farner 1993). Their slight year-to-year variability in the timing of gonadal recrudescence in spring and gonadal regression in summer (or autumn), as well as their initiation of reproductive activity well before the onset of the actual breeding season, characterises most temperate zone birds as regular seasonal breeders. Regular seasonal breeding can be interpreted as an adaptation to the pronounced seasonality of higher latitudes in which the occurrence of seasons favourable for breeding and non-breeding is highly predictable (e.g., Wingfield et al. 1992, 1993).
Opportunistic breeding is often regarded as the opposite of regular seasonal breeding, and as a strategy only rarely found among temperate zone birds. Opportunistic breeders are thought to lack regular seasonal cycles in gonadal development but instead maintain active or near active gonads throughout most of the year (Farner & Serventy 1960; Serventy 1971; Sossinka 1980). A prominent example for a temperate zone opportunist is the Red Crossbill Loxia curvirostra, which can be found breeding at any month of the year (Newton 1973; Benkman 1990). However, physiological studies of the Red Crossbill revealed that they do undergo seasonal changes in gonadal development (Berthold & Gwinner 1978; Hahn 1995). From more recent studies on the Red Crossbill by Hahn (1995), and the subtropical Zebra Finch Taeniopygia guttata by Zann (1996), it has become apparent that these prime examples of opportunistic breeding are not so fundamentally different from seasonal breeders (see Hahn et al. 1997). In fact, it is possible that both strategies are based on similar physiological mechanisms that regulate gonadal development, but differ in the degree to which they respond to the different environmental cues such as photoperiod and food (e.g., Hahn et al. 1997).
Proximate environmental factors that regulate reproduction in birds
Wingfield and co-workers (1992, 1993) have provided a framework to characterise the different types of environmental factors according to the information they contain. Here, we will concentrate on two categories of stimuli that are primarily responsible for the initiation and termination of reproductive activity (Wingfield 1980, 1983).
(1) Initial predictive factors provide reliable long-term information and regulate the transition between phases of reproductive activity and inactivity. The most important initial predictive factor is photoperiod, which relays long-term information about environmental conditions. (2) Supplementary factors supply short-term information about immediate environmental conditions and thereby help fine-tuning reproductive activity to local circumstances. Supplementary factors such as food, temperature, water, and nest sites may have both stimulatory and inhibitory effects (Immelmann 1973; Murton & Westwood 1977; Wingfield 1980; Wingfield et al. 1992, 1993; Ball 1993).
In most birds breeding in the temperate zones, photoperiod acts as the main initial predictive information for the initiation and termination of reproductive activity. Photoperiod can either act alone, 'driving' the seasonal cycles in gonadal development or it can synchronise an endogenous annual timing system which in turn controls reproductive activity (Murton & Westwood 1977; Farner & Follett 1979; Farner & Gwinner 1980; Follett 1984; Gwinner 1986; Wingfield & Kenagy 1991; Wingfield & Farner 1993).
Breeding strategies and environmental factors are interrelated with each other: a particular breeding strategy requires a certain set of environmental cues for its timing. For example, seasonal breeders might use initial predictive factors such as photoperiod as main predictors of the coming breeding season and therefore are able to physiologically initiate reproduction in advance of favourable environmental conditions (Murton & Westwood 1977; Farner & Follett 1979; Farner & Gwinner 1980; Follett 1984; Gwinner 1986; Wingfield & Kenagy 1991; Wingfield & Farner 1993). By doing so, they attain reproductive capacity before environmental conditions improve. In addition to initial predictive information, seasonal breeders use supplementary factors to evaluate current environmental conditions (Wingfield 1980, 1983). In contrast, opportunistic breeders rely mainly on supplementary information (Lofts & Murton 1968; Serventy 1971; Immelmann 1971, 1973). An adaptive explanation for this could be that they have no access to initial predictive factors (as has been proposed for many tropical birds because the changes in photoperiod are too small to be measured). Alternatively, initial predictive information is not reliably coupled to the occurrence of favourable environmental conditions. This seems to be the case when food availability is erratic and can not be predicted by the regular changes in photoperiod.
PROXIMATE REGULATION OF BREEDING IN TROPICAL BIRDS: A CASE STUDY ON SPOTTED ANTBIRDS
The breeding strategy of Spotted Antbirds
In the course of a two-year field study (1996-1997) we have investigated the breeding strategy of individual free-living Spotted Antbirds in a lowland moist forest in Soberanía National Park, Republic of Panama (9° N 79° W; Hau et al. 1998; Wikelski et al., unpubl.). We chose the Spotted Antbird as a study species, firstly, because the Antbird family (Thamnophilidae) presumably has a purely tropical phylogenetic history (Sibley & Monroe 1990). Secondly, Spotted Antbirds live in the understory of the forest, where climatic fluctuations are likely to be smaller than in any other terrestrial habitat.
Spotted Antbirds are small suboscine passerines (average body mass: 17 g; Hau et al. 1998) which defend a territory of about 4 ha. size year-round as a pair (Willis 1972; Sieving 1992; Robinson 1998). They are insectivorous and forage facultatively at army ant swarms. During the breeding season, the female lays two eggs in a cup-shaped nest in the forest understory and both parents incubate the eggs and care for the young (Willis 1972, pers. obs.).
We determined reproductive activity of Spotted Antbirds using physiological measures, since observational methods about reproductive, nesting and parental behaviour, or the occurrence of a brood patch do not allow us to unequivocally distinguish opportunists from seasonal breeders. We recorded the reproductive state of individuals repeatedly because population data could obscure the pattern shown by individual birds. We assessed gonad sizes of free-living birds by means of unilateral laparotomy (Wingfield & Farner 1976), which we conducted in the field under anaesthesia (Hau et al. 1998). Prior to each laparotomy, we took a small blood sample for subsequent determination of the titre of circulating reproductive hormones (Wikelski et al. 1999).
Our data confirm earlier observations (Willis 1972; Sieving 1992) that Spotted Antbirds have an extended breeding season of about 5-6 month and breed within the single rainy season in Panama from about May to October. We obtained conclusive evidence that Spotted Antbirds are regular seasonal breeders. When we examined the gonadal states of the birds we were surprised to find that individual Spotted Antbirds as well as the whole population showed regular cycles in gonadal development (Fig. 2A). Both males and females had tiny, regressed gonads from about November to February (end of the rainy season and the first two month of the dry season), increased gonads during the mid dry season (March-April) and had already fully developed gonads at the end of the dry season (beginning of May), before the rainy season started (Fig. 2; Hau et al. 1998; Wikelski et al., unpubl.). The seasonal pattern in gonadal development was similar in both years of our study and birds always initiated gonadal development well before the onset of the actual breeding season. Another unexpected finding was the close synchronisation of reproductive activity within the population. This corroborates the interpretation that the birds breed on a regular seasonal basis.
Apparently, seasonality in the forest in central Panama is pronounced and regular enough to make seasonal breeding an advantageous option for Spotted Antbirds. What environmental cues do Spotted Antbirds use to initiate reproductive activity on a regular seasonal basis? Do they have access to initial predictive information even in the forest understory in the deep tropics?
Environmental seasonality in the understory of a tropical moist forest in central Panama
Climatic records are available from many tropical areas. However, weather stations are usually located at exposed sites and may not reflect environmental conditions inside the forest. We therefore continuously monitored the changes in photoperiod, solar radiation, standard operative temperature (Bakken 1992), and rainfall in the understory of our study site (Wikelski et al., unpubl.).
Daylength (photoperiod) varied seasonally between 11.9 and 13.0 hours (Fig. 2B). Thus, the maximal seasonal change in photoperiod at this site was about one hour. Precipitation showed a strong seasonal pattern (Fig. 2C). A pronounced dry season with less than 100 mm rain per month is typical for Panama and lasts on average from late December to end of April (Leigh et al. 1996). The dry season is followed by a single wet season, which is characterised by an average monthly precipitation of 300 mm (Leigh et al. 1996). In 1996, the dry season was unusually wet with more than 300 mm rain in January. Moreover, in 1996 the rainy season began early (7 May), whereas in 1997 (the year in which the 1998 El Nino started) the dry season was prolonged and the wet season did not start before 7 June. Temperature showed no clear seasonal pattern but generally increased during the dry season and decreased at the beginning of the wet season. Solar radiation exhibited an inverse pattern to rainfall, with lower solar radiation during the rainy season (due to cloud cover) and higher radiation during the dry season.
What environmental factors provide temporal information to Spotted Antbirds?
We used cross-correlational statistics as a diagnostic tool to examine whether there were any associations between the five environmental factors and the gonadal development of male Spotted Antbirds (Wikelski et al., unpubl.). We only included data on male gonadal states in this analysis because testis sizes were easier to quantify. However, since the seasonal patterns in gonadal development were very similar in males and females, the results are probably also valid for females.
There was a strong and significant correlation of testis size with photoperiod (see also Fig. 2). The seasonal increase in photoperiod preceded the increase in testis size by about one month, suggesting that photoperiod could be used by Spotted Antbirds as initial predictive information. We also obtained a significant cross-correlation between photoperiod and rainfall, with the photoperiod cycle preceding the rainfall cycle by 3 months. This supports the idea that photoperiod might be a long-term predictor of the rainy (= breeding) season. Testis size also correlated significantly with rainfall, with testicular development preceding increased rainfall by about 1.5 months.
This analysis generated two hypotheses: firstly, photoperiod could provide initial predictive information for Spotted Antbirds and secondly, some stimuli associated with rainfall could serve as supplementary information.
Can Spotted Antbirds measure the changes in the tropical photoperiod?
From a temperate zone perspective, the seasonal changes in the tropical photoperiod are very small. Therefore, it has been suggested that tropical birds could not use the slight changes in photoperiod at tropical latitudes as a cue (e.g., Voous 1950; Miller 1959, 1965). However, this had not been experimentally tested. Instead, many authors have exposed tropical birds to the photoperiodic changes that occur in mid or high latitudes (e.g., Rollo & Domm 1943; Miller 1959, 1965; Wolfson & Winchester 1959; Disney et al. 1961; Epple et al. 1972; Chandola & Chakravorty 1982; Gwinner & Dittami 1985; Tewary & Dixit 1986). The surprising results were that many tropical birds are indeed capable of responding to photoperiod, although they were not supposed to ever experience any measurable photoperiodic changes in their environment.
We decided to experimentally test the sensitivity of Spotted Antbirds to photoperiod by exposing them to photoperiodic changes they encounter in nature (Hau et al. 1998). We set up two groups of birds: one group remained on a photoperiod of 12 hours light per day, the other was photostimulated with an abrupt one-hour increase in photoperiod (thus received 13 h of light per day). All birds had small, regressed gonads at the beginning of the experiment. As early as four weeks later, the gonad sizes of photostimulated males and females had increased strongly and significantly over those of control birds (Hau et al. 1998; Fig. 3 A,B). When the experiment was terminated after 7 weeks, testis volumes of males had increased 9-fold and follicle sizes of females had increased more than threefold over those of control birds. Song activity showed a 6-fold increase in the photostimulated group while the control group remained on a low level (Fig. 3C). These results demonstrated that Spotted Antbirds can measure a one-hour difference in photoperiod.
To find out whether Spotted Antbirds can measure even smaller differences in daylength, we exposed half of the former control group to an increase in photoperiod of only 17 min. (Hau et al. 1998). The other half of the control group remained under 12 h of daylight. The increase in photoperiod by only 17 min. was sufficient to stimulate significant gonadal growth, whereas control birds showed no change (Fig. 4). Song activity also increased strongly in the photostimulated group (Fig. 4 C).
These two experiments indicate that Spotted Antbirds possess both the sensitivity and the physiological mechanism to exploit the small changes in the tropical photoperiod as a long-term seasonal cue. Such a photoperiodic sensitivity is as yet unique for birds, but it is entirely possible that other tropical birds or even temperate zone birds are similarly sensitive to small-scale photoperiodic changes (Hau et al. 1998; see also discussion below). Although obtained in a different context, experiments on African Stonechats Saxicola torquata axillaris (Gwinner 1996), some temperate zone Fringillids (Wever 1967), House Sparrows Passer domesticus (Dawson 1991), and European Starlings Sturnus vulgaris (Schwab 1971; Hamner 1971) indicated a remarkable sensitivity to slight changes in photoperiod. Clearly, more experiments are needed to find out whether a high sensitivity to small-scale changes in photoperiod is characteristic only for tropical birds or whether temperate zone birds also possess this capacity.
The photoperiodic sensitivity of Spotted Antbirds opens many new questions. We now need to investigate whether Spotted Antbirds can adjust reproductive activity to the slight day-to-day changes in the natural photoperiod. Furthermore, we have to delimit the smallest photoperiodic difference that these birds can still measure. By doing so we could predict whether Spotted Antbirds that live right at the equator in western Ecuador (Hilty & Brown 1986) could also use photoperiodic cues as initial predictive information. In addition, it would be important to investigate the physiological mechanism that underlies the remarkable photoperiodic sensitivity in Spotted Antbirds. Do these birds make use of similar photoperiodic and circadian processes for the detection of daylength changes as temperate zone birds? Do Spotted Antbirds become photorefractory at a certain time of the year? By answering these type of questions we may also eventually find out where photoperiodicity has evolved: in the tropics and or in the temperate zones.
Do Spotted Antbirds use food stimuli as supplementary information?
Many tropical habitats exhibit a considerable year-to-year variability in the occurrence of seasons (e.g., in onset, end, duration, and severity of a particular season). A year-to-year variability, e.g., in the onset of the rainy season has been observed in central Panama (Windsor 1990; Leigh et al. 1996) and was also evident in the different durations of the dry seasons of 1996 and 1997 (see Fig. 2C). Thus, since Spotted Antbirds only breed during the rainy season, we expected them to incorporate information about short-term environmental conditions into their reproductive decisions.
Gonads of free-living male and female Spotted Antbirds started to grow at about the same time in the wet year of 1996 as in the dry year of 1997, but the rate of gonadal growth was slower in spring 1997 (coinciding with the prolonged dry season, Wikelski et al., unpubl.). This result suggested that Spotted Antbirds access short-term information about local environmental conditions. Two potential factors could be used as cues: rainfall per se, or some stimuli associated with rain. In the Panamanian forest, rainfall strongly influences the pattern of insect availability (Wolda 1978; Levings & Windsor 1996; Smythe 1996; Wolda 1996). Since food availability might also be an important ultimate factor causing seasonal breeding in tropical birds, we decided to experimentally test whether food stimuli can affect reproductive activity of Spotted Antbirds (Hau et al., unpubl.).
Two groups of Spotted Antbirds under a natural photoperiod were fed a regular diet of freshly made egg-food (following Gwinner et al. 1987) plus live mealworms (Tenebrio larvae). After an initial acclimatisation period, the food of one group was restricted in quantity such that the birds maintained their body mass but did not gain weight. The other group of birds had ad libitum access to the same food and additionally received live crickets Acheta domestica. As a consequence of the difference in food availability, birds from the 'food-stimulated' group increased their body mass by about 20% over their initial mass, whereas birds from the food-restricted group showed no change. Furthermore, song activity in the food-stimulated group increased threefold, whereas song activity in the control group did not increase. Most importantly, the bird adjusted gonadal growth to levels of food availability. Already after three weeks gonad sizes of food-stimulated birds had increased significantly over their initial sizes, whereas gonads of control birds remained unaffected (Hau et al., unpubl.).
These results strongly support the hypothesis that Spotted Antbirds use food-related cues as short-term environmental information. So far, we can only speculate about the specific nature of these cues. It could have been nutritional cues associated with cricket availability, food quantity, or some cue coming from the crickets per se. We therefore conducted a follow-up experiment in which the nutritional value of the food similar was kept as similar as possible. To do this, we offered either live crickets or dead crickets to captive male Spotted Antbirds and measured changes in song activity. Spotted Antbirds ate live and dead crickets alike but sang significantly less with access to only dead crickets (Hau et al., unpubl.). Hence, it appeared that some behavioural cue of catching or manipulating live crickets provided a positive stimulus to the birds. Although we can not exclude the possibility that some dietary compounds such as proteins or vitamins were missing in dead crickets, we consider nutritional stimuli an unlikely explanation for these results.
Taken together, the current data provide us with the following picture (see Fig. 5): Spotted Antbirds use photoperiod as a long-term cue to regulate reproductive activity. Short-term cues such as food availability modulate the rate of gonadal development and enable a fine-tuning of reproductive activity with local environmental conditions. However, some questions still remain open: Does food only act as a supplementary cue in Spotted Antbirds, i.e., can it modulate reproductive activity only once reproduction has been initiated by initial predictive cues? Are there other supplementary factors that Spotted Antbirds use to gather information about environmental conditions? What stimuli do live crickets provide to Spotted Antbirds?
DISCUSSION
How general is seasonal breeding among tropical land birds?
Many studies have reported seasonal nesting behaviour in tropical birds (here we only include studies on land birds conducted at latitudes within 15° of the equator, e.g., Moreau 1936; 1950; Skutch 1950; Voous 1950; Serventy & Marshall 1957; Fogden 1972; Snow 1976; Sinclair 1978; Fogden & Fogden 1979; Brown & Britton 1980; Bell 1982; Tye 1991; Chapman 1995; Komdeur 1996; Young 1996). However, since both opportunistic and seasonal breeders could potentially nest seasonally, the two strategies can only be distinguished on the basis of the associated gonadal patterns. A few studies investigated gonadal activity in tropical birds (Baker et al. 1940; Moreau et al. 1947; Miller 1955, 1959; Ward & Poh 1968; Ward 1969; Wolf 1969; Davis 1971; Frith et al. 1974; Davies 1977; Fogden & Fogden 1979; Stiles 1980; Wilkinson 1983; Dittami & Gwinner 1985; Dittami 1986, 1987; Dittami & Knauer 1986; Wingfield et al. 1991). However, most studies covered only one year, some even less. This precludes an analysis of the repeatability of the gonadal cycles between years and an evaluation of the effect of specific ecological and climatic conditions in a particular year. In addition, hardly any data are available on gonadal cycles of individual birds. Furthermore and maybe most importantly, all studies dealt with species inhabiting open, disturbed, or secondary habitats - with the exception of Stiles's (1980) study on Costa Rican hummingbirds. It could be argued that open-habitat species are exposed to other, and possibly more pronounced, ecological and climatic conditions than rainforest-interior birds.
Our data showed that even Spotted Antbirds, living in the rainforest understory under minor climatic fluctuations, were regular seasonal breeders. The seasonal pattern in gonadal development was clear and repeatable between two subsequent years, although the rate of gonadal development was affected by climatic conditions (i.e. the severity of the preceding dry season). Taken together with all previous studies, it seems likely that seasonal breeding in tropical areas is the rule rather than the exception. This notion is also supported by our recent study on the seasonal changes in gonad sizes of six additional bird species from central Panama (Wikelski, Hau, Robinson & Wingfield, unpubl.; see also Wikelski et al. 1999).
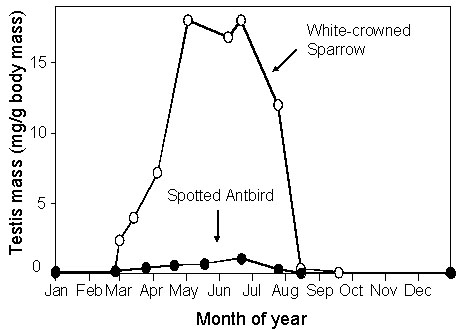
Hence, although there obviously is a substantial difference in ecological and climatic conditions between most temperate zone and tropical habitats (Fig. 1), the breeding strategies of the birds are remarkably similar (see Fig. 6, although there seem to exist clear differences in other aspects, such as maximal testis size). What conclusion can we draw from this? Apparently, the seasonal pattern in environmental conditions in many tropical areas is regular (or ‘predictable’, Colwell 1974; Wingfield et al. 1992) enough to render seasonal breeding an advantageous option. Our calculations showed that the predictability of their environment for Spotted Antbirds is similar to that of White-Crowned Sparrows (Zonotrichia leucophrys nuttalli) breeding in central California (Wikelski et al., unpubl.). In an environment with a high predictability of environmental conditions, it could be beneficial for birds to regress their gonads during the non-breeding season. By responding to long-term environmental cues they can then grow their gonads again in preparation of the coming breeding season.
Why do tropical birds breed seasonally?
One reason for a seasonal breeding strategy of Spotted Antbirds could be that the maintenance of active gonads is energetically costly (but see e.g., Kendeigh 1941; King 1973). Indeed, Spotted Antbirds had regressed gonads during the late rainy season and the beginning of the dry season, when food availability in the forest understory was minimal (Leigh et al. 1996). Costs other than energetic ones are also conceivable (see Ketterson et al. 1996, Wingfield et al. 1997). Gonadal activity, possibly mediated through hormones, could inhibit restorative processes after reproduction such as moult. In fact, the gonadal hormone testosterone has been shown to compromise moult in temperate zone passerines (Schleussner et al. 1985). Testosterone has also been implied in decreasing immune responses in birds (Folstad & Karter 1992; Zuk 1994; Hillgarth & Wingfield 1997). Finally, a regression of gonads during the late wet season could also prevent Spotted Antbirds from being mislead by short-term ameliorations in environmental conditions at a time of year at which a brood could not be successfully raised (which could pose detrimental short-term and long-term fitness effects, e.g., Stearns 1992). As a last possibility, seasonal breeding could simply be a phylogenetic relic (Owens & Bennett 1995).
Photoperiodism in tropical birds: relic or adaptive capability?
Many tropical birds that have been exposed to photoperiodic changes characteristic of temperate zone areas were able to respond physiologically by growing their gonads (e.g., Rollo & Domm 1943; Miller 1959, 1965; Wolfson & Winchester 1959; Disney et al. 1961; Epple et al. 1972; Chandola & Chakravorty 1982; Gwinner & Dittami 1985; Tewary & Dixit 1986). This capability has been puzzling, since these birds would never encounter such daylength differences in their natural habitat. Gwinner & Dittami (1985) proposed four possible reasons for photoperiodic responses of tropical birds: (1) to measure the slight seasonal changes in the tropical photoperiod, or in the twilight duration; (2) a 'side-effect' of a light-sensitive system that is not primarily designed to measure photoperiod; (3) a result of gene flow from higher latitude populations; (4) a phylogenetic relic.
For Spotted Antbirds, the latter two possibilities seem unlikely. We intentionally chose a study species whose distribution is restricted to the tropics and which presumably has also evolved in the tropics (Sibley & Monroe 1990). In contrast, our present data suggest that photoperiodic reactions may be adaptive for tropical birds, thus, we favour the first explanation of Gwinner & Dittami (1985). We would like to emphasise here that although seasonality in tropical habitats is relatively low (at least when compared to temperate zone habitats), seasonal fluctuations in the tropics might nevertheless have important consequences for reproductive success.
Use of supplementary information by tropical birds
Although many studies on reproduction of tropical birds have suggested that food acts as an important proximate factor, experimental evidence for this notion was still lacking. Our field and experimental data imply that Spotted Antbirds use food stimuli as supplementary cues. A fine-tuning with local environmental conditions could be quite important in the tropics, where a year-to-year variation in the occurrence of the seasons is often more pronounced than in many temperate zone habitats. In fact, it is possible that tropical birds have evolved a higher sensitivity to supplementary factors in order to cope with the seasonal variability in their environment. Clearly, more experiments are needed to characterise the response of tropical birds to short-term environmental information.
As a final remark we would like to emphasise that in order to understand how the majority of avian species cope with their environment, we need to collect more data on tropical birds. By making use of concepts like the ‘environmental predictability’ (Colwell 1974; Wingfield et al. 1992) we can quantify different environments and directly compare organismal responses between habitats. Furthermore, a thorough investigation of opportunistic breeders is urgently needed (see also Hahn et al. 1997). This will be the only way to find out which environments favour opportunistic breeding, how timing of breeding in general has evolved, which stimuli opportunistic breeders use to regulate reproduction, and how opportunistic breeding is physiologically realised.
ACKNOWLEDGEMENTS
We would like to thank many people for their support during this study, especially E. Gwinner, D.W. Robinson, T.R. Robinson, A.S. Rand, N.G. Smith, R. Urriola, the staff at the Smithsonian Tropical Research Institute in Panama, Armstrong Cricket Farm (West Monroe, LA), J. Habersetzer and L. Erckman. IN.RE.NA.RE kindly permitted our work in Soberanía National Park, Panama. E. Gwinner and A. Scheuerlein made valuable comments on a previous version of the manuscript. We appreciate the financial support of the Deutsche Forschungsgemeinschaft, the Deutsche Ornithologen-Gesellschaft, the Smithsonian Tropical Research Institute, and the Max-Planck-Institut für Verhaltensphysiologie (M.H.); the Alexander-von-Humboldt Gesellschaft, the Smithsonian Tropical Research Institute and the Max-Planck-Institut für Verhaltensphysiologie (M.W.); the National Science Foundation and the John Simon Guggenheim Foundation (J.C.W).
REFERENCES
Baker, J.R. 1938. The evolution of breeding seasons. In: De Beer, G.R. (ed.) Evolution: Essays on aspects of evolutionary biology. Oxford; Clarendon Press: 161-177.
Baker, J.R., Marshall, A.J. & Harrison, T.H. 1940. The seasons in a tropical rain-forest (New Hebrides). Part 5. Birds (Pachycephala). Journal of the Linnean Society London 41: 50-70.
Ball, G.F. 1993. The neural integration of environmental information by seasonally breeding birds. American Zoologist 33:185-199.
Bakken, G.S. 1992. Measurement and application of operative and standard operative temperatures in ecology. American Zoologist 32: 194-216.
Bell, H.L. 1982. A bird community of lowland rainforest in New Guinea. 2. Seasonality. Emu 82: 65-74.
Benkman, C.W. 1990. Foraging rates and the timing of crossbill reproduction. Auk 107: 376-386.
Berthold, P. & Gwinner, E. 1978. Jahresperiodik der Gonadengröße beim Fichtenkreuzschnabel (Loxia curvirostra). Journal für Ornithologie 119: 338-339.
Boag, P.T. & Grant, P.R. 1984. Darwin's finches (Geospiza) on Isla Daphne Major, Galápagos: breeding and feeding ecology in a climatically variable environment. Ecological Monographs 54: 463-489.
Brown, L.H. & Britton, P.L. 1980. The breeding seasons of East African birds. Nairobi; The East African Natural History Society: 164pp.
Chandola, A. & Chakravorty, K. 1982. Termination of seasonal breeding in the photoperiodic weaver bird. Journal of Experimental Zoology 222: 169-172.
Chapman, A. 1995. Breeding and moult of four birds species in tropical West Africa. Tropical Zoology 8: 227-238.
Cockrem, J.F. 1995. Timing of seasonal breeding in birds, with particular reference to New Zealand birds. Reproduction, Fertility & Development 7: 1-19.
Colwell, R.K. 1974. Predictability, constancy, and contingency of periodic phenomena. Ecology 55: 1148-1153.
Davies, S.J.J.F. 1977. The timing of breeding by the zebra finch Teniopygia castanotis at Mileura, Western Australia. Ibis 199: 369-372.
Davis, J. 1971. Breeding and molt schedules of the rufous-collared sparrow in coastal Peru. Condor 73: 127-146.
Dawson, A. 1991. Photoperiodic control of testicular regression and moult in male House Sparrows Passer domesticus. Ibis 133: 312-316.
Disney, H.J. de S., Lofts, B. & Marshall, A.J. 1961. An experimental study of the internal rhythm of reproduction in the red-billed dioch Quelea quelea by means of photostimulation, with a note on melanism induced in captivity. Proceedings of the Zoological Society London 136: 123-129.
Dittami, J.P. 1986. Seasonal reproduction, moult and their endocrine correlates in two Ploceidae species. Journal of Comparative Physiology B 156: 641-647.
Dittami, J.P. 1987. A comparison of breeding and moult cycles and life history strategies in two tropical starling species: the blue-eared glossy starling Lamprotornis chalybaeus and Rüppell's long-tailed glossy starling L. purpuropterus. Ibis 129: 69-85.
Dittami, J.P. & Gwinner, E. 1985. Annual cycles in the African stonechat Saxicola torquata axillaris and their relationship to environmental factors. Journal of Zoology, London 207: 357-370.
Dittami, J. & Knauer, B. 1986. Seasonal organization of breeding and molting in the Fiscal Shrike (Lanius collaris). Journal für Ornithologie 127: 79-84.
Drent, R.H. & Daan, S. 1980. The prudent parent: energetic adjustments in avian breeding. Ardea 68: 225-252.
Epple, A., Orians, G.H., Farner, D.S., Lewis, R.A. 1972. The photoperiodic testicular response of a tropical finch, Zonotrichia capensis costaricensis. Condor 74: 1-4.
Farner, D.S. 1985. Annual rhythms. Annual Review of Physiology 47: 65-82.
Farner, D.S. & Follett, B.K. 1979. Reproductive periodicity in birds. In: Barrington, J.W. (ed.) Hormones and Evolution. New York; Academic Press: 829-872.
Farner, D.S. & Gwinner, E. 1980. Photoperiodicity, circannual and reproductive cycles. In: Epple, A. & Stetson, M.H. (eds) Avian Endocrinology. New York; Academic Press: 331-366.
Farner, D.S. & Lewis, R.A. 1971. Photoperiodism and reproductive cycles in birds. In: Giese, A.C. (ed.) Photophysiology: 325-370.
Farner, D.S. & Serventy D.L. 1960. The timing of reproduction in birds in the arid regions of Australia. Anatomical Records 137: 354.
Fogden, M.P.L. 1972. The seasonality and population dynamics of equatorial forest birds in Sarawak. Ibis 114: 307-343.
Fogden, M.P.L. & Fodgen, P.M. 1979. The role of fat and protein reserves in the annual cycle of the Grey-backed camaroptera in Uganda (Aves: Sylvidae). Journal of Zoology, London 189: 233-258.
Follett, B.K. 1984. Birds. In: Lamming, G.E. (ed) Marshall's physiology of reproduction, Vol. 1. Edinburgh; Churchill Livingston: 283-350.
Folstad, I. & Karter, A.J. 1992. Parasites, bright males, and the immunocompetence handicap. American Naturalist 139: 603-622.
Frith, H.J., Braithwaite, L.W. & Wolfe, T.O. 1974. Sexual cycles of pigeons in a tropical environment. Australian Wildlife Research 1: 117-128.
Grant, R.B. 1996. Pollen digestion by Darwin's finches and its importance for early breeding. Ecology 77: 489-499.
Gwinner, E. 1986. Circannual rhythms. Heidelberg; Springer-Verlag: 154pp.
Gwinner E. 1996. Circadian and circannual programmes in avian migration. Journal of Experiment Biology 199: 39-48.
Gwinner, E. & Dittami J. 1985. Photoperiodic responses in temperate zone and equatorial stonechats: a contribution to the problem of photoperiodism in tropical organisms. In: Follett, B.K., Ishii, S. & Chandola, A. (eds) The Endocrine System and the Environment. Tokyo/Berlin; Japan Science Society Press/Springer-Verlag: 279-294.
Gwinner, E., Neusser, V., Engl, D., Schmidl, D. & Bals, L. 1987. Haltung, Zucht und Eiaufzucht afrikanischer und europäischer Schwarzkehlchen Saxicola torquata. Gefiederte Welt 111: 118-120 and 145-147.
Hahn, T.P. 1995. Integration of photoperiodic and food cues to time changes in reproductive physiology by an opportunistic breeder, the Red Crossbill Loxia curvirostra (Aves: Carduelinae). Journal of Experimental Zoology 272: 213-226.
Hahn, T.P., Boswell, T., Wingfield, J.C. & Ball, G.F. 1997. Temporal flexibility in avian reproduction. In: Nolan, V., jr., Ketterson, E.D. & Thompson, C. F. (eds) Current Ornithology. New York, London; Plenum Press: 39-80.
Hamner, W.M. 1971. On seeking an alternative to the endogenous reproductive rhythm hypothesis in birds. In: Menaker, M. (ed) Biochonometry.Washington, D.C.; National Academy of Sciences: 448-461.
Hau, M., Wikelski, M. & Wingfield, J.C. 1998. A neotropical forest bird can measure the slight changes in tropical photoperiod. Proceedings of the Royal Society London B 265: 89-95.
Hillgarth, N. & Wingfield, J.C. 1997. Parasite-mediated sexual selection: endocrine aspects. In: Clayton, D.H. & Moore, J. (eds) Host-parasite evolution: General principles and avian models. New York; Oxford University Press: 78-104.
Hilty, S.L. & Brown, W.L. 1986. Birds of Colombia. Princeton; Princeton University Press.
Immelmann, K. 1971. Ecological aspects of periodic reproduction. In: Farner, D.S. & King, J.R. (eds) Avian Biology. New York; Academic Press: 341-389.
Immelmann, K. 1973. Role of the environment in reproduction as source of ‘predictive’ information. In: Farner, D.S. (ed) Breeding biology of birds. Washington, D.C.; National Academy of Sciences: 121-157.
Jones, P.J. & Ward, P. 1976. The level of reserve protein as the proximate factor controlling the timing of breeding and clutch size in the red-billed quelea. Ibis 118: 547-574.
Kendeigh, S.C. 1941. Length of day and energy requirements for gonad development and egg-laying in birds. Ecology 22: 237-248.
Ketterson, E.D., Nolan, V. Jr., Cawthorn, M.J., Parker, P.G. & Ziegenfus, C. 1996. Phenotypic engineering: using hormones to explore the mechanistic and functional bases of phenotypic variation in nature. Ibis 138: 70-86.
King, J.R. 1973. Energetics of reproduction in birds. In: Farner, D.S. (ed.) Breeding biology of birds. Washington, D.C.; National Academy of Sciences: 78-120.
Komdeur, J. 1996. Seasonal timing of reproduction in a tropical bird, the Seychelles Warbler: a field experiment using translocation. Journal of Biological Rhythms 11: 333-346.
Lack, D. 1968. Ecological adaptation for breeding in birds. London; Methuen: 409pp.
Leigh, E.G., Rand, A.S. & Windsor, D.M. 1996. The ecology of a tropical forest: seasonal rhythms and long-term changes (2nd ed.). Washington, D.C; Smithsonian Institution Press: 503pp.
Levings, S.C. & Windsor, D.M. 1982. Seasonal and annual variation in litter arthropod populations. In: Leigh, E.G., Rand, A.S. & Windsor, D.M. (eds) The ecology of a tropical rainforest. Washington, D.C; Smithsonian Institution Press: 355-387.
Lofts, B. & Murton, R.K. 1968. Photoperiodic and physiological adaptations regulating avian breeding cycles and their ecological significance. Journal of Zoology, London 150: 249-272.
Martin, T.E. 1987. Food as a limit on breeding birds: a life-history perspective. Annual Review of Ecology and Systematics 18: 453-487.
Miller, A.H. 1955. Breeding cycles in a constant equatorial environment in Colombia, South America. Acta XI. Congressus Internationalis Ornithologici: 491-503.
Miller, A.H. 1959. Reproductive cycles in an equatorial sparrow. Condor 61: 344-347.
Miller, A.H. 1961. Adaptation of breeding schedule to latitude. Acta XII. Congressus Internationalis Ornithologici 12: 513-522.
Miller, A.H. 1965. Capacity for photoperiodic response and endogenous factors in the reproductive cyles of an equatorial sparrow. Proceedings of the National Academy of Sciences USA 54: 97-101.
Millington, S.J. & Grant, P.R. 1984. The breeding ecology of the Cactus finch Geospiza scandens on isla Daphne major, Galapagos. Ardea 72: 177-188.
Moreau, R.E. 1936. Breeding seasons of birds in East African Evergreen Forest. Proceedings of the Zoological Society London 1936: 631-653.
Moreau, R.E. 1950. The breeding seasons of african birds. - 1. Land birds. Ibis 92: 223-267.
Moreau, R.E., Wilk, A.L. & Rowan, W. 1947. The moult and gonad cycles of three species of birds at five degrees south of the equator. Proceedings of the Zoological Society London 117: 345-365.
Morton, E.S. 1971. Nest predation affecting the breeding season of the Clay-colored Robin. Science 181: 920-921.
Murton, R.K. & Westwood, N.J. 1977. Avian breeding cycles. Oxford: Clarendon Press: 594pp.
Nager, R.G. & van Nordwijk, A.J. 1995. Proximate and ultimate aspects of phenotypic plasticity in timing of great tit breeding in a heterogeneous environment. American Naturalist 146: 454-474.
Newton, I. 1973. Finches. Englewood Cliffs, N.J.; Taplinger: 288pp.
Owens, J.P.F. & Bennett, P.M. 1995. Ancient ecological diversification explains life-history variation among living birds. Proceedings of the Royal Society of London B 261: 227-232
Perrins, C.M. 1970. The timing of birds' breeding seasons. Ibis 112: 242-255.
Poulin, B., Lefebvre, G. & McNeil, R. 1992. Tropical avian phenology in relation to abundance and exploitation of food resources. Ecology 73: 2295-2309.
Robinson, D.W. 1998. Community structure, nesting success, and isolation effects in a tropical forest bird community. PhD Thesis, University of Illinois, Urbana-Champaign.
Rollo, M. & Domm, L.V. 1943. Light requirements of the weaver finch. I. Light period and intensity. Auk 60: 357-367.
Schleussner, G., Dittami, J.P. & Gwinner, E. 1985. Testosterone implants affect molt in male European starlings, Sturnus vulgaris. Physiological Zoology 58: 597-604.
Schwab, R.G. 1971. Circannian testicular periodicity in the European starling in the absence of photoperiodic change. In: Menaker, M. (ed) Biochonometry. Washington, D.C.; National Academy of Sciences: 428-447.
Serventy, D.L. 1971. Biology of desert birds. In: Farner, D.S., King, J.R. & Parkes, K.C. (eds) Avian biology. New York; Academic Press: 287-339.
Serventy, D.L. & Marshall, A.J. 1957. Breeding periodicity in Western Australian birds: with an account of unseasonal nestings in 1953 and 1955. Emu 57: 99-126.
Sibley, C.G. & Monroe, B.L., jr. 1990. Taxonomy and distrubution of the birds of the world. New Haven & London; Yale Univeristy Press: 1111pp.
Sieving, K.E. 1992. Nest predation and differential insular extinction among selected forest birds of central Panama. Ecology 73: 2310-2328.
Sinclair, A.R.E. 1978. Factors determining the food supply and breeding season of resident birds and movements of palaearctic migrants. Ibis 120: 480-497.
Skutch, A.F. 1950. The nesting season of central American birds in relation to climate and food supply. Ibis 92: 185-222.
Skutch, A.F. 1966. A breeding census and nesting success in central america. Ibis 108: 1-16.
Smythe, N. 1996. Seasonality of Homoptera on Barro Colorado Island. In: Leigh, E.G.,jr., Rand, A.S. & Windsor, D.M. (eds) The ecology of a tropical rainforest. Washington, D.C; Smithsonian Institution Press: 309-318.
Snow, D.W. 1976. The relationship between climate and annual cycles in the Cotingidae. Ibis 118: 366-401.
Snow, D.W. & Snow, B.K. 1964. Breeding seasons and annual cycles of Trinidad land-birds. Zoologica, New York 49: 1-39.
Sossinka, R. 1980. Ovarian development in an opportunistic breeder, the zebra finch, Poephila guttata castanotis. Journal of Experimental Zoology 211: 225-230.
Stearns, S.C. 1992. The evolution of life histories. Oxford; Oxford University Press: 249pp.
Stiles, G.F. 1980. The annual cycle in a tropical wet forest hummingbird community. Ibis 122: 322-343.
Svensson, E. 1995. Avian reproductive timing. Animal Behaviour 49: 1569-1575.
Tewary, P.D. & Dixit, A.S. 1986. Photoperiodic regulation of reproduction in subtropical female yellow-throated sparrows. Condor 88: 70-73.
Tye, H. 1991. Reversal of breeding season by lowland birds at higher altitudes in western cameroon. Ibis 134: 154-163.
Voous, K.H. 1950. The breeding seasons of birds in Indonesia. Ibis 92: 279-287.
Ward, P. 1969. The annual cycle of the Yellow-vented bulbul Pycnonotus goiavier in a humid equatorial environment. Journal of Zoology 157: 25-45.
Ward, P. & Poh, G.E. 1968. Seasonal breeding in an equatorial population of the Tree sparrow Passer montanus. Ibis 110, 359-363.
Wever, R. 1967. Zum Einfluss der Dämmerung auf die circadiane Periodik. Zeitschrift für vergleichende Physiologie 55: 255-277.
Wikelski, M., Hau, M., Robinson, D.W. & Wingfield, J.C. 1999. Seasonal endocrinology of tropical passerines - a comparative approach. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1224-1241. Johannesburg: BirdLife South Africa.
Wilkinson, R. 1983. Biannual breeding and moult-breeding overlap of the chestnut-bellied starling, Spreo pulcher. Ibis 125: 353-361.
Willis, E.O. 1972. The behavior of Spotted Antbirds. Ornithological Monographs 10: 1-157.
Windsor, D.M. 1990. Climate and moisture variability in a tropical forest: long-term records from Barro Colorado Island, Panamá. Washington, D.C.; Smithsonian Inst. Press: 145pp.
Wingfield, J.C. 1980. Fine temporal adjustment of reproductive functions. In: Epple, A. & Stetson, M.H. (eds) Avian Endocrinology. New York; Academic Press: 367-389.
Wingfield, J.C. 1983. Environmental and endocrine control of avian reproduction: an ecological approach. In: Mikami, S., Homma, K. & Wada, M. (eds) Avian Endocrinology. Tokyo/Berlin; Japan Science Society/Springer-Verlag: 265-288.
Wingfield, J.C. & Farner, D.S. 1976. Avian endocrinology - field investigations and methods. Condor 78: 571-573.
Wingfield, J.C. & Farner, D.S. 1993. Endocrinology of reproduction in wild species. In: Farner, D.S., King, J.R. & Parkes, K.C. (eds) Avian Biology. IX. Academic Press: 163-327.
Wingfield, J.C., Hahn, T.P., Levin, R. & Honey, P. 1992. Environmental predictability and control of gonadal cycles in birds. Journal of Experimental Zoology 261: 214-231.
Wingfield, J.C., Hahn, T.P. & Doak, D. 1993. Integration of environmental factors regulating transitions of physiological state, morphology and behaviour. In: Sharp, P.J. (ed) Avian Endocrinology. Bristol; Journal of Endocrinology: 111-122.
Wingfield, J.C., Hegner, R.E. & Lewis, D.M. 1991. Circulating levels of luteinizing hormone and steroid hormones in relation to social-status in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. Journal of Zoology 225: 43-58.
Wingfield, J.C., Jacobs, J. & Hillgarth, N. 1997. Ecological constraints and the evolution of hormone-behavior interrelationships. In: Carter, S.C., Lederhendler, I.I. & Kirkpatrick, B. (eds) The integrative neurobiology of affiliation. Vol. 807. Washington, D.C.; New York Academy of Sciences: 22-41.
Wingfield, J.C. & Kenagy, G.J. 1991. Natural control of reproduction. In: Pang, P.K.T. & Schreibman, M.P. (eds) Handbook of Comparative Endocrinology. New York; Academic Press: 181-241.
Wolda, H. 1978. Fluctuations in abundance of tropical insects. American Naturalist 112: 1017-1045.
Wolda, H. 1996. Seasonality of Homoptera on Barro Colorado Island. In: Leigh, E.G., jr., Rand, A.S. & Windsor, D.M. (eds) The ecology of a tropical rainforest. Washington, D.C; Smithsonian Institution Press: 319-330.
Wolf, L. 1969. Breeding and molting periods in a Costa Rican population of the Andean sparrow. Condor 71: 212-219.
Wolfson, A. & Winchester, D.P. 1959. Effect of photoperiod on the gonadal cycle in an equatorial bird, Quelea quelea. Nature 184: 1658-1659.
Worthington, A. 1982. Population sizes and breeding rhythms of two species of manakins in relation to food supply. In: Leigh, E.G., jr., Rand, A.S. & Windsor, D.M. (eds) The ecology of a tropical rainforest. Washington, D.C; Smithsonian Institution Press: 213-225.
Wrege, P.H. & Emlen, S.T. 1991. Breeding seasonality and reproductive success of white-fronted bee-eaters in Kenya. Auk 108: 673-687.
Young, B.E. 1994. The effects of food, nest predation and weather on the timing of breeding in tropical house wrens. Condor 96: 341-353.
Zann, R.A. 1996. The zebra finch. New York; Oxford University Press: 335pp.
Zuk, M. 1994. Immunology and the evolution of behavior. In: Real, L.A. (ed) Behavioral mechanisms in evolutionary ecology. Chicago; University of Chicago Press: 354-368.
Fig. 1. Seasonal changes in photoperiod, temperature, and precipitation in New York state (open symbols) and central Panama (closed symbols). N.Y. data were calculated from astronomical charts (for photoperiod) or gathered from climate records (from 1983, J.C. Wingfield, unpubl.), data from Panama were obtained during the present study (see text).

Fig. 2. Schematic representation of (A) seasonal changes in testis size of male Spotted Antbirds, (B) daylength, and (C) rainfall in the understory of our study site in central Panama. Vertical lines indicate the beginning of each year (redrawn from Wikelski et al., unpubl.).
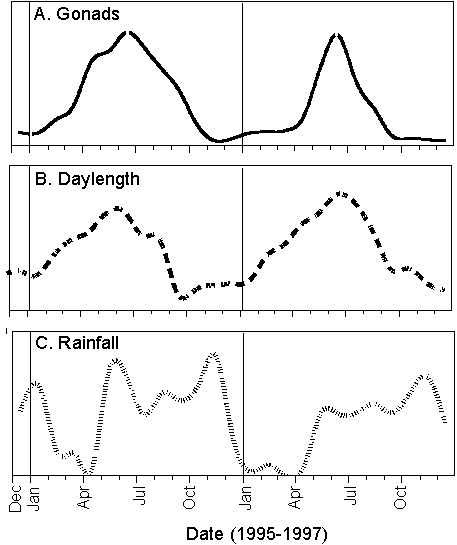
Fig. 3. Changes in (A) testis volume (mean ± S.E.), (B) follicle diameter (mean ± S.E.) and (C) song activity of Spotted Antbirds during the first photostimulation experiment. Open symbols: control group (12 hours light per day), closed symbols: photostimulated group (13 hours light per day). Vertical solid line indicates start of photostimulation (redrawn from Hau et al. 1998).
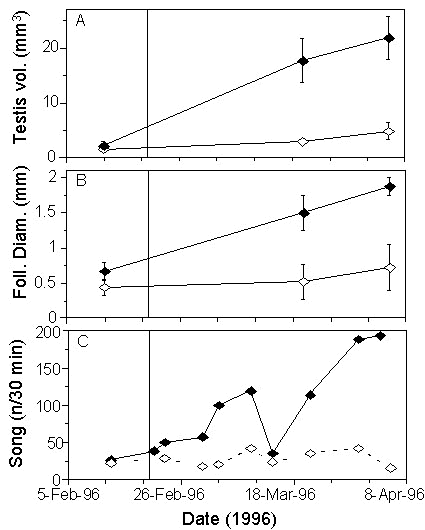
Fig 4. Changes in (A) testis volume, (B) follicle diameter and (C) song activity of individual Spotted Antbirds during the second photostimulation experiment. Open symbols, broken lines: control birds (12 hours light per day), closed symbols, solid lines: photostimulated birds (12 hours 17 minutes light per day, redrawn from Hau et al. 1998).
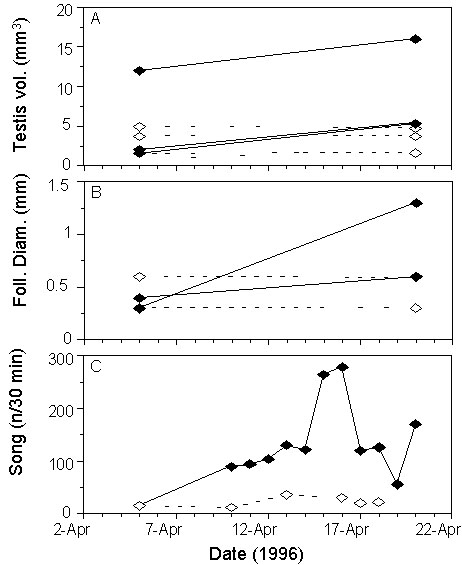
Fig. 5. Schematic summary of the environmental regulation of Spotted Antbird reproductive physiology (gonadal development) and behaviour (song).
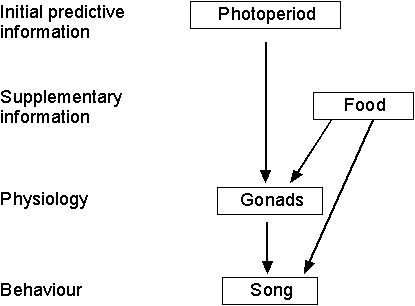
Fig. 6. Comparison of testis masses of Spotted Antbirds (filled symbols, solid line) and White-crowned Sparrows (open symbols, broken line). This comparison may be confounded by phylogeny but nevertheless illustrates that both species employ seasonal breeding strategies.